Abstract
Vitamin D status is inversely associated with the prevalence of metabolic syndrome (MetS). Whether this is true in the elderly without vitamin D deficiency is rarely investigated. Our data source is a cross-sectional survey of 1,966 community-dwelling elderly Taiwanese in 2012. An overnight fasting blood were obtained for biochemistry variables. Vitamin D deficiency was defined as serum 25-hydroxyvitamin D3 [25(OH)D] concentration <20 ng/mL. MetS is defined using modified ATP-III criteria. Of 523 participants without vitamin D deficiency (Men/Women = 269/254, age = 76.0 ± 6.2 years old [65–102 years old]), mean 25(OH)D was 44.0 ± 11.1 ng/mL, and the MetS prevalence of MS was 46.5%. Serum 25(OH)D was negatively associated with osteocalcin, the homeostatic model assessment insulin resistance (HOMA-IR) index, body mass index (BMI), and glycated hemoglobin A1c. Participants with more MetS features have lower serum 25(OH)D and osteocalcin. Binary logistic regression models showed that 25(OH)D, physical activity, and osteocalcin were negatively independent MetS factors, but that the HOMA-IR index, BMI, and being female were positively independent factors. The risk of MetS was progressively lower along with the increased 25(OH)D concentration, even above 60 ng/mL. In conclusion, a low 25(OH)D concentration is an independent risk factor for MetS in elderly people without vitamin D deficiency.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a cluster of abnormal metabolic markers: hypertension, insulin resistance, proinflammatory processes, dyslipidemia, and abnormal fat distribution. It is considered a major risk factor for type 2 diabetes mellitus (DM), cardiovascular disease (CVD), and all-cause mortality1,2. Because its prevalence increases with age, preventing it is important in aging societies.
Advanced age is also a known risk factor for vitamin D deficiency3,4. Vitamin D has been a focus of attention in recent decades because it is involved in bone metabolism and chronic diseases like MetS, type 2 DM, CVD, cancer, and cognitive dysfunction5. Several mechanisms linking vitamin D deficiency and MetS have been proposed: the alteration of the differentiation of preadipocytes, the inhibition of pancreatic β cell function, insulin resistance (IR), systemic inflammation, and elevated blood pressure induced by activating the renin-angiotensin-aldosterone system (RAAS)6. Several biomarkers have been evaluated to explain these mechanisms. The homeostatic model assessment insulin resistance (HOMA-IR) index is useful for estimating pancreatic β cell function and IR, and many studies have reported a negative association between 25(OH)D concentration and the HOMA-IR index7. However, one study8 of elderly Chinese found no association between IR and vitamin D status in patient with type 2 DM. High sensitivity C-reactive protein (hsCRP) is a commonly used biomarker for systemic inflammation, and several studies9 found an inverse relationship between vitamin D status and inflammation markers.
In addition to its role in bone mineralization and calcium ion homeostasis, osteocalcin induces pancreatic β cells to release more insulin and adipocytes to release adiponectin, which increases one’s sensitivity to insulin10. In the past decade, osteocalcin has been reported11 to be inversely associated with body adiposity and MetS, but it might predict MetS in the elderly.
Although most studies focus on the differences of metabolic aberrations between people with and without vitamin D deficiency, almost none focus on the elderly without vitamin D deficiency. The interrelationships among vitamin D, osteocalcin, the HOMA-IR index, hsCRP, and MetS are complicated and inadequately evaluated. Thus, we investigated these associations in the elderly without vitamin D deficiency, defined as 25-hydroxyvitamin D [25(OH)D] <20 ng/mL11. We hypothesized that there would be a consistently inverse association between 25(OH)D and MetS, even in the elderly without vitamin D deficiency.
Results
After excluding participants with vitamin D deficiency, we reviewed and analyzed 523 medical records (269 men, 254 women; mean age: 76.0 ± 6.2 years old; age range: 65–102 years) with complete data. The average 25(OH)D concentration was 44.0 ± 11.1 ng/mL (range: 20–70 ng/mL), and the prevalence of MetS was 46.5%. The Mini Nutrition Assessment (MNA) showed that 82.2% of the participants were well-nourished, and the Short Portable Mental Status Questionnaire (SPMSQ) showed that 89% had no or mild cognitive impairment. Participants with MetS were more often female, less literate, and had higher BMI, higher high sensitivity C-reactive protein (hsCRP), lower 25(OH)D, and lower osteocalcin levels (Table 1). Serum 25(OH)D concentrations were not significantly different between participants taking (40.1 ± 11.7; n = 5) and not taking (43.9 ± 11.2; n = 518) vitamin D supplements (Appendix 1). Vitamin D concentrations were more often relatively lower (20 ng/mL ≤ 25(OH)D < 30 ng/mL) in women, those living with a partner, with greater cognitive impairment, who smoked less, and who had a higher fasting glucose level, homeostasis model assessment insulin resistance (HOMA-IR) index, and osteocalcin level (Appendix 2). Similar findings were presented for lower 25(OH)D subjects defining as 20 ng/mL ≤ 25(OH)D < 40 ng/mL (Appendix 3).
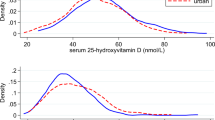
Serum 25(OH)D was significantly negatively associated with osteocalcin (r = 0.098, p < 0.05), the HOMA-IR index (r = −0.134, p < 0.01), intact parathyroid hormone (iPTH) (r = −0.156, p < 0.01), and body mass index (BMI) (r = −0.107, p < 0.05) (Table 2). Participants with more features of MetS tended to have relatively lower serum 25(OH)D (ptrend < 0.001) and osteocalcin levels (ptrend < 0.05); and a higher HOMA-IR index (ptrend < 0.001) and HbA1c levels (ptrend < 0.001) (Figs 1–4).
We used four logistic regression models to analyze the odds ratio (OR) of MetS. Model 1 showed that age (OR = 0.95, 95% CI: 0.92–0.98, p = 0.002), physical activity (OR = 0.57, 95% CI: 0.35–0.93, p = 0.026), serum 25(OH)D (OR = 0.98, 95% CI: 0.96–0.99, p = 0.007), and osteocalcin level (OR = 0.18, 95% CI: 0.07–0.46, p < 0.001) were negatively independent factors, but that being female (OR = 2.8, 95% CI: 1.59–4.92, p < 0.001) was a positive independent factor. In Models 2 and 3, the HOMA-IR index and BMI, respectively, were added for analysis. The significance of vitamin D and osteocalcin was lower but still significant. In Model 4, serum 25(OH)D was a significant negative independent factor (OR = 0.98, 95% CI: 0.96–0.99, p = 0.047), as were BMI and the adjusted HOMA-IR index (Table 3).
Using the same binary logistic regression models to analyze participants with either vitamin D > 32 ng/mL12 or vitamin D > 40 ng/mL showed that 25(OH)D was an independent factor for MetS (Appendices 4 and 5). We further categorized 25(OH)D concentration into 5 subgroups [20 ng/mL ≤ 25(OH)D < 30 ng/mL (n = 49), 30 ng/mL ≤ 25(OH)D < 40 ng/mL (n = 155), 40 ng/mL ≤ 25(OH)D < 50 ng/mL (n = 162), 50 ng/mL ≤ 25(OH)D < 60 ng/mL (n = 107), 25(OH)D ≥ 60 ng/mL (n = 50)]. The odds ratio (OR) showed a decreasing trend along with the increased 25(OH)D concentration (Fig. 5). The ORs for metabolic syndrome reached significance at 50 ng/mL ≤ 25(OH)D < 60 ng/mL (OR = 0.43, 95% CI: 0.20–0.94, p = 0.03) and 25(OH)D ≥ 60 ng/mL (OR = 0.25, 95% CI: 0.10–0.66, P = 0.005) in Model 1 (Appendix 6), and at 25(OH)D ≥ 60 ng/mL (OR = 0.25, 95% CI: 0.08–0.73, p = 0.012) in Model 4 (Appendix 6).
Discussion
Our findings that being female, a higher HOMA-IR index, lower physical activity, osteocalcin13, and vitamin D concentrations are independent risk factors of MetS are consistent with other studies1,2,7,14. Moreover, our study confirmed these relationships in elderly people without vitamin D deficiency or insufficiency.
Old age is a known risk factor for vitamin D deficiency3, but physical activity, exposure to sunlight, skin pigmentation, clothing, diet, and nutritional status might affect serum 25(OH)D concentrations14,15. Our participants lived in an area with abundant annual sunshine, and 44.3% of them were still active farmers. The MNA showed that they were robustly nourished, which might explain their relatively low prevalence of vitamin D deficiency or insufficiency and indicate a nature-nurture relationship between MetS and vitamin D.
The seasonal variation of serum 25(OH)D might be concerned but not consistent in different regions14,16,17. In a large Taiwan National Nutrition Survey18 and studies in Taiwan19,20, the seasonal variation of serum 25(OH)D was trivial (less than 3 ng/ml). As a randomized controlled trial found that vitamin D supplements were beneficial for lowering IR, especially when serum 25(OH)D concentration was ≥32 ng/mL (80 nmol/L)12. We used a similar binary logistic regression analysis in selected participants with serum concentrations of 25(OH)D ≥32 ng /mL or ≥40 ng/mL to reflect the possible effect of seasonal changes of vitamin D status. Vitamin D status was consistently an independent variable for MetS.
The core metabolic abnormality in MetS is IR. Other studies21,22,23 have reported that vitamin D modulates the effect of insulin by directly increasing the expression of insulin receptor, which increases insulin responsiveness for transporting glucose, or by indirectly regulating extracellular calcium in insulin-responsive skeletal muscle and adipose tissue. However, obesity might complicate the relationship between vitamin D and MetS. Obesity is associated with IR and with high blood pressure and dyslipidemia; thus, it is a significant risk factor for MetS1. There is a complex interrelationship between adiposity and vitamin D. Because vitamin D is fat-soluble, it can be sequestered, or diluted, in enlarged adipose compartment, thereby lowering serum 25(OH)D concentrations24. Most studies25,26 have reported that serum vitamin D status falls as adiposity increases. A large bidirectional genetic study27 hypothesized that a higher BMI leads to lower 25(OH)D, but that “any effects of lower 25(OH)D increasing BMI are likely to be small”. We found that vitamin D status negatively correlated with HOMA-IR and BMI. However, although we added both BMI and HOMA-IR to the analysis, vitamin D remained a significant independent negative factor for MetS. The regression models for patients with 25(OH)D concentrations ≥32 and ≥40 ng/mL were not significantly different (Appendices 4 and 5). This might indicate that there is an unidentified underlying mechanism other than increased IR and obesity that links vitamin D and MetS.
Other mechanisms linking vitamin D and MetS have been proposed5. The roles of vitamin D in immune system had been extensively studied in recent 10 years28,29. Vitamin D and its analogues inhibit the production of interleukin-2 and interferon-γ, and they stimulate the effects of T-helper type 2 lymphocytes, which leads to a reduction in matrix metalloproteinase and inhibits the progression of atherosclerotic plaque30. Because inflammation is an important component of MetS31 and vitamin D is anti-inflammatory, MetS might be linked to vitamin D deficiency. We chose hsCRP as the inflammation biomarker because it predicts cardiovascular risk32. Observational studies33,34,35,36,37 report inconsistent and variable results for the association of inflammatory markers and serum 25(OH)D. One study38 reported that bi-directional Mendelian randomization analyses showed no evidence of a causal relationship between high levels of 25(OH)D and low levels of CRP. One large observational study39 reported, in an asymptomatic general population, a significant inverse relation between 25(OH)D and serum CRP, independent of traditional cardiovascular risk factors, but only at concentrations <21 ng/mL; at concentrations ≥21 ng/mL, it was associated with an increase in serum CRP. The relatively higher 25(OH)D concentrations in our patients might have led to a nonsignificant association between 25(OH)D and hsCRP in our elderly participants. The relationship among 25(OH)D, CRP, and other inflammatory markers require additional investigations.
Although the association was not observed in our study, vitamin D deficiency was reported40,41 to be associated with high blood pressure, possibly through lack of suppression of the renin-angiotensin system42,43. The roles of vitamin D in atherogenic dyslipidemia has received less attention than has the mechanism discussed in the previous paragraph. In our study, the prevalence of hypertriglyceridemia and HDLC levels based on MetS criteria) were significantly lower in those with 25(OH)D concentrations ≥40 ng/mL. Most observational studies show that serum 25(OH)D is positively related to serum HDL-C and negatively related to total cholesterol (TC)/HDL-C and LDL-C/HDL-C ratios and TG44. In an animal study45, vitamin D receptor knockout mice expended more energy and consumed more oxygen than did wild-type mice; thus, they had less body fat and lower plasma TG and TC levels. In a recent study46, calcitriol suppressed hepatic triglyceride formation and reduced hepatic fat accumulation at above-physiological serum concentrations. More investigation is needed to clarify the role of 25(OH)D in lipid metabolism.
Osteocalcin is a novel IR biomarker of insulin resistance and believed to link bone and glucose metabolism. Serum osteocalcin levels are lower in middle-aged to elderly people with DM47 and elderly people with MetS48. We found that the total osteocalcin level was negatively associated with the components of the HOMA-IR index, MetS, and BMI. However, after HOMA-IR index had been adjusted for, osteocalcin was not a significant factor of MetS. Another study49 reported that weight loss and regular exercise significantly upregulated circulating osteocalcin. Because losing weight and exercise are important interventions against MetS and IR, osteocalcin might be a biomarker for MetS mediated by IR or obesity. Additional study is warranted.
The cutoff value of optimal serum 25(OH)D remains controversial. It is generally agreed that bone metabolism will be compromised once serum 25(OH)D falls <20 ng/mL11; optimal intestinal calcium absorption50 and suppression of serum iPTH occurs when serum 25(OH)D reaches about 30 ng/mL51. However, decades of debate, there is no consensus on the optimal level of 25(OH)D for predicting and preventing MetS, CVD, and other related chronic diseases. Nevertheless, it has been confirmed that patients with a lower, or even relatively adequate, 25(OH)D concentration have a higher risk of MetS. On the other hand, along with the increment of serum 25(OH)D concentration, the OR for metabolic syndrome shows a decreasing trend without an obvious ceiling effect (Fig. 5) as the highest 25(OH)D concentration in our study subjects is 70 ng/mL. Further investigation was needed to clarify the protective effect of high 25(OH)D concentration on metabolic risk.
In this study, serum 25(OH)D was an independent factor for MetS in the elderly without vitamin D deficiency. Interestingly, several meta-analyses7,24,52,53,54 have reported a limited effect of vitamin D supplementation on glucose homeostasis, diabetes prevention, adipokine levels, blood pressure control, and systemic inflammation. Studies7,55 have reported that the gene polymorphism of vitamin D synthesis (CYP1alpha), vitamin D transporter (DBP gene), and vitamin D receptor (ApaI, TaqI, BsmI, FokI) was associated with glucose intolerance and IR, and that it may influences the optimal effect of vitamin D supplements on preventing diabetes, IR, and glucose homeostasis. Or, perhaps vitamin D is a surrogate, like hsCRP in cardiovascular disease32, and is a biomarker for the integrated effect of metabolic abnormality which is not efficacious without the synchronized intervention of life style modification, exercise, weight control, etc. More studies of the beneficial effects of vitamin D supplementation on MetS risks are is warranted, especially in the elderly without a severe vitamin D deficiency.
Limitations
Our study has some limitations. First, this is a cross-sectional study, which cannot disclose causal relationships but only correlations. The actual seasonal variation of serum 25(OH)D was not determined; those variations might be trivial in Taiwanese18,19,20. Second, our participants were ambulatory and relatively healthy, but we were unable to recruit and enroll critically ill or disabled elderly. Therefore, our findings might not be applicable to people who are not ambulatory or who are bedridden. Our participants might have been relatively healthy elderly to whom the primary prevention of disease was especially important. Finally, our participants elderly Taiwanese living in a rural village, so that extrapolating our findings to the general population or to other ethnicities has to be made with caution.
Conclusion
In the elderly without vitamin D deficiency or insufficiency, serum 25(OH)D concentrations are inversely associated with metabolic syndrome risk.
Methods
Study participants
Tianliao District, located in Kaohsiung City in southern Taiwan, is a tropical, agricultural community at about 22°50′N latitude, like Hawaii (18° 55′N to 28° 27′N), and about 7° farther south than Florida (24°27′N to 31°00′N). The mean annual temperature is 25.1 °C (range: 19.3–29.2 °C) and mean amount of sunshine is 212.2 hours per month (longest: 221.4 hours in July; shortest: 161.8 hours in December). In the 2012 census report, the total population of Tianliao District was 7800, and 1966 (25.2%) participants were ≥65 years old. A cross-sectional survey56,57 targeted Tianliao’s elderly (≥65 years old) was conducted in July 2012. After we had excluded empty houses (n = 489) and deceased (n = 40), non-ambulatory (significantly disabled: n = 138), and unreachable residents (n = 201), we finally enrolled 285 men and 264 women using the whole-district sampling method. The response rate was 50% (549/1098).
Ethical approval
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital in compliance with the World Medical Association Declaration of Helsinki and good practice (GCP) guideline. Each participant signed an informed consent before the study began.
Data Collection: Questionnaire
Well-trained staff used a 20-minute structured questionnaire56,57 for individual face-to-face interviews with participants. The questionnaires asked about (a) sociodemographic characteristics: sex, age, occupational status, living status, and education; (b) lifestyle: cigarette smoking and alcohol drinking; (c) medical history: DM, hypertension, dyslipidemia, and medication; and (d) noninvasive assessment tools: the short-form International Physical Activity Questionnaires (IPAQ)58, the Mini Nutritional Assessment (MNA)59, and the 10-item Short Portable Mental Status Questionnaire (SPMSQ)60.
Cigarette smoking was quantified by packs/year (PYs), and habitual alcohol drinking was defined as “drinking alcohol more than once a week for more than half a year”. Physical activity was categorized by tertiles (low, middle, and high) levels)61. Nutritional status was categorized as normal (score: ≥24), at risk of malnutrition (score: ≥17 to <24), and malnutrition (score: <17) using the MNA62. Cognitive function was categorized as unimpaired to mildly impaired (score: 0–5) and moderately to severely impaired (score: 6–10)63.
Anthropometric variables
Body weight (BW) to the nearest 0.1 kg, and body height (BH) to the nearest mm (Solo® Eye-Level Clinical Scale; Detecto, Webb City, MO, USA) were measured with the participants wearing light clothing without shoes. The body mass index (BMI) kg/BH[m2]) was calculated. With the participant standing naturally, looking forward, and wearing only underwear, their waist circumference (WC) was measured to the nearest mm, using a standard tape (Gulick II; Country Technology, Inc., Gays Mills, WI, USA), midway between the lateral lower rib margin and the superior anterior iliac crest after a gentle breath expiration. Blood pressure (BP) was the average of two right-arm readings (HEM-7230; Omron, Tokyo, Japan) with the participant sitting down. Each variable was measured by the same trained staff.
Biochemistry variables
The laboratory data (fasting blood glucose, high-density lipoprotein cholesterol (HDL-C), triglyceride, fasting blood insulin, high sensitivity C-reactive protein (hsCRP), intact parathyroid hormone (iPTH), and osteocalcin) were obtained from blood samples after participants had fasted overnight. The homeostasis model assessment insulin resistance (HOMA-IR) was obtained analyzing fasting blood glucose and fasting blood insulin64. Serum 25(OH)D concentrations and insulin and osteocalcin levels were measured using competitive radioimmunoassay kits (Cobas®; Roche Vitamin D Total first generation assay (25OHD-I), Roche Diagnostics, Indianapolis, IN, USA). The coefficients of variation were 25(OH)D: 3.1%, insulin: 1.9%, and osteocalcin: 3.3%.
Diagnosis of MetS and vitamin D deficiency
MetS was diagnosed if a participant had three or more of the following five criteria defined by the Modified National Cholesterol Education Program Adult Treatment Panel III (ATPIII) Guideline for the Chinese population65: (a) fasting plasma glucose level ≥100 mg/dL or taking a hypoglycemic agent; (b) systolic blood pressure ≥130 mmHg, or diastolic blood pressure ≥85 mmHg, or taking an anti-hypertension medication; (c) serum HDL-C level <40 mg/dL (men) or <50 mg/dL (women); (d) serum triglyceride level ≥150 mg/dL; (e) WC ≥90 cm (men) or ≥80 cm (women). Although the cutoff of vitamin D deficiency and insufficiency is disputed because its high prevalence is disproportionate to actual illness, vitamin D deficiency is universally defined as a serum 25(OH)D concentration <20 ng/mL (50 nmol/L); vitamin D insufficiency is defined as a 25(OH)D concentration <30 ng/mL11.
Data analysis and statistical methods
All continuous variables (age, BMI, WC, and laboratory data) are expressed as means ± standard deviation (SD). Dichotomous data (sociodemographics, habitual alcohol drinking, PYs of cigarette smoking, physical activity, nutrition status, and cognitive function) are expressed as percentages. The log transformation method for HOMA-IR and osteocalcin was used to fit them to the normal distribution model. The differences in continuous and dichotomized variables between MetS− and MetS+ participants were analyzed using independent t tests or χ2 tests, respectively.
Pearson correlations of 25(OH)D concentration, log (osteocalcin), log (HOMA-IR), hsCRP, and BMI were calculated. The Ptrend of 25(OH)D concentration, osteocalcin, HOMA-IR and Hba1c with components of MetS were analyzed by using ANOVA. Binary logistic regression analysis was used to assess the independent contribution to the MetS by possible associated factors: age, sex, BMI, living and literacy statuses, current employment, habitual alcohol drinking, PYs of cigarette smoking, physical activity, nutrition status, cognitive function, 25(OH)D concentration (either as a continuous or as a categorical variable), log (osteocalcin), log (HOMA-IR), and hsCRP. The Nagelkerke pseudo R2 test was used to describe how well the model predicted MetS. SPSS 17 for Windows was used for all analyses. Significance was set at P < 0.05 (two-tailed).
References
Cornier, M. A. et al. The metabolic syndrome. Endocr. Rev. 29, 777–822 (2008).
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428 (2005).
Gallagher, J. C. Vitamin D and aging. Endocrinol. Metab. Clin. North Am. 42(2), 319–332 (2013).
Janssen, H. C., Samson, M. M. & Verhaar, H. J. Vitamin D deficiency, muscle function, and falls in elderly people. Am. J. Clin. Nutr. 75, 611–615 (2002).
Kulie, T., Groff, A., Redmer, J., Hounshell, J. & Schrager, S. Vitamin D: an evidence-based review. J. Am. Board Fam. Med. 22, 698–706 (2009).
Awad, A. B., Alappat, L. & Valerio, M. Vitamin D and metabolic syndrome risk factors: evidence and mechanisms. Crit. Rev. Food Sci. Nutr. 52, 103–112 (2012).
Alvarez, J. A. & Ashraf, A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 351385 (2010).
Luo, C. et al. Hypovitaminosis D in Chinese type 2diabetes: lack of impact on clinical metabolic status and biomarkers of cellular inflammation. Diab. Vasc. Dis. Res. 6, 194–199 (2009).
Jackson, J. L. et al. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J. Clin. Transl. Endocrinol. 5, 21–25 (2016).
Alfadda, A. A., Masood, A., Shaik, S. A., Dekhil, H. & Goran, M. Association between osteocalcin, metabolic syndrome, and cardiovascular risk factors: role of total and undercarboxylated osteocalcin in patients with type 2 diabetes. Int. J. Endocrinol. 2013, 197519 (2013).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
von Hurst, P. R., Stonehouse, W. & Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient–a randomised, placebo-controlled trial. Br. J. Nutr. 103(4), 549–555 (2010).
Oosterwerff, M. M., Schoor, N. M., Lips, P. & Eekhoff, E. M. Osteocalcin as a predictor of the metabolic syndrome in older persons: a population‐based study. Clin. Endocrinol. 78, 242–247 (2013).
Mithal, A. et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 20, 1807–1820 (2009).
Brock, K. et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid Biochem. Mol. Biol. 121, 462–466 (2010).
Levis, S. et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. J. Clin. Endocrinol. Metab. 90(3), 1557–1562 (2005).
Heidari, B. & Haji Mirghassemi, M. B. Seasonal variations in serum vitamin D according to age and sex. Caspian J. Intern. Med. 3(4), 535 (2012).
National survey of nutritional status in serum vitamin D level: from NAHIST 1993–1996 to 2005–2008. available online at https://obesity.hpa.gov.tw/TC/researchList.aspx?cid=163 (Accessed April 3, (2018).
Tsai, K. S. et al. Bone mineral density and bone markers in relation to vitamin D receptor gene polymorphisms in Chinese men and women. Bone 19(5), 513–518 (1996).
Hwang, J. S. et al. Vitamin D status in non-supplemented postmenopausal Taiwanese women with osteoporosis and fragility fracture. BMC Musculoskelet. Disord. 15(1), 257 (2014).
Pittas, A. G., Lau, J., Hu, F. B. & Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 92, 2017–2029 (2007).
Al-Shoumer, K. A. & Al-Essa, T. M. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J. Diabetes 6(8), 1057 (2015).
Strange, R. C., Shipman, K. E. & Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 6(7), 896 (2015).
Hyppönen, E. & Boucher, B. J. Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutr. Rev. 76(9), 676–692 (2018).
Parikh, S. J. et al. The relationship between obesity and serum 1, 25-dihydroxy vitamin D concentrations in healthy adults. J. Clin. Endocrinol. Metab. 89, 1196–1199 (2004).
Araghi, S. O. et al. BMI and body fat mass is inversely associated with vitamin D levels in older individuals. J. Nutr. Health Aging 19, 980–985 (2015).
Vimaleswaran, K. S. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 10(2), e1001383 (2013).
Prietl, B., Treiber, G., Pieber, T. R. & Amrein, K. Vitamin D and immune function. Nutrients 5(7), 2502–2521 (2013).
Hewison, M. Vitamin D and immune function: an overview. Proc. Nutr. Soc. 71(1), 50–61 (2012).
Andress, D. L. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 69(1), 33–43 (2006).
Sharma, P. Inflammation and the metabolic syndrome. Indian J. Clin. Biochem. 26(4), 317–318 (2011).
Danesh, J. et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 350, 1387–1397 (2004).
Peterson, C. A. & Heffernan, M. E. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25 (OH) D concentrations in healthy women. J. Inflamm. (Lond.) 5(1), 10 (2008).
Miller, R. R. et al. Association of serum vitamin D levels with inflammatory response following hip fracture: the Baltimore HipStudies. J. Gerontol. A Biol. Sci. Med. Sci. 62(12), 1402–1406 (2007).
Ewers, B., Gasbjerg, A., Zerahn, B. & Marckmann, P. Impact of vitamin D status and obesity on C-reactive protein in kidney-transplant patients. J. Ren. Nutr. 18(3), 294–300 (2008).
Clendenen, T. V. et al. Factors associated with inflammation markers, a cross-sectional analysis. Cytokine 56(3), 769–778 (2011).
de Oliveira, C., Biddulph, J. P., Hirani, V. & Schneider, I. J. C. Vitamin D and inflammatory markers: cross-sectional analyses using data from the English Longitudinal Study of Ageing (ELSA). J. Nutr. Sci 6, e1 (2017).
Liefaard, M. C. et al. Vitamin D and C-reactive protein: a Mendelian randomization study. PLoS One, 10(7), e0131740 (2015).
Amer, M. & Qayyum, R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006). Am. J. Cardiol. 109(2), 226–230 (2012).
Jorde, R., Figenschau, Y., Emaus, N., Hutchinson, M. & Grimnes, G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension 55(3), 792–798 (2010).
Scragg, R., Sowers, M. & Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 20(7), 713–719 (2007).
Li, Y. C. et al. Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 89, 387–392 (2004).
Zhou, C. et al. Calcium-independent and 1, 25 (OH) 2D3-dependent regulation of the renin-angiotensin system in 1α-hydroxylase knockout mice. Kidney Int 74(2), 170–179 (2008).
Jorde, R. & Grimnes, G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog. Lipid Res. 50(4), 303–312 (2011).
Wang, J. H. et al. Serum cholesterol and expression of ApoAI, LXRβ and SREBP2 in vitamin D receptor knock-out mice. J. Steroid Biochem. Mol. Biol. 113(3-5), 222–226 (2009).
Cheng, S. et al. Calcitriol reduces hepatic triglyceride accumulation and glucose output through Ca2+/CaMKKβ/AMPK activation under insulin-resistant conditions in type 2 diabetes mellitus. Curr. Mol. Med. 16(8), 747–758 (2016).
Kindblom, J. M. et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J. Bone Miner. Res. 24, 785–791 (2009).
Yeap, B. B. et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur. J. Endocrinol. 163, 265–272 (2010).
Fernández-Real, J. M. et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J. Clin. Endocrinol. Metab. 94, 237–245 (2009).
Heaney, R. P. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am. J. Clin. Nutr. 80(6), 1706S–1709S (2004).
Chapuy, M. C. et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 7(5), 439–443 (1997).
Seida, J. C. et al. Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 99, 3551–3560 (2014).
Beveridge, L. A. et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern. Med. 175, 745–754 (2015).
Agbalalah, T., Hughes, S. F., Freeborn, E. J. & Mushtaq, S. Impact of vitamin D supplementation on endothelial and inflammatory markers in adults: a systematic review. J. Steroid Biochem. Mol. Biol. 173, 292–300 (2017).
Sung, C. C., Liao, M. T., Lu, K. C. & Wu, C. C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 634195 (2012).
Liu, M. C. et al. Cyclophilin A is associated with peripheral artery disease and chronic kidney disease in geriatrics: the Tianliao Old People (TOP) study. Sci. Rep. 5, 9937 (2015).
Wu, C. H. et al. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr. Gerontol. Int. 14, 69–75 (2014).
Qu, N. & Li, K. Study on the reliability and validity of international physical activity questionnaire (Chinese Vision, IPAQ). Zhonghua Liu Xing Bing Xue Za Zhi 25, 265–268 (2004).
Guigoz, Y., Vellas, B. & Garry, P. J. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 54(S_1), 59 (1996).
Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 23, 433–441 (1975).
Chang, C. S. et al. Smoking, habitual tea drinking and metabolic syndrome in elderly men living in rural community: the Tianliao Old People (TOP) study 02. PLoS One 7, e38874 (2012).
Vellas, B. et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15, 116–122 (1999).
Stump, T. E., Callahan, C. M. & Hendrie, H. C. Cognitive impairment and mortality in older primary care patients. J. Am. Geriatr. Soc. 49, 934–940 (2001).
Hotta, Y. et al. Low leptin but high insulin resistance of smokers in Japanese men. Diab. Res. Clin. Pract. 81, 358–364 (2008).
Alberti, K. et al. Harmonizing the metabolic syndrome. Circulation 120, 1640–1645 (2009).
Acknowledgements
This work was supported by the Taiwan Ministry of Education under the NCKU Aim for the Top University Project (D101–35001), NCKUH-10304040, NCKUH-10605021, NCKUH-10709012, MOST-106-2314-B-006-064-MY2, and NCKUMCS2016028. We thank the staff of the Tianliao District Public Health Center for their generous support, Ms. Yu-Chen Shih for her administrative assistance and Bill Franke for the English editing.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: C.H.W., C.S.C. and Y.F.C. Sampling method: C.H.W. and C.J.C. Collected and analyzed the data: C.M.W., C.S.C., P.Y.L., Y.F.C., H.C.W., C.J.C., M.T.H. and C.Y.C. Biostatistics analysis: C.M.W. and C.S.C. Prepared the tables and figure: C.M.W. and C.H.W. Wrote the paper: C.M.W. and C.H.W. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, CM., Chang, CS., Chang, YF. et al. Inverse Relationship between Metabolic Syndrome and 25-Hydroxyvitamin D Concentration in Elderly People without Vitamin D deficiency. Sci Rep 8, 17052 (2018). https://doi.org/10.1038/s41598-018-35229-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35229-2
Keywords
This article is cited by
-
The risk of metabolic syndrome is associated with vitamin D and inflammatory status in premenopausal and postmenopausal Algerian women
Irish Journal of Medical Science (1971 -) (2024)
-
Preoperative Nutritional Deficiencies in Bariatric Surgery Candidates in Korea
Obesity Surgery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.