Abstract
The adaptive success of flowering plants is largely due to their ability to align floral production with optimal conditions. In Arabidopsis thaliana, MADS-box repressors of the FLC/MAF-clade prevent flowering under non-inductive conditions, although the role of some members is not yet clearly defined. Using a genetic strategy, we identified the KH-domain gene HEN4, previously shown to be involved in MADS-box floral homeotic gene regulation, as a modulator of flowering time. Loss-of-function hen4 mutants are early-flowering, and their response to low growth-temperature (16 °C) and day-length is altered. Interestingly, hen4 plants showed dramatic reduction of FLC and MAF4 transcripts, whereas other flowering repressors of the same clade (FLM, MAF2, MAF3, MAF5) remained unaltered. We also determined that hen4, partly due to loss of FLC, accelerates the vegetative phase-change. This report provides insight into flowering time control and highlights the potential of versatile regulators such as HEN4 to coordinate the juvenile-to-adult transition and floral timing.
Similar content being viewed by others
Introduction
After seedling emergence flowering plants undergo two successive developmental transitions, the vegetative phase-change (juvenile-to-adult transition) and the floral transition (vegetative-to-reproductive)1,2. During the vegetative phase-change, plants progress towards an adult state, acquiring reproductive competence1,2,3. In the model eudicot Arabidopsis thaliana (Arabidopsis hereafter), this transition is governed by a conserved regulatory circuit including two microRNA (miRNA) families and their targets. Initially abundant, miR156 levels decrease with plant age, allowing target SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes to activate miR172 that, in turn, downregulate APETALA 2 (AP2)-type transcriptional repressors, resulting in the promotion of the vegetative phase-change and subsequent floral induction4,5.
The floral transition must be aligned with optimal conditions to maximize reproductive success. To this end, plants have evolved a sophisticated network of flowering promotion pathways6,7. The aging pathway, defined by the miR156/SPL module4, together with the autonomous pathway monitor intrinsic developmental cues; the gibberellin (GA) pathway transduces hormonal information, whereas the photoperiod pathway perceives daylength and light quality7. Temperature is monitored by two distinct pathways. The vernalization pathway allows plants to adapt reproduction to seasonal variations (prolonged exposure to winter cold)8, and the thermosensory pathway enables plants to respond to changes in day-growth (ambient) temperature, accelerating or delaying flowering under warm or cold weather, respectively9,10.
All pathways ultimately converge in a common set of floral integrators such as FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) which, in turn, activate floral identity genes LEAFY (LFY) APETALA1 (AP1) and FRUITFULL (FUL)6,7,11. Floral integrators are counteracted by inhibitor activities that delay flowering under non-inductive conditions. Among them, the potent floral repressors FLOWERING LOCUS C (FLC) and SHORT VEGETATIVE PHASE (SVP) play predominant roles12,13. FLC and SVP form a complex that represses SOC1, FT and the FT homolog TWIN SISTER OF FT (TSF)14,15,16. In turn, the vernalization, autonomous, GA and thermosensory pathways downregulate FLC and SVP12,15,17,18,19.
Extensive crosstalk among different flowering pathways is mediated by shared regulatory factors. For example, DELLA proteins are transcriptional repressors that mediate the effects of GA and have been shown to interact with FLC to repress flowering, thus creating a hub for the vernalization, autonomous and GA pathways20. Likewise, the vegetative phase-change and the floral transition often do not appear clearly separated1. Thus, the SPL genes promote the transition to flowering5, and SVP and FLC delay the juvenile-to-adult progression13,21,22.
SVP is central to flowering thermoregulation18. This regulatory mechanism is of utmost importance since modest fluctuations in ambient temperature may result in significant variations in flowering time, being a crucial aspect of the impact of climate change on agriculture and ecosystems10. SVP interacts with additional floral repressors of the FLC-clade present in the Arabidopsis genome, FLOWERING LOCUS M/MADS AFFECTING FLOWERING 1 (FLM/MAF1) and MAF2-MAF523,24,25,26. The roles of FLM and MAF2 in thermosensory flowering are well documented. Both FLM and MAF2 produce temperature-dependent RNA splicing isoforms. One isoform, predominant at low temperatures, encodes an active polypeptide that heterodimerizes with SVP to form a potent repressor complex. By contrast, as temperature increases, alternative splicing variants accumulate at the expense of the former isoform19,27,28. Whether the main outcome of alternative variant production is encoding inactive polypeptides or RNA degradation via nonsense mediated decay is still a matter of debate19,27,29. In any case, the relative amount of the effective repressor complex decreases, hence adjusting flowering time to ambient temperature19,27,28. Furthermore, the stability of the SVP protein declines with increasing temperature, also resulting in decreasing levels of SVP-MAF repressive complexes19.
The contribution of the remaining MAF genes is less clear. MAF3 and MAF4 have been reported to respond to ambient temperature and their products interact with FLC, SVP, FLM and MAF2, likely assembling into flowering repressive complexes25. FLC also participates in flowering thermoregulation30 although its role was considered to be moderate compared to SVP or FLM19. In any case, flc and some maf single mutants are less sensitive to growth temperature than the wild type, whereas svp plants are essentially unresponsive, reflecting the central role of SVP in this process10,18,25.
As illustrated above, in addition to transcription, post-transcriptional mechanisms are major determinants for flowering time regulation. The HUA-PEP activity is composed of a functionally versatile group of genes encoding RNA-binding proteins (RBP) that control pre-mRNA processing of the MADS-box genes AGAMOUS (AG) and its clade members SHATTERPROOF1 (SHP1), SHP2 and SEEDSTICK (STK)31,32,33, crucial for flower and ovule morphogenesis34,35. Additionally, some HUA-PEP components also regulate FLC36,37. Here we identify HUA ENHANCER 4 (HEN4), a HUA-PEP member encoding a K-homology (KH) RBP, as a novel flowering time regulator. Strong hen4 mutants show reduced expression of FLC and its paralog MAF4 which correlates with early-flowering and reduced sensitivity to day-length and low ambient temperature (16 °C). Interestingly, other MAF genes remain unaffected in hen4 plants. We further demonstrate that HEN4, in line with a positive role in FLC regulation, also delays the vegetative phase-change. Our results add new insight into plant control of developmental timing. A multifaceted regulator such as HEN4 may be critical for orchestrating flowering responses and its characterization should facilitate a better understanding on how such coordination is achieved.
Results
The hen4 mutants are early-flowering
HEN4 encodes a polypeptide containing five KH RNA binding domains (Supplementary Fig. S1), involved in flower and ovule morphogenesis31,32,33. In addition, we observed that hen4 plants flowered earlier than the wild type. Therefore, we tested three available alleles to investigate the participation of HEN4 during the reproductive transition. The hen4-3 and hen4-4 alleles bear T-DNA insertions at introns three and six, respectively (Supplementary Fig. S1). Insertions within introns are occasionally transcribed and spliced out, yielding appreciable levels of wild-type transcripts. However, the hen4-2 allele carries a point mutation at the beginning of the fourth exon, generating a stop codon31 (Supplementary Fig. S1) and, very likely, the hen4-2 transcripts might be subject to degradation through the nonsense mediated decay pathway38. In line with this, hen4-2 presented lower levels of HEN4 transcripts than hen4-3 and hen4-4 plants as monitored by quantitative RT-PCR (qPCR) (Supplementary Fig. S1). We scored the flowering time for the three mutants. At 21 °C, both hen4-3 and hen4-4 did not show significant differences with the parental strain Col-0 (Supplementary Fig. S1). Conversely, hen4-2 plants flowered significantly earlier (P < 0.001) (Fig. 1a,b, and Supplementary Fig. S1). Therefore, we chose hen4-2 as the reference allele. These results are in agreement with previous reports indicating that hen4-2 is a strong loss-of-function allele31,32, and confirmed that HEN4 regulates flowering in Arabidopsis.
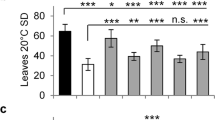
Early-flowering hen4-2 plants. (a) Flowering time of wild-type Col-0 and hen4-2 mutant plants at 21 °C and 16 °C, measured as the number of days (left) or rosette leaves at bolting (right). Bars indicate means ± SD (standard deviation) from three independent experiments with 21 plants per genotype each. (b) Representative 22-day-old (top) and 28-day-old (bottom) Col-0 and hen4-2 plants grown at 21 °C. Scale bar: 2 cm. (c) Relative expression of floral integrator genes monitored by qPCR, in Col-0 and hen4-2 plants grown at 21 °C and 16 °C. Bars indicate means ± SD. Significant differences with respect to Col-0 plants at the corresponding temperature are indicated: ***P < 0.001, ANOVA for panel (a) and Student’s t-test for panel (c).
We also observed that hen4-2 plants flowered earlier than the wild-type at lower ambient temperature. As the wild type, hen4-2 mutants flowered at 16 °C later than at 21 °C, but responsiveness clearly decreased, especially in terms of leaves (Fig. 1a). The hen4-2 plants produced on average 10 leaves at 21 °C vs 13 at 16 °C, whereas Col-0 plants generated about 13 and 22 leaves, respectively (Fig. 1a). Homozygous hen4-3 and hen4-4 mutants were also less sensitive than Col-0 plants to cool temperature, although not as much as hen4-2, further indicating that they represent weak alleles (Supplementary Fig. S1).
We measured the expression of the major floral integrators SOC1 and FT11, and the FT paralog TSF14. The three genes were highly expressed in hen4-2 at 16° and 21 °C as compared to the wild type, consistent with precocious flowering of hen4-2 plants at both temperatures (Fig. 1c). The expression of FT and SOC1 was much less affected in hen4-3 and hen4-4 plants, in consonance with their weaker phenotypes (Supplementary Fig. S2). We also measured the expression of LFY, a floral inducer whose expression in the meristem is a marker of floral commitment39. Consistently, precocious flowering of hen4-2 plants was also reflected in an increase of LFY mRNA at both temperatures (Fig. 1c). LFY mRNA also increased in hen4-3 and hen4-4 plants albeit much less than in hen4-2 mutants (Supplementary Fig. S2). Moreover, hen4-2 partially rescued the soc1-6 late flowering phenotype40 in the hen4-2 soc1-6 double mutant (Supplementary Fig. S3), further supporting the participation of HEN4 in flowering time regulation.
HEN4 is a positive regulator of FLC
FT, TSF, SOC1 and LFY integrate numerous endogenous and environmental stimuli11,41, hence remaining unclear the reason(s) whereby the loss of HEN4 accelerates flowering. We decided to analyse the FLC/MAF-clade repressors because HEN4 regulates structurally similar AG, SHPs and STK genes as part of the HUA-PEP activity, and HUA-PEP genes such as HUA2 and PEPPER (PEP) regulate FLC32,33,36,37.
We first examined FLC expression in the hen4-2 mutant. SOC1, FT and TSF are direct targets of FLC11,14 and FLC conveys in part the flowering response to low ambient temperature19. Consistently, FLC expression increased significantly in Col-0 plants grown at 16 °C as compared to those grown at 21 °C (Fig. 2a). This is congruent with previous results showing FLC downregulation in response to increasing temperatures9. In stark contrast, hen4-2 mutants showed only negligible levels of FLC at both temperatures (Fig. 2a) strongly suggesting that HEN4 is required for proper FLC expression. In consonance, FLC expression was also suppressed in the intermediate hen4-4 mutant whereas it remained unaffected in the much weaker hen4-3 background (Supplementary Fig. S2).
HEN4 activates FLC. (a) Relative FLC expression monitored by qPCR, in Col-0 and hen4-2 plants grown at 21 °C and 16 °C. (b) Representative 32-day-old Col-0 and mutant plants grown at 21 °C illustrating flk-2 late-flowering rescue by hen4-2. Scale bar: 4 cm. (c) Flowering time of wild-type Col-0 and diverse mutant strains at 21 °C and 16 °C, measured as the number of days (left) or rosette leaves at bolting (right). Data from two independent experiments with 21 plants per genotype each. (d) Relative FLC expression, monitored by qPCR, in Col-0 and indicated mutant plants grown at 21 °C. In panels (a,c,d) bars indicate means ± SD, and black asterisks denote significant differences with respect to Col-0 plants at the corresponding temperature. Red asterisks in panel (a) indicate significant variation between Col-0 plants at 21 °C and 16 °C. In panel (c) red asterisks indicate significant differences with respect to flk-2 at the corresponding temperature. ***P < 0.001. Student’s t-test, panels (a) and (d). ANOVA for panel (c).
To substantiate these results, we crossed hen4-2 with plants mutant for the autonomous pathway gene FLOWERING LOCUS K (FLK). Like other autonomous pathway mutants, flk plants flower late due to the accumulation of high levels of FLC mRNA42,43. Other hua-pep mutations such as hua2 and pep strongly reduce the FLC mRNA levels36,37 and consequently they partially rescue the flk late-flowering phenotype37. As expected, flk-2 null mutant plants42 flowered considerably later than the wild type at 21 °C and were highly responsive to temperature drop18 (Fig. 2b,c). Remarkably, the loss of HEN4 function masked the effect of flk-2 at 21 °C and 16 °C (Fig. 2b,c, Supplementary Fig. S4). In fact the hen4-2 flk-2 double mutants flowered faster than the wild type at both temperatures, being comparable to hen4-2 plants (Fig. 2c). These results indicate that hen4-2 is epistatic to flk-2 and strongly suggest that loss of FLC expression is an important factor to explain rapid flowering in hen4-2.
Loss of FLC abolishes the late-flowering phenotype of autonomous pathway mutants under long-day and short-day conditions44. Interestingly, hen4-2 plants showed a dramatic loss of sensitivity to day-length (Supplementary Fig. S5), reinforcing the notion of HEN4 as a positive FLC regulator.
As expected, FLC mRNA expression was strongly reduced in the hen4-2 flk-2 double mutant compared to flk-2 single mutant plants (Fig. 2d). However, it remained higher than in Col-0 and hen4-2 (Fig. 2d). These data indicate that rescue was not complete at the FLC mRNA level and hint at the existence of additional factors regulated by HEN4. In agreement with this hypothesis, the intermediate-strength hen4-4 allele, in which FLC expression was also reduced (Supplementary Fig. S2), also rescued the flk-2 late flowering phenotype, although to a much lesser extent than in hen4-2 flk-2 (Supplementary Fig. S6).
To complement the above observations we crossed the complete loss-of-function flc-3 mutant12 with hen4-2. As shown in Fig. 3, hen4-2 plants flowered earlier than flc-3 individuals, and hen4-2 flc-3 double mutants behaved essentially as hen4-2. These findings reinforce the notion that besides loss of FLC, additional factors are required to totally explain the hen4-2 early flowering phenotype.
hen4-2 is epistatic on flc-3. (a) Flowering time of wild-type Col-0 and diverse mutant strains at 21 °C and 16 °C, measured as the number of days (left) or rosette leaves at bolting (right). Data from three independent experiments with 21 plants per genotype each. Black asterisks denote significant differences with respect to Col-0 plants at the corresponding temperature. Red asterisks indicate significant differences with respect to flc-3 at the corresponding temperature. ***P < 0.001, ANOVA. (b) Representative Col-0 and mutant 24-day-old plants grown at 21 °C showing hen4-2 epistasis on flc-3. Scale bar: 2 cm.
MAF gene regulation by HEN4 is limited to MAF4
With the aim of identifying additional HEN4 targets, we analysed the expression of the additional five members of the FLC-clade present in the Arabidopsis genome, FLM and MAF2-MAF523,24,26. We first analysed FLM total gene expression as well as specific β and δ isoforms, encoding the active and inactive repressors, respectively19,27. Total FLM gene expression showed some reduction in hen4-2 plants only at 16 °C (Fig. 4a). However β isoform expression did not show statistically significant differences between hen4-2 and Col-0 plants at 16 °C or 21 °C (Fig. 4a), suggesting that HEN4 does not affect FLM. Differences observed in total gene expression at 16 °C could be very likely due to other non-functional RNA variants that are produced in addition to δ isoform29.
Early flowering of hen4 is FLM-independent. (a) Relative expression of total and specific FLM splicing RNA variants, β and δ19,27, at 21 °C and 16 °C, as monitored by qPCR. (b) Flowering time of wild-type Col-0 and various mutant strains at 21 °C and 16 °C, measured as the number of days (left) or rosette leaves at bolting (right). Data from two independent experiments with 21 plants per genotype each. (c) Representative 22-day-old Col-0 and mutant plants grown at 21 °C. Bars indicate means ± SD. Black asterisks denote significant differences with respect to Col-0 plants at the corresponding temperature. Red and blue asterisks in panel (b) indicate significant differences with respect to hen4-2 and flm-3 plants, respectively. *P < 0.05; ***P < 0.001. Student’s t-test for panel (a) and ANOVA for panel (b). Scale bar: 2 cm.
Next, we used the flm-3 null allele30 to build the hen4-2 flm-3 double mutant. We verified that flm-3 plants flowered at the same time as hen4-2 or even slightly faster (Fig. 4b). The hen4-2 flm-3 double mutant flowered earlier than either single mutant at 16 °C and 21 °C (Fig. 4b,c) indicating additive effects of both mutations and supporting the conclusion that HEN4 has little or no influence on FLM RNA production.
An alternative target to explain the attenuated response of hen4-2 to ambient temperature was MAF2. However, the expression analyses of MAF2 mRNA splicing variant 1, encoding the active repressor28, did not yield significant differences (Supplementary Fig. S7). Altogether, our data do not suggest that HEN4 modulates temperature-responsive flowering via FLM and/or MAF2 RNA regulation. Likewise, the expression levels of MAF3 and MAF5 in hen4-2, although responsive to low temperature (16 °C), were also very similar to those of Col-0 (Supplementary Fig. S7).
MAF4 was a notable exception. In the wild type, MAF4 expression significantly increased at 16 °C with respect to plants growing at 21 °C (Fig. 5a). Noticeably, in the hen4-2 mutant MAF4 expression decreased dramatically at both temperatures as compared to the wild type (Fig. 5a). This might contribute to hen4-2 precocious flowering and the attenuated response to temperature. Previous studies showed that maf4 mutants are partly insensitive to the growth temperature drop25. The control of flowering by ambient temperature is largely mediated by SVP and FLM whose products form MADS-domain complexes with FLC and other MAF genes, including MAF425,27. So, simultaneous loss of FLC and MAF4 might explain, at least in part, why hen4-2 plants are less sensitive to reduced (16°) growth temperature. Furthermore, MAF4 expression was not significantly altered in hen4-3 and hen4-4 (Supplementary Fig. S8). This might also explain why hen4-4 mutants, despite showing reduction of FLC expression, exhibit a flowering phenotype weaker than that of hen4-2 (Supplementary Figs S1 and S2).
MAF4 is upregulated by HEN4. (a) Relative MAF4 expression (qPCR) at 21 °C and 16 °C. Bars indicate means ± SD. (b) Flowering time of Col-0, maf4, and hen4-2 plants grown at 21 °C and 16 °C measured as the number of days (left) or rosette leaves at bolting (right). Bars indicate means ± SD where n = 21 plants per genotype. (c) Comparison of representative 24-day-old Col-0, maf4, and hen4-2 plants grown at 21 °C. Scale bar: 2 cm. (d) Relative MAF4 expression (qPCR) at 21 °C in Col-0 and diverse mutant backgrounds. Bars indicate means ± SD. In all panels black asterisks denote significant differences with respect to the wild type at the corresponding temperature. Red asterisks in (a) indicate significant differences between Col-0 plants grown at 21 °C and 16 °C. Red asterisks in (d), significant variation between flk-2 and hen4-2 flk-2. *P < 0.05; **P < 0.01; ***P < 0.001. Student’s t-test, panels (a) and (d). ANOVA, panel (b).
The genes MAF2-to-MAF5 are organized as a 22 kb tandem array extremely close to the HEN4 locus23,26, thus preventing the construction of hen4 maf double mutants by crossing. A null maf4 mutant (SALK_028506) flowered moderately earlier than the wild type at both 21 °C and 16 °C, although later than hen4-2 at both temperatures (Fig. 5b,c). Then, we leveraged the hen4-2 flk-2 double mutant to reinforce the notion of MAF4 regulation by HEN4. MAF4 mRNA expression was higher in flk-2 mutant plants than in the wild type, being reduced in the hen4-2 flk-2 double mutant to levels similar to those of hen4-2 plants (Fig. 5d). This finding suggests that the flk-2 late-flowering phenotype might be partly due to MAF4 overexpression, also contributing to explain why the hen4-2 flk-2 double mutants flower earlier than Col-0 plants in spite of expressing higher levels of FLC transcripts (see Fig. 2 above).
SVP is largely epistatic to HEN4
SVP plays a central role in the formation of MADS-box repressor complexes by which plants respond to ambient-temperature19,25,27,28. Therefore, to gain further insight into the role of HEN4 in flowering time regulation, we monitored SVP mRNA expression in hen4-2 plants. Unexpectedly, SVP RNA levels increased in this mutant at both 16 °C and 21 °C with respect to Col-0 (Fig. 6a). Although SVP response to ambient temperature is known to be dependent on the stability of the SVP protein19, this result was paradoxical. SVP RNA levels were unaltered in the weaker hen4-3 and hen4-4 mutants (Supplementary Fig. S9). Perhaps, SVP transcript levels increase in the stronger hen4-2 background as a feedback mechanism to compensate for the drop of SVP protein partners such as FLC and MAF4 in the repressor complexes15,25. In any case, early flowering of hen4-2 plants does not seem to be due to reduced levels of SVP transcripts.
SVP epistasis on HEN4. (a) Relative SVP expression at 21 °C and 16 °C, monitored by qPCR, in Col-0 and hen4-2 plants. Bars indicate means ± SD and asterisks indicate significant differences with the wild type. ***P < 0.001. (b) Flowering time of Col-0 and diverse mutant plants grown at 21 °C and 16 °C measured as the number of days (left) or rosette leaves at bolting (right). Bars indicate means ± SD where n = 21 plants per genotype. Significant differences with respect to the wild type, hen4-2 and svp-32, are indicated by black, red and blue asterisks, respectively. *P < 0.05; **P < 0.01; ***P < 0.001. (c) Representative 22-day-old plants of the genotypes shown in (b) grown at 21 °C. Scale bar: 2 cm. Student’s t-test (a) and ANOVA (b).
Next, we used the svp-32 loss-of-function allele18 to generate the hen4-2 svp-32 double mutant. As expected, svp-32 plants flowered earlier than hen4-2, and the response to growth temperature was greatly reduced18 (Fig. 6b). hen4-2 affects the expression of FLC and MAF4 (Figs 2, 5 and Supplementary Fig. S2) but the loss of SVP compromises the function of all FLC-clade repressors19,25,27,28. Therefore, it was not surprising that hen4-2 svp-32 plants flowered essentially at the same time as svp-32 single mutants, indicating that svp-32 is largely epistatic to hen4-2 (Fig. 6b). However, some minor but significant (***p < 0.001) differences between svp-32 and hen4-2 svp-32 at 16 °C in days to flowering (blue asterisks in Fig. 6b) hint at additional hen4-2 effects. Indeed, the hen4-2 svp-32 double mutant seemed to grow a bit faster than svp-32 plants (Fig. 6c).
HEN4 regulates the vegetative phase-change
FLC was shown to delay the vegetative phase-change21,22. To further confirm the role of HEN4 as an FLC regulator, we examined the juvenile-to-adult transit in the hen4-2 mutant. This transition is characterized by morphological changes (heteroblasty) between juvenile and adult leaves45. An easy-to-score differential trait is the presence of abaxial (lower) trichomes. Leaves produced during the juvenile phase develop trichomes exclusively on their adaxial (upper) surface. On the contrary, adult leaves typically present trichomes on both sides45. According to this morphological marker, the first adult leaf emerged in hen4-2, on average, more than two leaves earlier than in Col-0 (Fig. 7a,b), indicating a shortening of the juvenile vegetative phase. These results confirm that HEN4 affects the transition to reproductive maturity.
HEN4 delays the juvenile-to-adult vegetative transition. (a) Appearance of leaf abaxial trichomes in wild-type Col-0 and distinct mutant strains grown at 21 °C. The onset of abaxial trichomes is accelerated in flc-3, hen4-2 and hen4-2 flc-3, and delayed in flk-2. The number of juvenile leaves in hen4-2 flk-2 plants does not differ from that of hen4-2 individuals. Bars represent means ± SD. Data from two independent experiments with 21 plants per genotype. Significant differences with respect to the wild type, flc-3 and flk-2 are indicated by black, red and blue asterisks, respectively. (b) Representative examples of 6th (left) and 8th (right) rosette vegetative leaves in Col-0 and hen4-2. Red arrows point at the only two trichomes in the 6th hen4-2 leaf. Scale bars: 1 cm. (c) Relative expression at 21 °C of genes relevant to the progression of the vegetative phase as monitored by qPCR. Bars represent means ± SD. Asterisks indicate significant differences with Col-0. *P < 0.05; **P < 0.01; ***P < 0.001. ANOVA (a) and Student’s t-test (c).
We also analysed the flc-3 and hen4-2 flc-3 mutants. In line with previous reports21,22, abaxial trichomes appeared in flc-3 mutants earlier than in Col-0 plants (Fig. 7a). Remarkably, the phenotype of flc-3 plants was intermediate between Col-0 and hen4-2 plants whereas the hen4-2 flc-3 double mutant was essentially identical to hen4-2 plants. Moreover, flk-2 plants, which flower late due to FLC overproduction42 (Fig. 2d), exhibited a delayed phase transition (Fig. 7a). Interestingly, the hen4-2 mutation also rescued the flk-2 late phase transition, and no significant difference was found between hen4-2 and hen4-2 flk-2 plants (Fig. 7a). Overall, these results paralleled the flowering phenotypes shown above (Fig. 2, Supplementary Figs S2, S4, S6) and strongly suggest that HEN4 controls the vegetative phase transition via FLC regulation, but also suggest that additional factors might be required to explain completely the hen4-2 effect. MAF4 was an obvious candidate. Nevertheless, occurrence of abaxial trichomes in maf4 rosette leaves did not differ with respect to the wild type (Supplementary Fig. S10), suggesting a moderate (if any) contribution of MAF4 to the regulation of the vegetative phase-change, and also that additional HEN4 targets are yet to be found.
The influence of HEN4 on juvenile-to-adult transition was supported by expression of molecular markers of trichome formation such as TEMPRANILLO2 (TEM2) and GLABROUS INFLORESCENCE STEMS (GIS). TEM2 encodes an AP2-type transcription factor that represses the formation of trichomes, the vegetative phase-change, and the floral transition46 whereas GIS encodes a C2H2-domain transcription factor with opposite effects47. As shown in Fig. 7c, TEM2 and its paralog TEM1 were downregulated in hen4-2 whereas GIS expression was increased, nicely fitting the observed phenotypes. Likewise, SPL3, SPL8, SPL9 and SPL15, gene activities that promote the vegetative phase-change and the floral induction1,5, increased significantly in the hen4-2 background (Fig. 7c). SPL9 and SPL15 delay the rate of leaf initiation48,49. This might explain why flowering time difference between wild-type and hen4-2 plants is more pronounced in terms of leaves (Figs 1–6, Supplementary Fig. S1).
SPL genes activate transcription of MIR172 genes whose products, in turn, downregulate the AP2-EREBP floral repressors AP2, TARGET OF EARLY ACTIVATION TAGGED 1 (TOE1), TOE2, TOE3, SCHLAFMUTZE (SMZ) and SNARCHZAPFEN (SNZ), mainly at the translational level50,51,52. In line with this, the MIR172B and MIR172E genes were induced in hen4-2 vegetative leaves as compared to the wild type (Fig. 7c) whereas the mRNA levels of AP2 genes examined did not show significant changes (Supplementary Fig. S11). Altogether, these data support the role of HEN4 delaying the transition to the vegetative adult phase.
SPL3, SPL9 and SPL15 are repressed by miR1565. Nevertheless, the expression of MIR156 family members, including MIR156A and MIR156C, was unaltered in the mutant (Supplementary Fig. S12). We cannot exclude that a very small decrease in miR156/miR157 might lead to significant increment in SPL abundance, as recently reported53. However, SPL8, an SPL family member not targeted by miRNA156, was also upregulated in hen4-2 plantlets (Fig. 7c). SPL8 is induced by gibberellins (GA) which also activate the miR156-targeted SPL genes, thus promoting the vegetative transition and trichome differentiation1,54. HEN4 might affect GA activity via FLC. FLC and DELLA proteins can act as corepressors20, and SVP and FLC directly regulate GA metabolic and signalling genes55,56. In line with this, the expression of GIBBERELLIN-3-OXIDASE 2 (GA3OX2), encoding a key GA biosynthetic enzyme57 increased in the hen4-2 background whereas the catabolic activity encoded by GIBBERELLIN 2-OXIDASE 2 (GA2OX2)57 decreased with respect to the wild type, suggesting an increment of GA activity in the mutant (Supplementary Fig. S12). Moreover, the TEM genes are direct targets of FLC and SVP56 and also repress GA biosynthetic genes46,58. This is congruent with increased expression of the SOC1 paralog AGAMOUS-LIKE 42 (AGL42) in hen4-2 (Supplementary Fig. S12). AGL42 functions as a floral inducer primarily on the GA pathway59.
Discussion
The life cycle of flowering plants is characterized by successive developmental transitions governed by complex genetic programs and subject to endogenous and environmental stimuli. In this study, we reveal that HEN4 regulates the vegetative phase transition and floral induction, a dual role largely explained by upregulation of FLC (and its paralog MAF4). Several lines of evidence support this conclusion. FLC and MAF4 mRNA expression was drastically reduced in strong hen4-2 mutants. In consonance, hen4-2 rescued late-flowering and abaxial trichome appearance in the flk-2 background together with strong suppression of FLC and MAF4 overexpression. Also, in agreement with very low levels of FLC expression, hen4-2 plants flowered much earlier than the wild type under short-day conditions.
Given the structural similarity shared by MAF genes26, the HEN4 specificity towards MAF4 is intriguing but not unprecedented. The floral promoter AGL6 regulates negatively only MAF4 and MAF5 in addition to FLC60. Likewise, the RING-finger protein AtRING1A regulates flowering through repressing only MAF4 and MAF5 but not FLC61.
Many flowering regulators are multifaceted. For example, the autonomous and thermosensory pathways share several factors, and FLC participates in the vernalization, thermosensory and autonomous pathways7,9. Furthermore, FLC was recently described as a key modulator for adaptive plasticity against several environmental factors, including ambient temperature62. MAF4 has been also described as a floral repressor although its role is not yet as clearly defined25. FLC and MAF4 expression increased at low ambient temperature in the wild type (Fig. 8), and dramatically dropped in hen4-2 plants at both 16 °C and 21 °C. This is reminiscent of ACTIN RELATED PROTEIN 6 (ARP6), a component of the SWR1 chromatin remodelling complex that indirectly repress flowering by maintaining FLC, MAF3 and MAF4 expression in Arabidopsis63,64. Interestingly, the arp6 mutants phenocopy warm-growth plants and show a more rapid juvenile-to-adult transition63,64.
Schematic representation of HEN4 influence on the vegetative phase-change and the floral transition. HEN4 and low temperatures (16 °C) upregulate FLC and MAF4 whose products assemble into MADS-box repressor complexes (curved boxes) including SVP (also stabilized by low ambient temperature) and other MAF-clade members. These complexes then repress SPLs, MIR172, GA activity genes and floral integrators (yellow)5,56, contributing to slow down the progression to vegetative adult state (competence to flower) and delaying the floral transition. The MADS-box repressor complexes also activate the TEM genes that cooperate delaying both developmental transitions. The hen4 mutant phenotype is largely but not totally explained by loss of FLC and/or MAF4, thus possible FLC/MAF4 independent regulation by HEN4 of known, or as yet unidentified, factors (denoted by ?) that promote juvenility and/or delay flowering is tentatively represented by direct interactions. Positive regulation is represented by black arrows whereas negative interactions are depicted as red blunt-ended lines. Dashed lines indicate putative interactions. For simplicity, some players and interactions have been deliberately omitted in the figure.
The hen4-2 mutant rescued flowering delay under short-day conditions, which is consistent with reduced FLC expression44. The role of HEN4 in flowering time control was also strongly evidenced in hen4 flk double mutants. Unlike other autonomous pathway mutants that also function in thermosensory flowering, late-flowering flk plants do not show elevated SVP expression and are clearly responsive to temperature drop, blooming considerably later at 16 °C18. Most remarkably, hen4-2 flk-2 plants flowered earlier than the wild type at both temperatures. Suppression of flowering delay in hen4-2 flk-2 correlated with mRNA expression decrease for both FLC and MAF4.
SVP and all members of the FLC/MAF-clade interact with each other25 likely assembling into tetrameric complexes65. Most probably, drastic reduction of FLC and MAF4 contribution to such protein complexes is reflected in the reduced sensitivity to growth temperature of the hen4-2 mutant.
How does HEN4 regulate FLC and MAF4? HEN4 might regulate its target transcripts directly. Not mutually exclusive, HEN4 might interact with FLK, encoding a three KH-domain RBP that represses FLC42,43. The mechanism whereby FLK performs this function remains unknown. Interestingly, we found that HEN4 and PEP (also encoding KH-domain RBPs) interact with FLK at the protein level32,66. So, it is tempting to speculate that the HEN4 protein might interfere with the FLK repressive action on FLC, although this scenario does not fit with hen4-2 epistasis over flk-2. Contrariwise, FLK might also repress FLC via protein inhibition of FLC activators such as HEN4. Further work is required to elucidate if some of these possibilities are correct.
As part of the HUA-PEP activity, HEN4 participates in flower and ovule morphogenesis, regulating AG, SHPs and STK pre-mRNAs31,32,33. More than a decade ago, structurally similar AG and FLC genes were hypothesized to share common post-transcriptional regulatory mechanisms31,36. Indeed, other HUA-PEP members such as HUA2 and PEP were shown to activate FLC and delay flowering36,37. Now, our genetic and molecular analyses describe HEN4 as a new regulator of flowering and vegetative phase-change, broadening the scope of developmental processes governed by members of the HUA-PEP regulatory module. This is in agreement with our recent finding that the HUA-PEP activity retains the ability to regulate floral MADS-box homeotic genes in vegetative leaves33.
In the hen4-2 mutant, reduced FLC expression resulted in precocious occurrence of leaf abaxial trichomes, indicating a shortening of the juvenile vegetative phase. The role of FLC delaying the juvenile-to-adult transition is firmly established21,22. However, our genetic data suggested the involvement of additional factors regulated by HEN4 beyond FLC. MAF4 was a candidate. However, the MAF4 contribution to the vegetative transition is unclear since leaf abaxial trichome occurrence in maf4 seedlings was wild-type. Loss of MAF4 might influence the progression of the vegetative phase but not to the extent of generating a morphological phenotype on its own, likely masked by redundant activities.
SVP and FLC directly regulate GA metabolic and signalling genes55,56 (Fig. 8). In hen4-2, increased GA activity was suggested by variations in GA3OX2 and GA2OX2 genes. Higher GA activity in hen4-2 might explain in part the elevated expression of SPL genes. This notion was supported by upregulation of the miRNA156-independent SPL8 gene54. SPL genes promote trichome differentiation and the vegetative transition, and directly activate FT and SOC13,49 (Fig. 8), also contributing to explain precocious flowering in the hen4 mutants. Additionally, TEM1 and TEM2 were downregulated in hen4-2. The TEM genes are direct targets of FLC and SVP56, acting as floral inhibitors that repress FT and TSF, but also genes related to GA biosynthesis and the trichome program, being described as regulators of juvenility46,58,67,68,69 (Fig. 8). FLC and GA signalling interact to modulate flowering20,21. For instance SPL15, which is directly repressed by FLC and upregulated in hen4-221(Fig. 8), coordinates flowering by integrating GA signalling and diverse environmental cues3. Additional HEN4 targets that, independently of FLC (and MAF4), impinge on flowering thermoregulation and/or vegetative phase transition, still await to be characterized (? symbol in Fig. 8). Investigations to elucidate the identity of such factors are currently underway.
In conclusion, we have functionally characterized HEN4 as a factor connecting the vegetative phase and flowering through FLC and MAF4 positive regulation. Repressors such as FLC and its clade-members are crucial to prevent premature developmental transitions and facilitate reproduction under favourable conditions. Understanding how plants integrate signals to regulate reproductive development is vital to cope with variations driven by environmental phenomena such as climate change.
Materials and Methods
Plant material and phenotypic analyses
All plants used in this study were in the Arabidopsis thaliana (L.) Heynh., Columbia (Col-0) background except hen4-2, originally isolated in Ler31 and backcrossed five times into Col-0. soc1-640(SALK_138131C), flm-330, maf4 (SALK_028506C), svp-3218, hen4-3 (SAIL_364_H12; this work) and hen4-4 (SAIL_874_G03; this work) were obtained from the Nottingham Arabidopsis Stock Centre (NASC). Other lines used were flk-242 and flc-312. All double mutants were generated by crossing. PCR-based genotyping was used to identify homozygous lines. Information about primer sequences and molecular genotyping is listed in Supplementary Table S1.
Seeds were surface-sterilized, stratified for 2 days at 4 °C and grown on Murashige & Skoog (MS) plates or soil under short-day (8 h day and 16 h night) or continuous light (130 mol m−2 s−1) generated by cool white light fluorescent tubes (Sylvania standard F65W) as previously described66,70. Floral timing was scored as the number of days and rosette leaves produced from sowing to bolting. In all experiments at least 20 plants were analysed per genotype and treatment. Replicated experiments were carried out in the same growth chamber using different seed batches. Disposition of trays was randomized in order to minimize position effects inside the chamber66,70. The onset of abaxial trichome occurrence was measured observing rosette leaves under a stereomicroscope and photographed with an IDS digital camera (UI-1490SE-C), operated by the uEye 4.90 program. Growing plants were photographed with a Canon digital camera 1000D. Data were subjected to analysis of variance (ANOVA) to determine significant differences (*P < 0.05; **P < 0.01; ***P < 0.001) among genotypes and temperature treatments. Standard deviation (SD) was calculated from aggregate data from independent experiments.
Quantitative PCR
Quantitative reverse transcriptase-polymerase chain reaction (qPCR) was carried out according to Rodríguez-Cazorla et al.33 with minor modifications. 5 μg of total RNA was extracted from 12-day-old rosettes grown at 21 °C, or 15-day-old rosettes when grown at 16 °C, treated with DNase I, and used for cDNA synthesis with an oligo (dT) primer and RevertAid Reverse Transcriptase (ThermoFisher) following the manufacturer’s instructions. Subsequently, for each qPCR reaction, 0.5 μl of the cDNA was used as template. Relative changes in gene expression levels were determined using the LightCycler 1.5 system with the Maxima SYBR Green qPCR Master Mix kit according to the manufacturer (ThermoScientific). RNA levels were normalized to the constitutively expressed gene OTC (ORNITHINE TRANSCARBAMYLASE), and the corresponding wild-type levels, as previously reported32. Each experiment was undertaken using three biological replicates with three technical replicates each. Statistical significance was estimated by the Student’s t-test according to Pfaffl et al.71 (*P < 0.05; **P < 0.01; ***P < 0.001). PCR primer sequences are listed in Supplementary Table S2.
Data Availability
All data generated or analysed during this study are included in this published article and its Supplementary files.
References
Huijser, P. & Schmid, M. The control of developmental phase transitions in plants. Development 138, 4117–4129 (2011).
Poethig, R. S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 105, 125–152 (2013).
Hyun, Y., Richter, R. & Coupland, G. Competence to flower: age-controlled sensitivity to environmental cues. Plant Physiol. 173, 36–46 (2017).
Wang, J. W., Czech, B. & Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 (2009).
Spanudakis, E. & Jackson, S. The role of microRNAs in the control of flowering time. J. Exp. Bot. 65, 365–380 (2014).
Fornara, F., de Montaigu, A. & Coupland, G. SnapShot: control of flowering in Arabidopsis. Cell 141(550), 550.e1–2 (2010).
Srikanth, A. & Schmid, M. Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 68, 2013–2037 (2011).
Bouché, F., Woods, D. P. & Amasino, R. M. Winter memory throughout the plant kingdom: different paths to flowering. Plant Physiol. 173, 27–35 (2017).
Blázquez, M. A., Ahn, J. H. & Weigel, D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33, 168–171 (2003).
Capovilla, G., Schmid, M. & Pose, D. Control of flowering by ambient temperature. J. Exp. Bot. 66, 59–69 (2015).
Lee, J. H. et al. Integration of floral inductive signals by flowering locus T and suppressor of overexpression of Constans 1. Physiol. Plant. 126, 475–483 (2006).
Michaels, S. D. & Amasino, R. M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956 (1999).
Hartmann, U. et al. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 21, 351–360 (2000).
Yamaguchi, A., Kobayashi, Y., Goto, K., Abe, M. & Araki, T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 46, 1175–1189 (2005).
Li, D. et al. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell 15, 110–120 (2008).
Jang, S., Torti, S. & Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 60, 614–625 (2009).
Sheldon, C. C. et al. The FLF MADS-box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11, 445–458 (1999).
Lee, J. H. et al. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 21, 397–402 (2007).
Lee, J. H. et al. Regulation of Temperature-Responsive Flowering by MADS-Box Transcription Factor Repressors. Science. 342, 628–632 (2013).
Li, M. et al. DELLA proteins interact with FLC to repress flowering transition. J. Integr. Plant Biol. 58, 642–655 (2016).
Deng, W. et al. FLOWERINGLOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. 108, 6680–6685 (2011).
Willmann, M. R. & Poethig, R. S. The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development 138, 677–685 (2011).
Ratcliffe, O. J., Nadzan, G. C., Reuber, T. L. & Riechmann, J. L. Regulation of flowering in Arabidopsis by an FLC homologue. Plant Physiol. 126, 122–132 (2001).
Scortecci, K. C., Michaels, S. D. & Amasino, R. M. Identification of a MADS-box gene, FLOWERING LOCUS M, that represses flowering. Plant J. 26, 229–236 (2001).
Gu, X. et al. Arabidopsis FLC clade members form flowering-repressor complexes coordinating responses to endogenous and environmental cues. Nat. Commun. 4, 1947 (2013).
Ratcliffe, O. J., Kumimoto, R. W., Wong, B. J. & Riechmann, J. L. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15, 1159–1169 (2003).
Posé, D. et al. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503, 414–417 (2013).
Airoldi, C. A., McKay, M. & Davies, B. MAF2 is regulated by temperature-dependent splicing and represses flowering at low temperatures in parallel with FLM. PLoS One 10, e0126516 (2015).
Sureshkumar, S., Dent, C., Seleznev, A., Tasset, C. & Balasubramanian, S. Nonsense-mediated mRNA decay modulates FLM-dependent thermosensory flowering response in Arabidopsis. Nat. Plants 2, 16055 (2016).
Balasubramanian, S., Sureshkumar, S., Lempe, J. & Weigel, D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLOS Genet. 2, e106 (2006).
Cheng, Y., Kato, N., Wang, W., Li, J. & Chen, X. Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS pre-mRNA in Arabidopsis thaliana. Dev. Cell 4, 53–66 (2003).
Rodríguez-Cazorla, E. et al. K-homology nuclear ribonucleoproteins regulate floral organ identity and determinacy in Arabidopsis. PLOS Genet. 11, e1004983 (2015).
Rodríguez-Cazorla, E. et al. Ovule identity mediated by pre-mRNA processing in Arabidopsis. PLOS Genet. 14, e1007182 (2018).
Pinyopich, A. et al. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424, 85–88 (2003).
Favaro, R. et al. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell 15, 2603–2611 (2003).
Doyle, M. R. et al. HUA2 is required for the expression of floral repressors in Arabidopsis thaliana. Plant J. 41, 376–385 (2005).
Ripoll, J. J. et al. Antagonistic interactions between Arabidopsis K-homology domain genes uncover PEPPER as a positive regulator of the central floral repressor FLOWERING LOCUS C. Dev. Biol. 333, 251–262 (2009).
Peccarelli, M. & Kebaara, B. W. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Eukaryot. Cell 13, 1126–1135 (2014).
Adrian, J., Torti, S. & Turck, F. From decision to commitment: the molecular memory of flowering. Mol. Plant 2, 628–642 (2009).
Chen, J. et al. Suppressor of overexpression of CO 1 negatively regulates dark-induced leaf degreening and senescence by directly repressing pheophytinase and other senescence-associated genes in Arabidopsis. Plant Physiol. 173, 1881–1891 (2017).
Andrés, F. & Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13, 627–639 (2012).
Mockler, T. C. et al. Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc. Natl. Acad. Sci. 101, 12759–12764 (2004).
Lim, M.-H. et al. A new Arabidopsis gene, FLK, encodes an RNA binding protein with K homology motifs and regulates flowering time via FLOWERING LOCUS C. Plant Cell 16, 731–740 (2004).
Michaels, S. D. & Amasino, R. M. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13, 935–41 (2001).
Telfer, A., Bollman, K. M. & Poethig, R. S. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124, 645–654 (1997).
Matías-Hernández, L. et al. TEMPRANILLO reveals the mesophyll as crucial for epidermal trichome formation. Plant Physiol. 170, 1624–1639 (2016).
Gan, Y. et al. GLABROUS INFLORESCENCE STEMS modulates the regulation by gibberellins of epidermal differentiation and shoot maturation in Arabidopsis. Plant Cell 18, 1383–1395 (2006).
Wang, J.-W., Schwab, R., Czech, B., Mica, E. & Weigel, D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 20, 1231–1243 (2008).
Wu, G. et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009).
Aukerman, M. J. & Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741 (2003).
Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science. 303, 2022–2025 (2004).
Yamaguchi, A. & Abe, M. Regulation of reproductive development by non-coding RNA in Arabidopsis: to flower or not to flower. J. Plant Res. 125, 693–704 (2012).
He, J. et al. Threshold-dependent repression of SPL gene expression by miR156/miR157 controls vegetative phase change in Arabidopsis thaliana. PLOS Genet. 14, e1007337 (2018).
Zhang, Y., Schwarz, S., Saedler, H. & Huijser, P. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol. Biol. 63, 429–439 (2007).
Andrés, F. et al. SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proc. Natl. Acad. Sci. 111, 627–639 (2014).
Mateos, J. L. et al. Combinatorial activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C define distinct modes of flowering regulation in Arabidopsis. Genome Biol. 16, 31 (2015).
Hedden, P. & Thomas, S. G. Gibberellin biosynthesis and its regulation. Biochem. J. 444, 11–25 (2012).
Osnato, M., Castillejo, C., Matías-Hernández, L. & Pelaz, S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat. Commun. 3, 808 (2012).
Dorca-Fornell, C. et al. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. Plant J. 67, 1006–1017 (2011).
Yoo, S. K., Wu, X., Lee, J. S. & Ahn, J. H. AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis. Plant J. 65, 62–76 (2011).
Shen, L. et al. The putative PRC1 RING-finger protein AtRING1A regulates flowering through repressing MADS AFFECTING FLOWERING genes in Arabidopsis. Development 141, 1303–1312 (2014).
Méndez-Vigo, B. et al. Environmental and genetic interactions reveal FLOWERING LOCUS C as a modulator of the natural variation for the plasticity of flowering in Arabidopsis. Plant. Cell Environ. 39, 282–294 (2016).
Martin-Trillo, M. et al. EARLY IN SHORT DAYS 1 (ESD1) encodes ACTIN-RELATED PROTEIN 6 (AtARP6), a putative component of chromatin remodelling complexes that positively regulates FLC accumulation in Arabidopsis. Development 133, 1241–1252 (2006).
Kumar, S. V. & Wigge, P. A. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147 (2010).
Theissen, G. & Saedler, H. Plant biology: floral quartets. Nature 409, 469–471 (2001).
Ripoll, J. J., Ferrándiz, C., Martínez-Laborda, A. & Vera, A. PEPPER, a novel K-homology domain gene, regulates vegetative and gynoecium development in Arabidopsis. Dev. Biol. 289, 346–359 (2006).
Sgamma, T., Jackson, A., Muleo, R., Thomas, B. & Massiah, A. TEMPRANILLO is a regulator of juvenility in plants. Sci. Rep. 4, 3704 (2015).
Marín-González, E. et al. SHORT VEGETATIVE PHASE up-regulates TEMPRANILLO2 floral repressor at low ambient temperatures. Plant Physiol. 169, 1214–1224 (2015).
Matías-Hernández, L., Aguilar-Jaramillo, A. E., Cigliano, R. A., Sanseverino, W. & Pelaz, S. Flowering and trichome development share hormonal and transcription factor regulation. J. Exp. Bot. 67, 1209–1219 (2016).
Zavala-Gonzalez, E. A. et al. Arabidopsis thaliana root colonization by the nematophagous fungus Pochonia chlamydosporia is modulated by jasmonate signaling and leads to accelerated flowering and improved yield. New Phytol. 213, 351–364 (2017).
Pfaffl, M. W., Horgan, G. W. & Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36 (2002).
Acknowledgements
This research was funded by grant BIO2014-56321-P from the Spanish Ministry of Economy and Competitiveness to A.V. and A.M-L. The NASC is acknowledged for supplying seeds.
Author information
Authors and Affiliations
Contributions
A.V., A.M.-L., S.O.-M. and E.R.-C. designed the research; S.O.-M. and E.R.-C. performed the experiments; A.V., A.M.-L., S.O.-M., E.R.-C. and E.A.Z.-G. analysed data; E.A.Z.-G. provided technical assistance; A.V. wrote the manuscript; with contributions of all the authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ortuño-Miquel, S., Rodríguez-Cazorla, E., Zavala-Gonzalez, E.A. et al. Arabidopsis HUA ENHANCER 4 delays flowering by upregulating the MADS-box repressor genes FLC and MAF4. Sci Rep 9, 1478 (2019). https://doi.org/10.1038/s41598-018-38327-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38327-3
This article is cited by
-
Comparative transcriptomic analysis of male and females in the dioecious weeds Amaranthus palmeri and Amaranthus tuberculatus
BMC Plant Biology (2023)
-
Identification and characterization of MADS box gene family in pigeonpea for their role during floral transition
3 Biotech (2021)
-
The Arabidopsis KH-domain protein FLOWERING LOCUS Y delays flowering by upregulating FLOWERING LOCUS C family members
Plant Cell Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.