Abstract
With the dissemination of extremely drug resistant bacteria, colistin is now considered as the last-resort therapy for the treatment of infection caused by Gram-negative bacilli (including carbapenemase producers). Unfortunately, the increase use of colistin has resulted in the emergence of resistance as well. In A. baumannii, colistin resistance is mostly caused by the addition of phosphoethanolamine to the lipid A through the action of a phosphoethanolamine transferase chromosomally-encoded by the pmrC gene, which is regulated by the two-component system PmrA/PmrB. In A. baumannii clinical isolate the main resistance mechanism to colistin involves mutations in pmrA, pmrB or pmrC genes leading to the overexpression of pmrC. Although, rapid detection of resistance is one of the key issues to improve the treatment of infected patient, detection of colistin resistance in A. baumannii still relies on MIC determination through microdilution, which is time-consuming (16–24 h). Here, we evaluated the performance of a recently described MALDI-TOF-based assay, the MALDIxin test, which allows the rapid detection of colistin resistance-related modifications to lipid A (i.e phosphoethanolamine addition). This test accurately detected all colistin-resistant A. baumannii isolates in less than 15 minutes, directly on intact bacteria with a very limited sample preparation prior MALDI-TOF analysis.
Similar content being viewed by others
Introduction
Currently, antimicrobial resistance is on top of the agenda for scientists and governments, while pan-resistant organisms are fast emerging. Any lack of swift commitment and action to improve diagnostic and prevention would irremediably take us back to the dark age of dreadful and devastating epidemics. The pipeline of new antibiotics is very limited, and colistin is now considered as the last resort therapy for the treatment of infection caused by multidrug resistant (MDR) Gram-negative bacteria, such as carbapenemase-producing Acinetobacter baumannii1. Unfortunately, with an increase in the use of colistin to treat carbapenem-resistant Acinetobacter baumannii infections, colistin resistance is emerging2.
In Gram-negative bacteria, acquired resistance to polymyxins results mostly from modifications of the drug target, i.e. the lipopolysaccharide (LPS). These modifications correspond to addition(s) of cationic groups such as 4-amino-L-arabinose (L-Ara4N) and/or phosphoethanolamine (pETN) on the lipid A, the anchor of the LPS. Unlike Enterobacteriaceae, A. baumannii lacks all the genes required for L-Ara4N biosynthesis. Accordingly, colistin resistance is caused by the addition of pETN to the lipid A on position 1 or 4’ by an EptA-like phosphoethanolamine transferase chromosomally-encoded by the pmrC gene. As well, mutations in the chromosome-encoded pmrA and pmrB genes result in a constitutive activation of the PmrA/PmrB two-component system, which in turn upregulates the expression of pmrC3,4,5. Recently, plasmid-mediated resistance to polymyxin, named MCR, was described in Enterobacteriaceae. The mcr genes (mcr-1, -2, -3, -4, -5, -6, -7 and -8) also encode a phosphoethanolamine transferase involved in addition of pETN on the lipid A6. Although mcr genes were found to have disseminated in Enterobacteriaceae (mostly Escherichia coli), they are not currently reported in Acinetobacter for which resistance to polymyxin is still restricted to chromosome-encoded resistance5,6,7.
Rapid detection of resistance is one of the key issues to improve the treatment of patient infected with MDR bacteria. However, detection of colistin resistance in Acinetobacter relies on minimal inhibition concentration determination using broth microdilution which is the gold standard for polymyxins susceptibility testing8. This method has been chosen as the unique reference by The Clinical Laboratory Standard Institute (CLSI) and by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), recently gathered in a joint subcommittee9, which ruled out methods classically used for determination of antimicrobial susceptibility, such as agar dilution, disk diffusion, gradient diffusion (Etest) and automated systems (MicroScan®, Vitek® 2, BD Phoenix™) for which high rates of false susceptibility were observed. Consequently, results can only be obtained up to 24 h after bacterial isolation10. Recently, a biochemical test, the rapid polymyxin NP test, that detects bacterial growth in the presence of a defined concentration of a polymyxin (3.5 mg/L) has been developed11. With this colorimetric test, the bacterial growth, or absence of, is tracked on the basis of carbohydrate metabolism12. The acid formation resulting from carbohydrate metabolism during the continuous growth of colistin resistant bacteria even in presence of polymyxin, is detected by the color change of a pH indicator. Also this test was validated for fermenting bacteria, such as Enterobacteriaceae, it cannot conceptually be applied to non-fermenters such as Acinetobacter.
Recently, we developed a rapid technique using MALDI-TOF able to detect colistin resistance directly on intact bacteria in less than 15 minutes, the MALDIxin test13. This cutting-edge method has been validated for E. coli for which it can detect not only polymyxin resistance but also discriminated chromosome- and plasmid-encoded resistance (i.e. mcr). This method is based on the MALDI-TOF on-target extraction of the free lipid A directly from bacterial colonies14. Indeed, this technique does not required prior time-consuming processes for the whole extraction of the LPS and hydrolysis into its lipid A using highly hazardous reagents14. In this study, the peaks corresponding to pETN-modified Lipid A are detected in all colistin-resistant E. coli isolates13. In addition, a specific peak corresponding to the addition of pETN associated with dephosphorylation of the native lipid A was found only in MCR-producing E. coli strains13.
Here, we evaluated the ability of the MALDIxin test to detect colistin-resistance in A. baumannii.
Results and Discussion
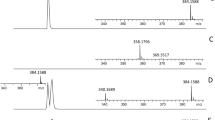
In polymyxin susceptible Acinetobacter baumannii, the mass spectrum is dominated by 2 set of peaks centred at m/z 1728.1 and m/z 1910.3 (Fig. 1a), assigned to bis-phosphorylated hexa-acyl and bis-phosphorylated hepta-acyl lipid A, with acyl chain ranging from 12 to 14 carbons in length, respectively15,16. In colistin resistant strains, the mass spectrum is dominated by 2 set of peaks centred at m/z 1935.3 and m/z 2033.3, corresponding to the previously observed m/z +25 and m/z +123 shifts of mass unit of the native bis-phosphorylated hepta-acyl lipid A at m/z 1910.3 (Fig. 1b). These peaks were assigned to pETN-modified-bis-phosphorylated hepta-acyl lipid A with acyl chain of 12 carbons in length (m/z 2033.3)15, and pETN-modified-mono-phosphorylated hepta-acyl lipid A with acyl chain of 12 carbons in length (m/z 1935.3)13, As previously observed with MCR-producing E. coli, the addition of a pETN moiety onto the phosphate group at position 1 lead to a global shift of +123 m/z of the peak corresponding to the native lipid A, while the addition of a pETN moiety onto the 4′ of native lipid A is concomitant with loss of the phosphate group on position 1 leading to a global shift of +25 m/z of the peak corresponding to the native lipid A13. Since EptA-like and MCR-like enzymes are both phosphoethanolamine transferases, we expected to observe the appearance of two peaks at +25 m/z and +123 m/z related to pETN modified lipid A compare to the native lipid A, as it was previously shown with all MCR-producing E. coli13. Of note, these two specific peaks at m/z 1935.3 and m/z 2033.3 were also observed for the three resistant isolates for which the molecular mechanism remains unknown (Ab-R2, Ab-R4 and Ab-R6) (Fig. 1b, Table 1). However, although no mutation in pmrA, pmrB and pmrC was identified, overexpression of the pmrC/eptA gene (encoding the pETN transferase) was systematically observed in these three isolates using qRT-PCR. As shown in Table 1 the peaks corresponding to the pETN modified lipid A (m/z 1935.3 and m/z 2033.3) were not observed with colistin susceptible isolates, while they were systematically in all colistin resistant isolates.
Results of the MALDIxin test on A. baumannii. Representative spectra of a polymyxin-susceptible A. baumannii isolate (Ab-S1) (panel a) and a colistin-resistant A. baumannii isolate (Ab-R1) (panel b). Peaks of interest are indicated. The peaks at m/z 1728.1 m/z and 1910.3 m/z corresponds to the native lipid A of A. baumannii, the peak at m/z 1935.3 likely corresponds to the addition of pETN on the phosphate group at position 4’ of the native lipid A of A. baumannii with concomitant loss of the phosphate group on position 1, and the peak at m/z 2033.3 corresponds to the addition of one pETN on the phosphate group at position 1 of the native lipid A of A. baumannii.
Here, we demonstrated that the MALDIxin test is a rapid, accurate and cost effective technique based on MALDI-TOF for the detection of colistin resistance in A. baumannii. As previously described13, the routine use of the MALDIxin test will require switching the MALDI-TOF-MS machine to the negative ion mode due to the inherent negative charge of lipid A. This negative mode is not currently used for bacterial identification and thus it is locked in the MALDI-TOF MS machines commonly used in diagnostic. However, preliminary data indicate that MALDIxin test might be implemented on classical MALDI-TOF MS machines where this negative mode was unlocked (data not shown). In addition, one of the limitations of this study resides in the low number of tested isolates. However, the resistance mechanisms included in this study represent the most prevalent causes of colistin resistance in A. baumannii (mostly modification/mutation in PmrA or PmrB)5,7,17. Compared to the unique rapid detection method available for the detection of colistin resistance, the Polymyxin NP test, the MALDIxin test can work on a large panel of Gram negative bacteria, including non-fermenters (e.g. A. baumannii). In addition, this study paves the way for the future development of a rapid diagnostic test that could detect colistin resistance in all Gram-negative bacteria, including Enterobacteriaceae (E. coli, K. pneumoniae, Salmonella, …), Acinetobacter spp. and Pseudomonas aeruginosa. However, optimizations are still needed to allow the direct detection of L-Ara4N-modified lipid A which remains the main cause of colistin resistance in K. pneumoniae and P. aeruginosa.
Methods
Bacterial strains
A collection of 17 A. baumannii isolates including 9 colistin-resistant and 8 colistin-susceptible isolates were subjected to the MALDIxin test. The 9 polymyxin-resistant isolates, of which 5 harboured modifications of PmrB and one is mutated in PmrA (Table 1). For all colistin-resistant isolates, the modifications (mutation, deletion, disruption) in phoP, phoQ, pmrA, pmrB and pmrC were verified by whole genome sequencing (Illumina).
Susceptibility testing
Colistin MIC was determined by broth microdilution according to the Clinical Laboratory Standard Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines9. Results were interpreted using EUCAST breakpoints as updated in 2018 (http://www.eucast.org/clinical_breakpoints/).
MALDIxin test
The MALDIXin procedure was performed as previously described13. Briefly, a single colony cultured on Mueller-Hinton agar (bioMérieux, La Balme-les-Grottes, France) was resuspended in 200 μl of distilled water, washed three times with double distilled water and resuspended in 100 μl of double distilled water. 0.4 μL of the bacterial solution was loaded onto the target and immediately overlaid with 0.8 μL of a 2, 5-dihydroxybenzoic acid (DHB) matrix (Sigma Aldrich, Gillingham, United-Kingdom) used at a final concentration of 10 mg/mL in chloroform/methanol (CHCl3/MeOH) 90:10 v/v. Bacterial solution and matrix were mixed directly on the target by pipetting and the mix was dried gently under a stream of air (less than one minute). MALDI-TOF MS analysis was performed on a 4800 Proteomics Analyzer (Applied Biosystems, Foster City, USA) using the reflectron mode. Samples were analyzed by operating at 20 kV in the negative ion mode using an extraction delay time set at 20 ns. Mass spectrometry data were analyzed using Data Explorer version 4.9 (Applied Biosystems).
Expression of pmrC/eptA
The three colistin-resistant A. baumannii that gave a positive signal for the presence of a peak related to the production of a phosphoethanolamine transferase activity, but were negative for modification in pmrA, pmrB and pmrC genes (Ab-R2, Ab-R4 and Ab-R6) were subjected to qRT-PCR to assess the expression of pmrC/eptA gene as previously described16. The expression of pmrC/eptA was normalized using 16 S RNA encoding gene. The fold change expression of pmrC/eptA was related to the basal expression of pmrC/eptA in the colistin susceptible Ab-S4 isolate.
References
Xie, R., Zhang, X. D., Zhao, Q., Peng, B. & Zheng, J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerging microbes & infections 7, 31, https://doi.org/10.1038/s41426-018-0038-9 (2018).
Qureshi, Z. A. et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clinical infectious diseases 60, 1295–1303, https://doi.org/10.1093/cid/civ048 (2015).
Adams, M. D. et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrobial agents and chemotherapy 53, 3628–3634, https://doi.org/10.1128/AAC.00284-09 (2009).
Cai, Y., Chai, D., Wang, R., Liang, B. & Bai, N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67, 1607–1615, https://doi.org/10.1093/jac/dks084 (2012).
Jeannot, K., Bolard, A. & Plesiat, P. Resistance to polymyxins in Gram-negative organisms. International journal of antimicrobial agents 49, 526–535, https://doi.org/10.1016/j.ijantimicag.2016.11.029 (2017).
Partridge, S. R. et al. Proposal for assignment of allele numbers for mobile colistin resistance (mcr) genes. J Antimicrob Chemother, https://doi.org/10.1093/jac/dky262 (2018).
Poirel, L., Jayol, A. & Nordmann, P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clinical microbiology reviews 30, 557–596, https://doi.org/10.1128/CMR.00064-16 (2017).
Jayol, A., Nordmann, P., Lehours, P., Poirel, L. & Dubois, V. Comparison of methods for detection of plasmid-mediated and chromosomally encoded colistin resistance in Enterobacteriaceae. Clinical microbiology and infection 24, 175–179, https://doi.org/10.1016/j.cmi.2017.06.002 (2018).
Clinical and Laboratory Standard Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) joint subcommittee. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group (2017).
Hindler, J. A. & Humphries, R. M. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. Journal of clinical microbiology 51, 1678–1684, https://doi.org/10.1128/JCM.03385-12 (2013).
Nordmann, P., Jayol, A. & Poirel, L. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerging infectious diseases 22, 1038–1043, https://doi.org/10.3201/eid2206.151840 (2016).
Hugh, R. & Leifson, E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. Journal of bacteriology 66, 24–26 (1953).
Dortet, L. et al. Rapid detection and discrimination of chromosome- and MCR-plasmid-mediated resistance to polymyxins by MALDI-TOF mass spectrometry in Escherichia coli: The MALDIxin test. The Journal of antimicrobial chemotherapy. (2018). In press.
Larrouy-Maumus, G., Clements, A., Filloux, A., McCarthy, R. R. & Mostowy, S. Direct detection of lipid A on intact Gram-negative bacteria by MALDI-TOF mass spectrometry. Journal of microbiological methods 120, 68–71, https://doi.org/10.1016/j.mimet.2015.12.004 (2016).
Arroyo, L. A. et al. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrobial agents and chemotherapy 55, 3743–3751, https://doi.org/10.1128/AAC.00256-11 (2011).
Beceiro, A. et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrobial agents and chemotherapy 55, 3370–3379, https://doi.org/10.1128/AAC.00079-11 (2011).
Olaitan, A. O., Morand, S. & Rolain, J. M. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Frontiers in microbiology 5, 643, https://doi.org/10.3389/fmicb.2014.00643 (2014).
Jaidane, N. et al. Genomic analysis of in vivo acquired resistance to colistin and rifampicin in Acinetobacter baumannii. International journal of antimicrobial agents 51, 266–269, https://doi.org/10.1016/j.ijantimicag.2017.10.016 (2018).
Garcia-Quintanilla, M. et al. Lipopolysaccharide loss produces partial colistin dependence and collateral sensitivity to azithromycin, rifampicin and vancomycin in Acinetobacter baumannii. International journal of antimicrobial agents 46, 696–702, https://doi.org/10.1016/j.ijantimicag.2015.07.017 (2015).
Acknowledgements
LD has received funding from the People ProGramme (Marie Skłodowska-Curie Actions) of the European Union’s Horizon 2020 under REA grant agreement n° [654909] and from the University Paris Sud. GLM received funding from an MRC Confidence in Concept Award (Wellcome Trust: ISSF Wellcome Trust 105603/Z/14/Z).
Author information
Authors and Affiliations
Contributions
L.D. and G.L.M. had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: L.D., G.L.M. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: L.D., G.L.M. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding authors
Ethics declarations
Competing Interests
L.D., A.F. and G.L.M. are co-inventors of the MALDIxin test for which a patent has been filed by Imperial Innovations.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dortet, L., Potron, A., Bonnin, R.A. et al. Rapid detection of colistin resistance in Acinetobacter baumannii using MALDI-TOF-based lipidomics on intact bacteria. Sci Rep 8, 16910 (2018). https://doi.org/10.1038/s41598-018-35041-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35041-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.