Abstract
Coffee is believed to prevent postoperative ileus. This systematic review and meta-analysis was undertaken to determine the effectiveness of coffee consumption in stimulating gastrointestinal function after abdominal surgery. A number of databases for randomized controlled trials comparing coffee consumption following abdominal surgery versus water drinking or no intervention were searched. Cochrane’s Risk of Bias tool was used to assess risk of bias in included studies. Six trials involving 601 participants were included. All studies had high risk of performance bias. Three studies had an unclear risk of selection bias. Postoperative coffee consumption reduced time to first defecation (mean difference (MD), −9.98 hours; 95% CI, −16.97 to −2.99), time to first flatus (MD, −7.14 hours; 95% CI, −10.96 to −3.33), time to first bowel sound (MD, −4.17 hours; 95% CI, −7.88 to −0.47), time to tolerance of solid food (MD, −15.55 hours; 95% CI, −22.83 to −8.27), and length of hospital stay (MD, −0.74 days; 95% CI, −1.14 to −0.33). Benefits increased with increasing complexity of the procedure. None of the included studies reported adverse events associated with coffee consumption. Postoperative coffee consumption is effective and safe for enhancing the recovery of gastrointestinal function after abdominal surgery.
Similar content being viewed by others
Introduction
Although surgical techniques and perioperative care have improved with time, postoperative ileus (POI) remains a frequent complication following abdominal surgery1. POI is a transient impairment of coordinated bowel motility in response to the trauma of surgery thus leading to accumulation of gastrointestinal secretions within the lumen of the gut1,2. Symptoms of POI include failure to pass stool or flatus, nausea, vomiting, and abdominal pain and usually lasts 2 to 4 days after surgery1. Factors associated with an increased risk of POI following abdominal surgery include colorectal surgery, open abdominal surgery, emergency operations, prolonged operative time, amount of opioid analgesic use, longer nasogastric catheter use, and smoking history1,3,4. POI contributes to delay patient recovery, prolonged hospital stay, and increased post-operative morbidity and healthcare costs1,5.
The pathophysiology of postoperative ileus is multifactorial1,2. There are three proposed mechanisms involved in the development of POI, namely neurogenic, inflammatory and pharmacological mechanisms. The neurogenic mechanisms contribute to the immediate phase of POI occurring during operation by inhibiting sympathetic stimulation and increasing adrenergic motor neuronal activity. The second phase of POI, which typically occurs 3–4 h after operation, is mediated through the inflammation process caused by the release of pro-inflammatory cytokines and chemokines. This inflammatory response then activates the phagocytes residing throughout the gut to release nitric oxide and prostaglandins which reduce bowel mobility by inhibiting smooth muscle contractility1,2. Several pharmacological agents can diminish gastrointestinal peristalsis. The use of a crystalloid solution can result in intestinal edema and stretching. Common anesthetic agents such as halothane, enflurance, and atropine prolong gastric emptying times which increase risks of postoperative nausea and vomiting. Postoperative narcotic analgesia is associated with decreased gastrointestinal mobility by activating opioid receptors1,2.
Coffee is one of the most popular beverages consumed worldwide. Coffee consists of several biologically active compounds, such as caffeine, diterpenes, and chlorogenic acids, which can positively affect human health6. Coffee consumption mitigates the risks of cardiovascular and metabolic disorders by its antioxidant activity6. Coffee is associated with a reduction in the incidence of diabetes, liver disease, and Parkinson’s disease6. In addition, coffee consumption trends to reduce mortality6.
Coffee also induces bowel movement7,8. Coffee stimulates motor activity of the large intestine within a few minutes after intake, particularly in the transverse and descending colon7,8. Based on this finding as well as its popularity as beverage and low cost, coffee might be a promising option to hasten the recovery of gastrointestinal function after abdominal surgery. Therefore, it is crucial to establish strong evidence as to whether postoperative coffee consumption is effective and safe for alleviating POI. Accordingly, this systematic review and meta-analyses of randomized controlled trials was conducted to determine the influence of postoperative coffee consumption in enhancing the early recovery of gastrointestinal function following abdominal surgery.
Methods
The details of the protocol for this systematic review were registered with PROSPERO (CRD42018085090). This meta-analysis was performed and reported according to the PRISMA statement9.
Criteria for considering studies for this review
Randomized controlled trials (RCTs) comparing coffee consumption following any kind of abdominal surgery versus drinking water or no intervention irrespective of language of publication, publication status, year of publication, or sample size were included.
Types of outcome measures
The primary outcome was the time to first defecation. Secondary outcomes included the time to first passage of flatus, the time to first bowel movement sound, the time to tolerance of solid food, possible adverse effects of postoperative coffee intake, and length of hospital stay.
Search methods for identification of studies
To identify potential eligible studies, a systematic literature search was conducted using the major electronic databases including MEDLINE, Scopus, ISI Web of Science, Pubmed, CINAHL, and Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to March 2018. Reference lists of articles were retrieved by the search and authors of the trials contacted to obtain additional data if necessary. In addition, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (www.who.int/ictrp) were searched for unpublished, planned and ongoing trial reports. Open Grey (www.opengrey.eu) was searched for grey literature. To ensure the comprehensive searches, the titles of all relevant articles were identified on Google Scholar and then a further search was made related to these studies focusing on the first 50 records identified10.
Study selection and data extraction
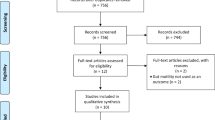
Titles and abstracts of studies retrieved by electronic searching were screened independently by two review authors. Those studies where their titles and abstracts clearly did not meet the inclusion criteria were excluded. The full texts of potentially eligible studies were retrieved and independently assessed by two review authors. Figure I presents the PRISMA flow diagram. The risk of bias of included studies was independently evaluated by two authors using the Cochrane Risk of Bias Tool for Randomized Controlled Trials11. Data were extracted independently onto a data abstraction form specifically designed for the review. Any disagreements were resolved through discussion with a third person.
Statistical analysis
Statistical analysis using the Cochrane Review Manager (RevMan) software was performed12. The random-effects model with inverse variance weighting for all meta-analyses as between-study heterogeneity that was expected was applied13,14. For continuous outcomes, e.g. time to first defecation, time to first flatus, time to first bowel sound, time to tolerance of solid food, and length of hospital stay, the mean differences (MD) with their 95% confidence intervals (CI) were calculated. If continuous outcomes were expressed as median and range, the study author was contacted to obtain sample mean and standard deviation (SD) data. If this was not possible, the data were converted to mean and SD using the formulae proposed by Wan et al.15. For dichotomous outcomes (postoperative nausea), the risk ratios (RR) and the 95% CI were calculated.
Statistical heterogeneity in each meta-analysis using the I² statistic was assessed: values of I2 greater than 50% were indicative of significant heterogeneity and the Chi² tests, where a cut off of p value < 0.1 indicating whether there was statistical evidence of heterogeneity were considered14. Subgroup analysis was carried out according to the types of operative procedure. Sensitivity analyses to assess the robustness of the findings by repeating the analysis excluding studies judged to be at high risk or unclear risk of selection bias was performed. A leave-one-out sensitivity analysis was also performed by iteratively excluding one included study with largest effect size to confirm that our findings were not driven by any single study.
Results
Characteristics of included studies
A broad search yielded 88 references from the electronic database searches. Two additional references from other sources were identified. After de-duplication, 29 references were screened and 20 references were excluded that obviously did not meet the inclusion criteria. Of the nine studies that potentially met the review inclusion, six studies were included after reviewing the full texts involving 601 participants (Supplementary Table S1). Figure 1 displays the PRISMA flowchart for study selection. Five included studies published in peer-reviewed journals16,17,18,19,20. One included study was only available in an academic search engine (Supplementary Table S1).
Of the six included studies, a study of Dulskas et al.18 divided the participants into three groups to receive regular coffee, decaffeinated coffee, and water. Participants who received two different kinds of coffee in this included study were then combined into a single group to create a single pair-wise comparison. All participants in a study of Göymen et al.17 received decaffeinated coffee. The remaining four included studies used regular coffee. Göymen et al.17 reported their continuous outcomes as median and interquartile range. Two groups of participants in a study of Göymen et al.17 who received no intervention or water were combined into a single control group.
Of the six included studies, three evaluated the effects of coffee consumption after cesarean delivery. The remaining three included studies were undertaken among participants undergoing colorectal (2 studies) and gynecologic cancer surgery (1 study). Of the two included studies that were undertaken among participants undergoing colorectal surgery, one study evaluated the effects of coffee consumption following laparoscopic left-sided colectomy. Approximately 61% of participants in the remaining study underwent laparotomy for colectomy. All participants in the study of gynecologic cancer surgery underwent the laparotomy approach. The operations performed in all included studies were elective procedures (Supplementary Table S1).
All of studies included in this review do not clearly state the use of the Enhanced Recovery after Surgery (ERAS) protocol or other ‘fast-track’ protocols during postoperative care.
Four ongoing trials which are determining the coffee consumption after small or large bowel resections were identified (Supplementary Table S2).
Risk of bias in included studies
Figure 2 presents the summary of the risk of bias for each included study. All included studies had high risk of performance bias as they were unable to blind the participants about the intervention allocated. Three included studies were determined as to be of unclear risk of selection bias (Supplementary Table S1).
Effects of interventions
Time to first defecation
Meta-analyses assessing 601 participants indicated that postoperative coffee consumption reduced the time to first defecation when compared to water or no intervention (MD, −9.98 hours; 95% CI, −16.97 to −2.99 hours). Subgroup analysis, however, revealed that the significant effect of coffee consumption in reducing the time to first defecation was observed in either colorectal or gynecologic cancer surgeries but not in cesarean delivery (Fig. 3).
Time to first flatus
Coffee consumption after operation significantly decreased the time to first flatus when compared to water or no intervention (MD, −7.14 hours; 95% CI, −10.96 to −3.33 hours). The significant impact of coffee in reducing the time to first flatus was observed across the three different types of surgical procedures, with the largest magnitude of effect in participants undergoing gynecologic cancer surgery (Fig. 4).
Time to first bowel sound
The time to the first audible bowel sound was only evaluated in 434 participants who underwent cesarean delivery or gynecologic cancer surgery. Overall, participants allocated to the coffee group had a shorter time to first audible bowel sound when compared to those in the control group (MD, −4.17 hours; 95% CI, −7.88 to −0.47 hours). In subgroup analysis, however, the significant effect was solely observed among participants undergoing gynecologic cancer surgery (Fig. 5).
Time to tolerance of solid food
Meta-analysis assessing 476 participants showed that participants with coffee consumption had a shorter time to tolerance of solid food than those given water or no intervention (MD, −15.55 hours; 95% CI, −22.83 to −8.27 hours). The significant shorter time to tolerance of solid food after coffee consumption was noted across the three different types of operations (Fig. 6).
Postoperative nausea
Postoperative nausea was assessed in 359 participants undergoing cesarean delivery and gynecologic cancer surgery (Fig. 7). Overall, there was no significant difference in the risk of postoperative nausea between the participants assigned to the coffee group and those in the control group (RR, 0.61; 95% CI, 0.27 to 1.36). There was, however, a significantly lower risk of postoperative nausea in participants undergoing gynecologic cancer surgery who received coffee than that in the control group (RR, 0.21; 95% CI, 0.05 to 0.95).
Length of hospital stay
Meta-analysis assessing 476 participants indicated a shorter length of hospital stay among the participants in the coffee group than those assigned to the control group (MD, −0.74 days; 95% CI, −1.14 to −0.33 days), with the largest benefit in gynecologic cancer surgery (MD, −1.30; 95% CI, −2.11 to −0.49 days; Fig. 8).
Sensitivity analysis
After excluding three studies that were judged to have an unclear risk of selection bias17,18,20, the magnitude of favorable effects of postoperative coffee consumption in shortening the time to first defecation, time to first flatus, and time to first audible bowel sounds was substantially increased with a more precise estimate of their corresponding 95% CIs. The overall effects on the time to tolerance of solid food, postoperative nausea, and length of hospital stay, however, tended to be unchanged (Table 1). Leave-one-out analyses which removed a study of Gungorduk et al.19 showed no marked difference in the results excepting time to first bowel sound movement which may indicate that the most of the pooled estimates were not solely driven by one single study (Table 1).
Discussion
Main findings
Evidence from this review is based on six RCTs with 601 participants. It was found that postoperative coffee consumption significantly reduced the time to first defecation, time to first flatus, time to first audible bowel sound, and time to tolerance of solid food. Postoperative coffee consumption slightly reduced the length of hospital stay. The effect sizes appeared to be directly associated with the complexity of surgical procedures with the largest benefit in gynecologic cancer surgery and smallest in cesarean delivery. However, the results observed in the patients with gynecologic cancer were based on a single small study, future RCTs with a sufficiently sample size is required to confirm these promising results.
Sensitivity analyses applying risk of selection bias and leave-one out basis showed no clinically important changes in the findings. None of the six included studies reported adverse events associated with coffee consumption.
Strengths and limitations of the study
This systematic review has some strengths. First, investigators are not aware of any previous systematic reviews determining the effectiveness of postoperative coffee consumption with the aim of enhancing the recovery of gastrointestinal functions following surgery. Second, this review restricted included studies to RCTs in order to use only the strongest evidence. Third, this systematic review indicates the benefits of coffee consumption in promoting the recovery of gastrointestinal functions that were observed across different types of abdominal surgeries. In addition, the robustness of the review findings was reaffirmed by sensitivity analyses.
This review has some limitations. As it was not feasible to blind the participant to intervention received, all included studies were rated at high risk of performance bias which may influence some treatment effect estimates. The operations performed in all included studies were elective procedures. Therefore, generalization of the results to the patients undergoing emergency operations may be limited. A limited number of included studies that where minimally invasive surgery was performed precluded the ability to carry out a subgroup analyses to assess the impact of the types of surgical approaches.
Interpretation in light of other evidence
Controversy still exists about whether the effects of decaffeinated coffee on the recovery of gastrointestinal functions are comparable to regular coffee. In one of the included studies that was conducted among women undergoing cesarean delivery, all participants allocated in the intervention group received decaffeinated coffee. The findings of this study found no significant differences in the recovery of gastrointestinal functions after decaffeinated coffee consumption in terms of time to first defecation (MD, −1.27 hours; 95% CI, −3.95 to 1.42), time to first flatus (MD, −1.90 hours; 95% CI, −4.33 to 0.54), and time to first audible bowel sound (MD, −0.80; 95% CI, −3.26 to 1.65) when compared to the control group17. In contrast, another included study in which two of the three groups of participants received regular coffee (28 participants) and the third decaffeinated coffee (28 participants) showed comparable benefits in stimulating gastrointestinal functions following colorectal surgery between these two comparison groups in terms of the time to defecation (3.75 versus 3.00 hours), time to first flatus (1.57 versus 1.47 hours), and time to tolerance of solid food (2.60 versus 1.85 hours)18. These available data were too sparse to permit drawing meaningful conclusions.
Although there were no reported adverse events associated with postoperative coffee consumption in any included study in this review, coffee is not without potential adverse effects; for instance, coffee drinking acutely raises blood pressure and heart rate which are secondary to an increased circulating concentrations of norepinephrine21,22.Thus hypertensive patients may be discouraged from postoperative coffee consumption22. In addition, coffee drinking may have adverse sleep-related consequences including decreased total sleep time, difficulty falling asleep, increased nocturnal awakenings, and daytime sleepiness23,24. Although while the negative impact of coffee on sleep patterns in healthy individuals are mostly mild, it may lead to increased worrying and complicate the well-being in postoperative patients.
Chewing gum is another promising intervention for enhancing the early recovery of gastrointestinal functions after operation. The Cochrane systematic review assessing the effectiveness of chewing gum after surgery reported some evidence for the benefit of postoperative chewing gum in improving recovery of gastrointestinal functions by reduction of time to first flatus (−10.4 hours; 95% CI: −11.9, −8.9), time to bowel movement (−12.7 hours; 95% CI: −14.5, −10.9), time to first bowel sound (−5.0 hours; 95% CI: −6.4, −3.7). In addition, use of chewing gum slightly reduced length of hospital stay (−0.7 days; 95% CI: −0.8, −0.5)25. Similar to the present findings, the effect sizes of intervention on outcomes were directly related to the complexity of the surgical procedure. The benefits were substantial in colorectal surgery but only minimal in cesarean delivery25. Interestingly, this review suggested that the effect of chewing gum on outcomes was generally reduced in the analysis of studies conducted within an ERAS context25.
Implications for review findings
This present review highlights that postoperative coffee consumption is an innovative intervention to hasten the recovery of gastrointestinal function following abdominal surgery given that it is generally well tolerated by individuals, low-cost, widely available and easy to implement. Based on these promising results, clinicians may consider advising patients to drink coffee during postoperation period as appropriate.
More studies are needed to explore the effects of coffee consumption after a variety of different surgical settings e.g. emergency operations and a minimally invasive surgical approach. Additionally, as the ERAS protocol, an evidence-based care improvement process, is becoming more widespread throughout various fields in surgery26,27, the usefulness of postoperative coffee consumption within an ERAS context is a further relevant question to be addressed.
Conclusions
Postoperative coffee consumption is effective for stimulating the recovery of gastrointestinal function after abdominal surgery. This intervention also reduces the length of hospital stay. The benefits appear to increase with an increased complexity of the surgical procedure.
References
Bragg, D., El-Sharkawy, A. M., Psaltis, E., Maxwell-Armstrong, C. A. & Lobo, D. N. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr 34, 367–376 (2015).
Luckey, A., Livingston, E. & Tache, Y. Mechanisms and treatment of postoperative ileus. Arch Surg 138, 206–214 (2003).
Sugawara, K. et al. Perioperative Factors Predicting Prolonged Postoperative Ileus After Major Abdominal Surgery. J Gastrointest Surg 22, 508–515 (2018).
Chapman, S. J., Pericleous, A., Downey, C. & Jayne, D. G. Postoperative ileus following major colorectal surgery. Br J Surg 105, 797–810 (2018).
Iyer, S., Saunders, W. B. & Stemkowski, S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm 15, 485–494 (2009).
Cano-Marquina, A., Tarin, J. J. & Cano, A. The impact of coffee on health. Maturitas 75, 7–21 (2013).
Rao, S. S., Welcher, K., Zimmerman, B. & Stumbo, P. Is coffee a colonic stimulant? Eur J Gastroenterol Hepatol 10, 113–118 (1998).
Sloots, C. E., Felt-Bersma, R. J., West, R. L. & Kuipers, E. J. Stimulation of defecation: effects of coffee use and nicotine on rectal tone and visceral sensitivity. Scand J Gastroenterol 40, 808–813 (2005).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009).
Haddaway, N. R., Collins, A. M., Coughlin, D. & Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLoS One 10, e0138237 (2015).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March: The Cochrane Collaboration; 2011. Available from, Cochrane handbook.org, www.cochrane-handbook.org 2011).
Cochrane Collaboration. Review manager (Version 5.3) [Computer Software]. Copenhagen, Denmark: The Nordic Cochrane Centre, Cochrane Collaboration; 2014.
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 45, 139–145 (2015).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14, 135 (2014).
Muller, S. A. et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg 99, 1530–1538 (2012).
Göymen, A. et al. Effect of gum chewing and coffee consumption on intestinal motility in caesarean sections. J Clin Anal Med (2016).
Dulskas, A., Klimovskij, M., Vitkauskiene, M. & Samalavicius, N. E. Effect of coffee on the length of postoperative ileus after elective laparoscopic left-sided colectomy: a randomized, prospective single-center study. Dis Colon Rectum 58, 1064–1069 (2015).
Gungorduk, K. et al. Effects of coffee consumption on gut recovery after surgery of gynecological cancer patients: a randomized controlled trial. Am J Obstet Gynecol 216, 145 e1–e7 (2017).
Rabiepoor, S., Yas, A., Navaei, J. & Khalkhali, H. R. Does coffee affect the bowel function after caesarean section? Eur J Obstet Gynecol Reprod Biol 220, 96–99 (2018).
Riksen, N. P., Rongen, G. A. & Smits, P. Acute and long-term cardiovascular effects of coffee: implications for coronary heart disease. Pharmacol Ther 121, 185–191 (2009).
Palatini, P. et al. Coffee consumption and risk of cardiovascular events in hypertensive patients. Results from the HARVEST. Int J Cardiol 212, 1131–137 (2016).
Calamaro, C. J., Mason, T. B. & Ratcliffe, S. J. Adolescents living the 24/7 lifestyle: effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics 123, e1005–10 (2009).
Chaudhary, N. S., Grandner, M. A., Jackson, N. J. & Chakravorty, S. Caffeine consumption, insomnia, and sleep duration: Results from a nationally representative sample. Nutrition 32, 1193–1199 (2016).
Short, V. et al. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst Rev 2, CD006506 (2015).
Ljungqvist, O., Scott, M. & Fearon, K. C. Enhanced Recovery After Surgery: A Review. JAMA Surg 152, 292–298 (2017).
Kleppe, K. L. & Greenberg, J. A. Enhanced Recovery After Surgery Protocols: Rationale and Components. Surg Clin North Am 98, 499–509 (2018).
Acknowledgements
Professor Emeritus James A. Will from the University of Wisconsin edited the manuscript via the Medical Faculty Publication Clinic KKU, Thailand.
Author information
Authors and Affiliations
Contributions
N.E. and C.K. designed the study, collected the data, performed the statistical analysis; interpretation of the results, and wrote the manuscript; N.J. and C.N. contributed to the statistical analysis, and interpretation of the results; S.K. and P.L. contributed to discussion and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eamudomkarn, N., Kietpeerakool, C., Kaewrudee, S. et al. Effect of postoperative coffee consumption on gastrointestinal function after abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Sci Rep 8, 17349 (2018). https://doi.org/10.1038/s41598-018-35752-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35752-2
Keywords
This article is cited by
-
Gum Chewing and Coffee Consumption but not Caffeine Intake Improve Bowel Function after Gastrointestinal Surgery: a Systematic Review and Network Meta-analysis
Journal of Gastrointestinal Surgery (2023)
-
Short-term outcome of diverting loop ileostomy reversals performed by residents: a retrospective cohort prognostic factor study
International Journal of Colorectal Disease (2023)
-
The effect of coffee/caffeine on postoperative ileus following elective colorectal surgery: a meta-analysis of randomized controlled trials
International Journal of Colorectal Disease (2022)
-
Bacillus coagulans TBC169 probiotics for the recovery of intestinal function after gynecological laparoscopic surgery: a randomized, placebo-controlled trial
International Journal of Clinical Pharmacy (2022)
-
Comparison of treatment to improve gastrointestinal functions after colorectal surgery within enhanced recovery programmes: a systematic review and meta-analysis
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.