Abstract
Children weight gain is mostly due to fat-free mass than fat mass, but the changes in body composition dynamics related to child growth can be attributed to the obesity epidemic. We aimed to assess changes in measures of body composition from 6 to 11 years of age according to sex, and to examine whether changes in these measures are associated with sociodemographic characteristics. A longitudinal study using data from the 2004 Pelotas Birth Cohort was conducted, and assessed body composition and fat distribution through measures of BMI, fat mass index, fat-free mass index, and android and gynoid fat mass percentages from DXA. Changes in body fatness were calculated as the difference between measures collected at 6 and 11 years of age, and linear regression models were used to assess changes in body composition according to sociodemographic characteristics. An increase in mean BMI z-score from 6 to 11 years was observed only in boys and obesity prevalence reached one out of four boys and one out of five girls. There was an increase in fat mass percentage, fat mass index and android fat mass, with this effect more accentuated in boys when compared to girls. Maternal BMI was the most consistent factor associated with change in body fatness. Children from mothers with obesity showed larger increases in fat mass percentage, fat mass index and android fat mass. There was an increase in body fatness and a centralisation of body shape, mostly associated with male sex and maternal obesity. These results may indicate an early risk of non-communicable diseases in children from the Pelotas 2004 Birth Cohort.
Similar content being viewed by others
Introduction
Children weight gain is usually based on fat-free mass rather than fat mass, as the proportion of fat mass tends to decline during childhood1,2. Accentuated increases in body fat percentage is only seen after the onset of puberty, when sex differences in overall and regional body composition become more visible1,2,3,4. Body composition dynamics related to children’s growth, however, may be changing due to the high obesity prevalence in young populations worldwide.
In Latin America, approximately 20 million children are either overweight or obese5, though most investigations use body mass index (BMI) and waist circumference (WC) to assess overall obesity and central body shape. Despite being easy to apply, interpret, and compare with other studies, both BMI and WC do not provide information on fat mass and fat-free mass6,7,8. As such, this limitation does not allow us to know to what extent excessive weight gain in children is exclusively due to excessive fat mass or if there is an interactive and proportional growth of fat mass, lean mass and bone mineral content.
High proportions of total and central fat in adults is associated with disease risks and mortality9,10,11. If the body composition dynamics related to children’s growth are changing due to the childhood obesity epidemic, we can expect higher amounts of fat tissue at earlier ages. As a result, these higher concentrations of total and central fat mass earlier than the onset of puberty might further increase health risks in the short- and long- term12,13.
In the 2004 Pelotas Birth Cohort Study, we have information about total and regional body composition assessed when children were 6 and 11 years of age14. With these data, we can investigate the body composition dynamics related to children’s growth from childhood to early adolescence. Therefore, our objective was to assess the change in measures of overall and regional body composition from 6 to 11 years of age according to sex, and to examine whether changes in these measures are associated with socioeconomic and demographic characteristics.
Methods
Subjects
In 2004, a third birth cohort study started in Pelotas, Brazil. Pelotas is a medium-sized city (330,000 inhabitants), located around 150 km away from the Brazilian south border. Its economy is based on agriculture and commerce, and compared to the whole country, Pelotas has a lower Gross Domestic Product per capita, lower illiteracy and a higher Human Development Index.
The 2004 Pelotas Birth Cohort Study recruited 4231 new-borns in the city’s maternity hospitals, representing 99.2% of total births from mothers living in urban areas. Trained interviewers assessed mothers and their babies within 24-hours after delivery and applied a structured questionnaire containing information about the family, mother, current pregnancy, birth and child.
At the ages of 3 months, and 1, 2, 4, 6 and 11 years, the whole cohort were followed-up, and specifically trained field-workers collected information about anthropometric measures, health status, dietary intake, child development, housing conditions and socioeconomic position (SEP). Complete information about the perinatal study and all follow-up waves have been previously published14,15,16. Retention rates in all follow-ups were greater than 80%.
The Research Ethics Committee from the Medical School of Federal University of Pelotas approved all follow-ups, and the mother or legal guardian gave written informed consent to participate in the study. All methods employed in follow-ups of the 2004 Pelotas Birth Cohort Study were performed in accordance with relevant guidelines and regulations.
Anthropometry, body composition and distribution of body fat at 6 and 11 years
When children averaged 6.8 and 10.9 years of age, they visited our research clinic centre and anthropometric and body composition assessments were performed. In anthropometric evaluation, weight was measured using a high precision scale (0.01 Kg) coupled to a BodPod machine (Cosmed, Italy, http://goo.gl/7jzfLc), while height was collected using a Harpender metal stadiometer (Holtain, Crymych, UK). We then calculated BMI by dividing weight (Kg) by height (m2). After, we standardized BMI using the 2007 World Health Organization (WHO) growth reference17, and classified children as ‘normal’ (−2 to ≤+1 s.d.), ‘overweight’ (>+1 to ≤+2 s.d.) or ‘obese’ (>+2 s.d.).
We performed body composition assessment in both follow-ups using dual-energy X-ray absorptiometry (DXA). In DXA examinations conducted at 6 and 11 years of age in our research clinic centre (Dr. Amilcar Gigante Epidemiology Research Center), children remained in supine position, barefoot and wearing light and tight-fitting clothes, with no earrings, piercings or any metallic objects. Trained field-workers conducted DXA examinations and assessed the quality of exams using the same hardware (GE Lunar Prodigy densitometer) and software (enCORE v15) in both follow-ups. The equipment was calibrated at the beginning of each working day, following the manufacturer’s recommendations.
To assess total body composition, we used information on the BMI z-score as well as the fat mass and fat-free mass index from DXA. Fat mass and fat-free mass indexes were calculated as following:
-
Fat mass index = [Total fat mass (Kg)/height (m2)];
-
Fat-free mass index = [Total fat-free mass (Kg)/height (m2)];
Children were classified according to their level of fat mass index using the gender-specific curves proposed by Khadilkar et al.18, in order to identify children with high (between >85th and 95th percentile) and very high (>95th percentile) fat mass index. These cut-offs were defined to align with those used by WHO growth charts for overweight and obesity.
Due to the importance of body fat distribution to disease risks and mortality, we also used data from regional body fat measured by DXA. Android and gynoid fat mass percentage were used as indicators of central and peripheral body shape, respectively. We calculated android and gynoid fat mass percentages as following:
-
Android fat mass (%) = [android fat mass (Kg)/total fat mass (Kg)] * 100;
-
Gynoid fat mass (%) = [gynoid fat mass (Kg)/total fat mass (Kg)] * 100.
Independent variables
To assess the socioeconomic and demographic information associated with changes in body fatness from 6 to 11 years of age, we used information about SEP at birth, based on National Wealth Index (IEN); an index which classifies individual’s SEP according to household goods and the household head’s education19. We also used information about maternal education (0–4 years, 5–8 years and 9 or more), maternal age at birth (<18, 18–35 and >35 years), maternal BMI three months after birth (normal BMI, overweight and obese), and maternal reported skin colour (white, brown and black) used here as a proxy of ethnicity.
Statistical analyses
Means and 95% confidence intervals are presented for BMI z-scores, fat mass and fat-free mass indexes, and android and gynoid fat mass percentages. Changes in these measures from 6 to 11 years of age were calculated as the difference between measures collected at 11 years and 6 years.
We used linear regression models to check whether or not changes in measures of body fatness (BMI z-score and fat mass index) and body fat distribution (android fat mass and gynoid fat mass percentages) were associated with socioeconomic and demographic characteristics (SEP at birth, maternal education, maternal age at birth, maternal BMI and maternal reported skin colour).
In these linear regression models, the calculated difference between measures collected at 11 and 6 years was the dependent variable and the socioeconomic or demographic factor analysed was the independent variable. All analyses were adjusted for SEP at birth as this variable is well associated with all the other independent characteristics included in our study (except when SEP at birth was the independent variable).
As we tested different scenarios, we used Bonferroni corrected confidence intervals to address the multiple testing issue in the linear regression models. Variation Inflation Factor was also checked to assess for multicollinearity. All analyses were stratified by sex and performed using Stata 13.1.
Results
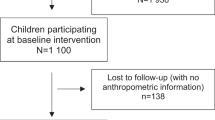
We included 3135 children who were followed at 6 and 11 years of age with available information on anthropometric and DXA measures in both follow-ups. Children in the study were more likely to be boys (51.1%) and to have low SEP at birth (1st or 2nd quintile). More than 40% of their mothers had at least 9 years of formal education, almost 10% of mothers were adolescents at the time of birth and around 45% were classified as overweight or obese three months after birth. Most mothers reported white as their children’s skin colour (Table 1). Comparing these children with those lost to follow-up, the latter were poorer and delivered by younger mothers. They did not differ in terms of maternal education, maternal BMI and mother reported skin colour (data not shown).
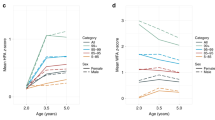
The mean of BMI z-scores increased from +0.71 s.d. (CI 95% 0.64 to 0.79) at 6 years to +0.83 s.d. (CI95% 0.76 to 0.90) at 11 years in boys and remained stable in girls (+0.68 s.d. at 6 and +0.67 at 11 years). Regarding obesity status, while less than 20% of boys were classified as obese at 6 years, the prevalence of obesity at 11 years of age increased to 25%. In girls, obesity status increased only 1.8 percentage points, from 17.5% at 6 years to 19.3% at 11 years (Table 2).
Fat mass and fat-free mass indexes increased in both boys and girls from 6 to 11 years, however the increase in fat mass index was more accentuated; from 4.2 to 6.3 Kg/m2 in girls and from 3.3 to 5.5 Kg/m2 in boys. At both time-points, girls presented a higher fat mass index while boys presented a higher fat-free mass index (p-value < 0.001) (Table 2). When we assessed fat mass index classification according to Khadilkar et al. (2013) percentiles, we observed similar results to BMI status. There was no difference in fat mass index classification according to gender at 6 years then at 11 years of age, the proportion of children being above the 85th percentile was higher in boys (45% vs. 35%) (Table 2).
Regarding body fat distribution, android fat mass percentage increased from 7.2% (CI95% 7.1 to 7.3) to 7.4% (CI 95% 7.3 to 7.4) in girls, and from 6.7% (CI 95% 6.6 to 6.7) to 7.2% (CI 95% 7.2 to 7.3) in boys (Table 2). On the other hand, the proportion of gynoid fat mass decreased from 6 to 11 years in both sexes. Girls presented 22.5% (CI 95% 22.3 to 22.6) of gynoid fat mass at 6 years and 21.1% (CI 95% 20.9 to 21.2) at 11 years; boys presented 23.8% (CI95% 23.7 to 24.0) of gynoid fat mass at 6 years and 21.4% (CI 95% 21.2 to 21.5) at 11 years (Table 2).
Table 3 shows changes in BMI z-scores and fat mass indexes from 6 to 11 years according to the independent variables included in our study. Compared to the less-affluent children, boys and girls with higher SEP at birth presented smaller increases in BMI z-score from 6 to 11 years. Children from overweight and obese mothers presented larger increases in fat mass index, independent of SEP at birth. Finally, black girls presented a larger increase in BMI z-score from 6 to 11 years of age when compared to the white girls (Table 3).
Table 4 shows changes in android and gynoid fat mass percentage from 6 to 11 years according to the same co-variables. Boys and girls from overweight and obese mothers presented larger increases in android fat mass percentage, independent of SEP at birth. Android and gynoid fat mass percentage from 6 to 11 years did not differ at all according to the other factors included in this analysis (Table 4).
Discussion
Our study showed changes in overall and regional body fatness from 6 to 11 years of age in a population-based sample from Pelotas, Brazil. At 6 years of age, there was no difference in BMI z-scores according to sex, however when children were 11 years old, BMI z-scores became higher in boys. In addition, one out of four boys and one out of five girls were classified as obese when they were 11 years old.
Recent estimates from Brazil and other settings have already shown an increase in obesity prevalence in all age groups20,21,22. In Brazil as a whole, the last national representative survey showed a prevalence of obesity in 5 to 9 year-old children of 17% and 12% in boys and girls, respectively23. Despite the prevalence of obesity having increased in Brazilian children, the proportion of obesity in children from the 2004 Pelotas Birth Cohort is higher, mainly in boys, reaching one quarter of the sample. Furthermore, a notable number of children from our cohort were classified with high fat mass indexes, even when referenced against Indian children who are known to have higher amounts of body fatness than Western children18.
Children included in our study had not started the onset of puberty or, at best, were in the early stages of it. Due to this reason, we might expect that gain in total body mass would be mostly due to increases in fat-free mass than fat mass, as before the onset of puberty, weight gain is supposed to be based on fat-free mass rather than fat mass1. We observed, however, that the fat mass index increased in the whole cohort, independent of socioeconomic and demographic characteristics.
The most consistent factor associated with increases in body fatness from 6 to 11 years was maternal BMI. These results are consistent with other reports of maternal BMI and offspring’s body composition in this cohort and other studies24,25,26. An investigation using data from the 2004 Pelotas cohort study showed a positive association between pre-pregnancy BMI and offspring body composition at 6 years of age as measured by air-displacement plethysmography24. Furthermore, Pacce et al.26 and Zalbahar et al.25 also found a positive relationship between maternal BMI and offspring’s body composition. Despite cross-sectional association between pre-pregnancy maternal BMI and offspring’s body composition having been well reported in the literature, our study went further and showed that children from mothers with obesity presented larger increases in BMI z-scores, fat mass indexes and android fat mass percentages from late childhood to early adolescence.
But what would be the reasons involved in high prevalence of obesity and higher storage of fat mass from childhood to early adolescence? Unhealthy feeding habits may be one aspect related to this process. Recently published research using data from this cohort showed that more than 40% of daily energy intake at 6 years came from ultra-processed foods27. It is an interesting finding as other studies have shown that ultra-processed foods are associated with a higher risk of obesity28,29. Additionally, as physical activity levels decrease considerably from childhood to adolescence30,31,32, sedentarism might be another factor associated with body fatness increases in this cohort. Nevertheless, we did not address this association in our study and further investigations would be interesting to test this hypothesis.
Our sample also presented a centralisation of body fatness from 6 to 11 years, despite the modest increase in measures of android fat mass (0.5 percentage point in boys and 0.2 percentage point in girls). Hoffman et al.33, using data from a Brazilian cohort, showed an increase in trunk fat mass percentage in children entering puberty, indicating a centralisation of fatness. Nonetheless, despite fat mass centralisation observed in our cohort, the decrease in peripheral body fat was a little bit more accentuated, reaching more than two percentage points in boys. This may be associated with the prevalence of obesity, but may also be part of the maturing process faced by these children. Independent of the reasons for body fat centralisation, higher amounts of central body fat is associated with disease risks and mortality, and an early exposure to android body shape can increase these risks even more10,12,34.
Large sample size and low attrition rates at both 6-year and 11-year follow-ups can be considered a strength of our study. A further strength is the comprehensive body composition assessment at both time-points using DXA, which allowed us to look at the evolution of children’s body fatness from late childhood to early adolescence beyond BMI.
Descriptive analyses with no adjustment for potential confounders can be treated as a limitation of our study, since associations observed here may be confounded by other factors. In addition, the unavailability of pubertal stage information for boys meant we were unable to address the potential role of pubertal stage on the evolution of body fatness/body fat distribution in this study. Finally, the use of multiple analyses in our study may be considered another limitation, increasing the probability of type-I error. Nevertheless, the use of Bonferroni’s correction and the good power of our sample helped in addressing this matter.
In conclusion, there was an increase in overall body fatness as well as a centralisation of body shape from late childhood to early adolescence, associated with male sex and maternal obesity soon after the delivery. These results indicate that children from the 2004 Pelotas Birth Cohort Study may be at a higher risk of non-communicable diseases in the mid- and long-term, and actions to address this problem are needed.
Data Availability
Due to confidentiality conditions, the authors were only allowed to publish analytic results from the data, but not the data itself.
References
Wells, J. C. K. The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol. Rev. 81, 183 (2006).
Wells, J. C. K. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 21, 415–430 (2007).
A. H. Kissebah, G. R. K. Regional adiposity and morbidity. Physiological Reviews 761–811 (1994).
Pulit, S. L., Karaderi, T. & Lindgren, C. M. Sexual dimorphisms in genetic loci linked to body fat distribution. Biosci. Rep. 37, BSR20160184 (2017).
Rivera, J. Á. et al. Childhood and adolescent overweight and obesity in Latin America: a systematic review. Lancet Diabetes Endocrinol. 2, 321–332 (2014).
Deurenberg-Yap, M., Chew, S. K. & Deurenberg, P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes. Rev. 3, 209–215 (2002).
Jackson, A. et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. 26, 789–796 (2002).
Snijder, M., van Dam, R., Visser, M. & Seidell, J. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 35, 83–92 (2006).
Cerhan, J. R. et al. A Pooled Analysis of Waist Circumference and Mortality in 650,000 Adults. Mayo Clin. Proc. 89, 335–345 (2014).
Yang, M. et al. Truncal and leg fat associations with metabolic risk factors among Chinese adults. Asia Pac. J. Clin. Nutr. 25, 798–809 (2016).
Hübers, M., Geisler, C., Plachta-Danielzik, S. & Müller, M. J. Association between individual fat depots and cardio-metabolic traits in normal- and overweight children, adolescents and adults. Nutr. Diabetes 7, e267 (2017).
Chen, F. et al. Association between Childhood Obesity and Metabolic Syndrome: Evidence from a Large Sample of Chinese Children and Adolescents. PLoS ONE 7, e47380 (2012).
Abrams, P. & Levitt Katz, L. E. Metabolic effects of obesity causing disease in childhood. Curr. Opin. Endocrinol. Diabetes Obes. 18, 23–27 (2011).
Santos, I. S. et al. Cohort Profile Update: 2004 Pelotas (Brazil) Birth Cohort Study. Body composition, mental health and genetic assessment at the 6 years follow-up. Int. J. Epidemiol. 43, 1437–1437f (2014).
Barros, A. J. D. et al. Coorte de nascimentos de Pelotas, 2004: metodologia e descrição. Rev. Saúde Pública 40, 402–413 (2006).
Santos, I. S. et al. Cohort Profile: The 2004 Pelotas (Brazil) Birth Cohort Study. Int. J. Epidemiol. 40, 1461–1468 (2011).
de Onis, M. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85, 660–667 (2007).
Khadilkar, A. V., Sanwalka, N. J., Chiplonkar, S. A., Khadilkar, V. V. & Pandit, D. Body fat reference percentiles on healthy affluent Indian children and adolescents to screen for adiposity. Int. J. Obes. 37, 947–953 (2013).
Barros, A. J. D. & Victora, C. G. Indicador econômico para o Brasil baseado no censo demográfico de 2000. Rev. Saúde Pública 39, 523–529 (2005).
Trends in adult body-mass index in 200 countries from 1975 to 2014. : a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. The Lancet 387, 1377–1396 (2016).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet https://doi.org/10.1016/S0140-6736(17)32129-3 (2017).
Malta, D. C., Andrade, S. C., Claro, R. M., Bernal, R. T. I. & Monteiro, C. A. Trends in prevalence of overweight and obesity in adults in 26 Brazilian state capitals and the Federal District from 2006 to 2012. Rev. Bras. Epidemiol. 17, 267–276 (2014).
Brazilian Institute of Geography and Statistics. Consumer expenditure survey 2008-2009: Anthropometry and nutritional status of children, adolescents and adults in Brazil. (2010).
Castillo, H., Santos, I. S. & Matijasevich, A. Relationship between maternal pre-pregnancy body mass index, gestational weight gain and childhood fatness at 6-7 years by air displacement plethysmography: Maternal pre-pregnancy BMI and childhood fatness. Matern. Child. Nutr. 11, 606–617 (2015).
Zalbahar, N. et al. Association of parental body mass index before pregnancy on infant growth and body composition: Evidence from a pregnancy cohort study in Malaysia. Obes. Res. Clin. Pract. 10, S35–S47 (2016).
Pacce, S. et al. Impact of maternal nutritional status before and during pregnancy on neonatal body composition: A cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 10, S7–S12 (2016).
Bielemann, R. M. et al. Early feeding practices and consumption of ultraprocessed foods at 6 y of age: Findings from the 2004 Pelotas (Brazil) Birth Cohort Study. Nutrition 47, 27–32 (2018).
Mendonca, R. D. D. et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 104, 1433–1440 (2016).
Louzada, M. L. et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. 81, 9–15 (2015).
Troiano, R. P. et al. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 40, 181–188 (2008).
Baptista, F. et al. Prevalence of the Portuguese Population Attaining Sufficient Physical Activity. Med. Sci. Sports Exerc. 44, 466–473 (2012).
da Silva, I. C. et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int. J. Epidemiol. 43, 1959–1968 (2014).
Hoffman, D. J., Martins, P. A., Roberts, S. B. & Sawaya, A. L. Body fat distribution in stunted compared with normal-height children from the shantytowns of São Paulo, Brazil. Nutrition 23, 640–646 (2007).
Lotta, L. A. et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49, 17–26 (2016).
Acknowledgements
We are thankful for all cohort members and their mothers/legal guardians for have accepted and contributed with 6 and 11-year-old follow-ups. The 2004 birth cohort study is supported by the Wellcome Trust through the scheme called ‘Major Awards for Latin America on Health Consequences of Population Change’ (Grant Number 086974/Z/08/Z). The World Health Organization (Grant Number 03014HNI), Brazilian National Research Council (CNPq) (Grant Numbers 481012-2009-5; 484077-2010-4; 470965-2010-0; and 481141- 2007-3), and Brazilian Ministry of Health (Grant Number 25000.105293/2004-83) have supported previous phase of the study.
Author information
Authors and Affiliations
Contributions
This study was conducted by all authors. L.P.S. proposed the idea, performed the statistical analyses and drafted the manuscript. A.J.D.B. supervised all the statistical analyses process and helped in drafting the manuscript. A.M. and I.S.S. helped in interpreting results and in drafting the manuscript. A.J.D.B., A.M. and I.S.S. participated in the design and conduction of the original cohort study as well as in the 6- and 11-year-old follow-ups. All authors read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos, L.P., Santos, I.S., Matijasevich, A. et al. Changes in overall and regional body fatness from childhood to early adolescence. Sci Rep 9, 1888 (2019). https://doi.org/10.1038/s41598-019-38486-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38486-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.