Abstract
The closely related yeasts Ogataea polymorpha and O. parapolymorpha differ drastically from each other by sensitivity to the toxic phosphate analog vanadate. Search for genes underlying this difference revealed two genes, one designated as ABV1 (Alcian Blue staining, Vanadate resistance), which encodes a homologue of Saccharomyces cerevisiae Mnn4 responsible for attachment of mannosylphosphate to glycoside chains of secretory proteins, and the other designated as its S. cerevisiae homologue PHO87, encoding the plasma membrane low affinity phosphate sensor/transporter. The effect of Pho87 on vanadate resistance was bidirectional, since it decreased the resistance on phosphate-depleted medium, but was required for pronounced protection against vanadate by external phosphate. This highlights the dual function of this protein as a low affinity phosphate transporter and an external phosphate sensor. Involvement of Pho87 in phosphate sensing was confirmed by its effects on regulation of the promoter of the PHO84 gene, encoding a high affinity phosphate transporter. The effect of Abv1 was also complex, since it influenced Pho87 level and enhanced repression of the PHO84 promoter via a Pho87-independent pathway. Role of the identified genes in the difference in vanadate resistance between O. polymorpha and O. parapolymorpha is discussed.
Similar content being viewed by others
Introduction
Inorganic phosphate (Pi) is a crucial nutrient for living cells. As the plasma membrane is impermeable to this cation, its uptake depends on special transporters. Saccharomyces cerevisiae possesses five Pi transporters: four plasma membrane transporters Pho84, Pho87, Pho89, and Pho90, which provide Pi uptake from environment in different conditions, and one vacuolar transporter Pho91, which is probably required for supplying the cytosol with phosphate from the vacuolar pool (for the review see1,2). The high affinity Pi transporters Pho84 and Pho89 differ from each other by pH optimum and cation requirements. Particularly, transport through Pho84 depends on protons and reaches the highest rate at pH 4.5, while Pho89 is a Pi:Na+-coupled symporter with an optimum at pH 9.53. The PHO84 and PHO89 genes are repressed at high external Pi concentration and activated upon Pi limitation3,4. Pho87, Pho90, and Pho91 are low affinity Pi transporters5. Unlike genes for the high affinity transporters, the PHO87, PHO90, and PHO91 genes do not undergo tight regulation depending on Pi availability6. However, at low external Pi concentration, the presence of the plasma membrane low affinity Pi transporters can be detrimental to the cell, and this effect can be alleviated by targeting these proteins for vacuolar degradation via the endocytic pathway7.
The transcriptional response to changes in cytosolic Pi concentration is mediated by a complex regulatory circuit referred to as PHO pathway (for a review see8), which controls expression of certain genes through the activity of transcription factors Pho4 and Pho29. Beside transcription factors, the core of this circuit involves a complex consisting of cyclin-dependent protein kinase (CDK) Pho85, one of its cyclins Pho80, and the CDK inhibitor Pho81. Upon Pi limitation conditions, Pho4 is relocalized to the nucleus where it is able to bind specific sites in promoter regions in cooperation with Pho2. Elevation of intracellular phosphate level relieves inhibition of Pho85 by Pho8110,11, leading to phosphorylation of Pho4 at multiple sites. This prevents Pho4 interaction with Pho2 and causes its redistribution from the nucleus to the cytosol12,13,14,15. Apart from this circuit, regulation of PHO84, which depends on the low affinity Pi transporters and does not depend on internal Pi concentration, has also been elucidated16. The high affinity transporter Pho84 and low affinity transporter Pho87 were shown to be Pi sensors allowing cells to respond to changes in external Pi availability via the protein kinase A dependent signaling pathway17.
Vanadate (orthovanadate, VO43−) is a toxic Pi analog, which has multiple targets within the cell. It efficiently inhibits many different enzymes, whose substrates carry a phosphate moiety18. One can assume that multiple targets can be protected from this inhibitor only by prevention of its uptake or by rapid conversion into less toxic compounds. Indeed, vanadate resistant mutants isolated in Candida albicans19 and Neurospora crassa20,21 were defective in the Pi transport system. Interestingly, in the conventional yeast S. cerevisiae, an increase in vanadate resistance can be achieved by alterations in the Golgi apparatus protein glycosylation22. At the same time no mutations in the Pi transport system conferring this phenotype have been reported in this yeast.
Some yeast species can exhibit much higher resistance to vanadate than others. For example, compared to S. cerevisiae, Ogataea (Hansenula) polymorpha is much more resistant to this compound23, while its closest relative O. parapolymorpha24 is not25. Here we used these two yeast species to gain insight into the mechanisms involved in vanadate resistance and its relation to Pi transport and protein glycosylation.
Results
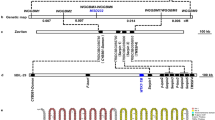
Inactivation of YPT6 or a homologue of S. cerevisiae MNN4 decrease vanadate resistance in O. polymorpha but not in O. parapolymorpha
To find genes responsible for high vanadate resistance in O. polymorpha, vanadate sensitive clones were selected among transformants obtained using linearized plasmids supposed to randomly integrate within the genome. Clones with pronounced vanadate sensitivity were subjected to identification of the plasmid integration loci. In two cases, involvement of the identified genes in vanadate resistance was confirmed by their targeted inactivation. One of them (accession number MH286258) codes for a homolog of the S. cerevisiae Ypt6 GTPase involved in retrograde vesicle transport between different compartments of the secretory pathway and endosomes26. Inactivation of this gene in O. polymorpha led to increased sensitivities to SDS and caffeine as well as to decrease in the carboxypeptidase Y level (Supplementary Fig. S1). In O. parapolymorpha this mutation caused hypersensitivities to caffeine, high salt concentration and elevated temperature (Supplementary Fig. S1). All these phenotypes have been previously observed in S. cerevisiae ypt6 mutants26,27. Based on these observations, this gene was also designated as YPT6.
Another identified gene (accession number MH286257) encodes a homolog of S. cerevisiae Mnn4, which was shown to be involved in the attachment of mannosylphosphate to protein glycoside chains in the secretory pathway28. O. polymorpha cells were efficiently stained with the Alcian blue dye (Fig. 1), indicating presence of mannosylphosphate in the glycoside chains of the cell wall29, while inactivation of the identified gene led to inability to bind Alcian blue indicating drastic decrease or absence of mannosylphosphate in the cell wall. Designation ‘MNN4’ for the identified gene seemed to be objectionable, since O. polymorpha30 and O. parapolymorpha31 genomes possess another ORF, which codes for a protein (accession number XP_013933535.1) showing even higher similarity to S. cerevisiae Mnn4 since its sequence alignment reveals 195 identical positions of 780 amino acid sequence compared to 150 of 783 residues in the identified gene. To avoid this ambiguity, the gene was designated ABV1 (Alcian Blue staining, Vanadate resistance), according to the observed mutant phenotypes.
Staining of yeast cells with the Alcian blue dye. “Opa” and “Opo”, O. parapolymorpha and O. polymorpha, respectively; “WT”, transformants possessing ABV1 and YPT6 wild-type alleles; “ypt6-Δ” and “abv1-Δ”, transformants disrupted for YPT6 or ABV1, respectively. “OpoABV1” and “OpaABV1” indicate presence of plasmids with the corresponding genes. Numbers in brackets correspond to designation of transformants in the Supplementary Table S1.
Inactivation of YPT6 also led to some decrease in Alcian blue binding (Fig. 1), which might lead one to suggest that its effect on the vanadate sensitivity might be related to alteration of phosphomannosylation of glycoside chains in the secretory pathway. However, this was not supported by further experiments.
Inactivation of YPT6 in the sensitive species O. parapolymorpha did not noticeably affected vanadate resistance, while effect of ABV1 inactivation was opposite to that in O. polymorpha, since this mutation increased vanadate resistance of the former species (Fig. 2, O. polymorpha and O. parapolymorpha strains labeled “WT”, “abv1-Δ” and “ypt6-Δ”).
Dependence of vanadate sensitivity on phosphate concentration in culture medium. Numbers in brackets correspond to designation of transformants in the Supplementary Table S1. Overnight saturated cultures grown in the phosphate depleted medium supplemented with 0.5 mM Na-Pi buffer were 500-fold diluted and spotted onto plates with the phosphate depleted medium supplemented with different concentrations of Na3VO4 and Na-Pi buffer pH 7.2.
O. parapolymorpha vanadate resistance depends on ABV1 gene dosage
As O. polymorpha and O. parapolymorpha genomes are almost identical, one could suggest that functions of only one or several genes may determine their difference in vanadate resistance. To search for O. polymorpha genes, which are able to improve vanadate resistance in O. parapolymorpha, the O. parapolymorpha DL1-L strain was transformed with an O. polymorpha genomic library. Several vanadate resistant transformants were selected. All of them possessed plasmids containing the ABV1 gene. This led us to conclude that function of ABV1 is insufficient in this yeast. However, the untransformed O. parapolymorpha cells were able to bind Alcian blue, while inactivation of ABV1 abolished the staining. At the same time, the staining of O. parapolymorpha wild-type cells seemed not to be as intensive as in O. polymorpha (Fig. 1).
The ABV1 gene products of these two yeast species are not completely identical (7% of mismatches), which might lead one to suggest that O. parapolymorpha Abv1 is functional in the phosphomannosylation of cell wall proteins, but does not target a specific subset of proteins including ones involved in increasing vanadate resistance. However, the autonomously replicating plasmid with O. parapolymorpha ABV1 was able to increase vanadate resistance like its O. polymorpha orthologue in both, O. polymorpha abv1-Δ and O. parapolymorpha (Supplementary Fig. S2). This indicates that ability to increase vanadate resistance is not due to some special property of O. polymorpha Abv1, but rather depends on its production level.
Introduction of a multi copy plasmid with ABV1 into O. parapolymorpha cells led to only a moderate increase in vanadate resistance, which was still far from that of the vanadate resistant species O. polymorpha, indicating that this gene is not the only player that determines this difference between the two yeast species. According to our previous experience, the genomic library we used in this approach is representative enough to cover the whole O. polymorpha genome. Indeed, using it, we have always been able to clone target genes, some of which were toxic in Escherichia coli, possibly restricting their representation in the library. This led us to suggest that lower vanadate resistance in O. parapolymorpha compared to O. polymorpha is prompted by increased activity of some proteins(s) and the current approach does not allow identification of additional player(s) determining the difference in vanadate resistance between these yeasts.
Plasma membrane low affinity phosphate transporter/sensor Pho87 modulates vanadate resistance in O. parapolymorpha
One could expect that inactivation of genes, whose increased expression is responsible for the lower vanadate resistance of O. parapolymorpha, should improve the resistance. To find such genes we mutagenized O. parapolymorpha by random integration of a linearized plasmid (see Materials and Methods) and selected vanadate resistant clones. Plasmid integration in one of the selected clones, which showed pronounced and stable vanadate resistance, occurred in the gene coding for a homologue of the S. cerevisiae plasma membrane low affinity Pi transporters Pho87 and Pho90 (accession number XP_013932315). In contrast to S. cerevisiae, O. parapolymorpha31 and O. polymorpha30 genomes possess only one gene coding for such transporter, since only two ORFs with sufficient similarity to S. cerevisiae PHO87 can be revealed: one of which is the identified gene, while another one (accession number XP_013933495) codes for the homologue of the vacuolar Pi transporter Pho91. This and additional data described below allowed us to designate the identified gene like its S. cerevisiae homologue PHO87. Interestingly, this gene is likely to be essential in O. polymorpha, since we were unable to inactivate PHO87 in a strain carrying only one allele of this gene, while we could do this in transformants with an integrative plasmid bearing O. parapolymorpha PHO87 (OpaPHO87).
As was shown in S. cerevisiae, low affinity Pi transporters support phosphate uptake at a relatively high external phosphate concentration, while their abundance at low external Pi concentrations can be deleterious32. This is also probably true in O. parapolymorpha since a drastic drop in Pho87 amount was observed when cells were incubated in phosphate depleted medium (Fig. 3a). This was not accompanied by substantial repression of the OpaPHO87 promoter (Table 1) indicating that the amount of this protein might be regulated by vacuolar degradation, like in S. cerevisiae7.
Immunoblotting of Pho87 (upper panels) and Coomassie stained gels as loading controls (lower panels). Lanes cropped from different parts of the same gel or blot are divided by spaces. Positions of the marker bands are shown at the left. In Coomassie-stained gels the NEB marker (Cat. #P7702) was used; the prestained marker (ThermoFisher Scientific, cat. #sm0441) was used in immunoblots. Despite the affinity purification, some non-specific bands (NS1 and NS2) migrating close to the full-length Pho87 band were still revealed by this antibody. To resolve them, in case of blots presented in panels a, c, d, and f electrophoresis was run until the 85 kDa pre-stained marker band came to end of the gel. O. parapolymorpha pho87-Δ strain (#9 in the Supplementary Table S1) was used in all cases. (a) Analysis of Pho87 content in O. parapolymorpha grown in medium repleted with specified phosphate concentrations. “PHO87”, stain #6 (Supplementary Table S1); “pho87-Δ ”, the negative control strain, which was grown in the medium repleted with 1 mM Pi. (b) Comparison of Pho87 content in O. polymorpha and O. parapolymorpha cells grown in regular YPD. “Opo”, “Opa” and “pho87-Δ” correspond to strains ## 58, 6, 9 in the Supplementary Table S1, respectively. “1/8”, “1/4”, “1/2” and “1” indicate that sample was 8-, 4-, 2-fold diluted, or undiluted, respectively. (c) Expression of the plasmid-borne OpaPHO87 and OpoPHO87 in O. polymorpha pho87-Δ strain. Lanes are marked with the strain numbers according to the Supplementary Table S1. Two expositions (9′ and 30′) of the immunoblot are presented. (d) Effects of ABV1 and YPT6 deletions in O. polymorpha. WT, abv1-Δ, ypt6-Δ, and pho87-Δ, strains designated as #58, 59, 62, and 9 in the Supplementary Table S1, respectively. (e) Effects of ABV1 and YPT6 deletions in O. parapolymorpha. pho87-Δ, WT, abv1-Δ, and ypt6-Δ, strains designated as #9, 6, 7, and 8 in the Supplementary Table S1, respectively. (f) Effect of ABV1 extra copies in O. parapolymorpha. pho87-Δ, WT, ABV1, and ABV1↑, strains designated as #9, 6, 14, and 17 in the Supplementary Table S1, respectively.
Importantly, vanadate resistance depended on the PHO87 gene dosage since introducing a plasmid with this gene made O. polymorpha and O. parapolymorpha cells more sensitive to vanadate (Fig. 2), at least at low Pi concentration. One could assume that the difference in vanadate resistance between these yeasts is determined by a difference in PHO87 expression levels. In agreement with this, amount of Pho87 in the resistant species O. polymorpha was much lower than in O. parapolymorpha if cells were incubated in regular YPD medium (Fig. 3b). It was also possible that OpoPho87 is less efficient as a transporter than OpaPho87. Indeed, introduced into the O. parapolymorpha pho87-Δ strain, the resistant species gene OpoPHO87 conferred less sensitivity than its native gene, OpaPHO87, not only in clones with approximately the same expression levels (Figs 2 and 3c; transformant 3 vs. transformant 4), but even when OpoPHO87 was more highly expressed (transformants 2 and 3 vs. transformant 1 having the wild-type OpaPHO87 locus). Interestingly, OpaPHO87 expression level in transformant 4 was noticeably lower than in 5, but their vanadate sensitivities were similar, indicating that when Pho87 content reaches a certain level its alteration is unable to noticeably affect vanadate sensitivity.
Inactivation of PHO87 in O. parapolymorpha did not increase vanadate resistance to the same level as in O. polymorpha wild-type cells (Fig. 2) suggesting the presence of an additional mechanism underlying this difference. One can suggest that vanadate, being a phosphate analog, enters the cell via phosphate transporters. In this case, vanadate toxicity should depend on the phosphate concentration in culture medium. In agreement with this suggestion, increasing phosphate concentration rescued cells from vanadate toxicity, though this effect became much less pronounced after inactivation of PHO87 (Fig. 2). Transformants overexpressing OpaPHO87 or OpoPHO87 showed reduced vanadate resistance on phosphate depleted medium and efficient protection against vanadate by increased phosphate concentration. This dual effect of Pho87 on the sensitivity could originate from its two functions, namely a low affinity phosphate transporter and external phosphate sensor. In terms of this hypothesis, Pho87 confers vanadate uptake as a phosphate transporter and inhibits it as an external phosphate sensor by repressing another plasma membrane transporter, which is also responsible for vanadate uptake.
Based on the homology with S. cerevisiae high affinity plasma membrane phosphate transporters, O. polymorpha and O. parapolymorpha genomes also possess genes for these transporters – one for Pho84 and one for Pho89 (accession numbers XP_013935929 and XP_013933062, respectively). Importantly, as it was shown in S. cerevisiae, Pho87 and Pho84 have higher activity at lower pH, while Pho89 is active at higher pH and requires Na+ cations33. Analyzing 32Pi uptake in O. parapolymorpha we observed that at pH4.5 its rate was higher (approx. 5.0 fold in strain possessing wild-type PHO87 locus and approx. 2 fold in the Δpho87 strain) than at pH8.0 and in presence of 10 mM NaCl (Supplementary Table S2). This indicates that like in S. cerevisiae17, O. parapolymorpha Pho84 has more impact on phosphate uptake than Pho89.
To study PHO84 transcription regulation we fused its promoter to the ORF encoding Renilla luciferase. Indeed, PHO84 promoter provided significantly different gene expression levels at high and low phosphate concentration in culture medium. This regulation apparently depended on PHO87, since its deletion affected the promoter repression and activation at high and low phosphate concentration, respectively, while its overexpression decreased PHO84 promoter activity in both cases (Table 2). In addition, production of acid phosphatase, which is known to depend on internal phosphate concentration34, was studied (Table 2). In contrast to the luciferase produced under control of the PHO84 promoter, regulation of production of acid phosphatase was almost unaffected by inactivation or extra copies of PHO87. In particular, acid phosphatase production was efficiently repressed in the wild type strain at high phosphate concentration, while both inactivation and extra copies of PHO87 led to very slight derepression.
ABV1 affects regulation of phosphate homeostasis
Unlike Pho87, which could be directly involved in vanadate uptake as a phosphate transporter and sensor, the role of mannosylphosphate attachment to glycoproteins catalyzed by Abv1 in modulation of vanadate resistance was not obvious. It was possible to suggest that this modification somehow influences regulation of phosphate homeostasis. To test this hypothesis, we studied effects of both ABV1 deletion and dosage increase on vanadate sensitivities (Fig. 2) and regulation of PHO84, and PHO5 genes (Table 2) in relation to phosphate concentration in culture medium.
Importantly, transformants bearing extra copies of ABV1 were obtained by two different approaches: (i) targeting of single copy plasmid integration into the LEU2 locus and (ii) using a special autonomously replicating vector allowing selection of subclones that arose due to integration of several plasmid copies into the genome (see Materials and Methods).
In the O. parapolymorpha strain with the PHO87 wild-type allele, multiple (but not single copy) integration of an OpoABV1 containing plasmid led to some derepression of the PHO5 gene at high phosphate concentration in culture medium indicating reduction of cytosolic phosphate content (Table 2). Despite this difference, the effects of multiple and single copy integration on vanadate resistance were undistinguishable. At the same time, in a strain with disrupted PHO87, one extra copy of ABV1 led to some PHO5 derepression at high phosphate concentration and had more impact on vanadate resistance than the multi copy plasmid. This correlated with stronger repression of the PHO84 promoter. These results demonstrate that ABV1 is able to affect response to external phosphate and to modulate phosphate uptake in absence of PHO87.
Effect of the ypt6-Δ mutation on the PHO84 promoter regulation was opposite to the effect of abv1-Δ (Table 2), despite similar effects on vanadate resistance. This indicates that ypt6-Δ may affect vanadate resistance independently of ABV1.
In contrast to observations in the resistant species O. polymorpha, inactivation of ABV1 in the sensitive species O. parapolymorpha led to a noticeable increase in the vanadate resistance, which even exceeded the resistance increase in response to ABV1 extra copies. This mutation decreased amount of Pho87 in both species (Fig. 3d,e), while one extra copy of ABV1 could even slightly increase it (Fig. 3f; Table 1), despite similar effects of these modifications on vanadate resistance. This demonstrates that, in addition to the Pho87-independent effects, Abv1 influences phosphate homeostasis via affecting Pho87 level.
Summarizing the data presented in this section, it can be noted that ABV1 expression affects regulation of PHO84 and PHO87. The ABV1 effect on the phosphate homeostasis was also revealed by observing acid phosphatase derepression in a strain with increased ABV1 dosage.
Discussion
In this work, we have identified and characterized three genes which influence vanadate resistance in O. polymorpha and O. parapolymorpha. One of these genes, designated as its S. cerevisiae orthologue, PHO87, encodes a protein with a dual function, namely the low affinity plasma membrane Pi transporter and sensor of external Pi concentration. Involvement of Pho87 in modulation of vanadate resistance indicates that vanadate, as a Pi analog, enters the cell via the Pi transport system. Being a Pi transporter, Pho87 mediates vanadate uptake, while, being an external Pi sensor, it can provide protection against vanadate by repressing high affinity phosphate transporters. Indeed, inactivation of PHO87 led to a substantial increase of vanadate resistance at low Pi concentrations, but narrowed down the effect of external Pi in rescuing cells from vanadate toxicity. This correlates with the effect of PHO87 inactivation on regulation of the PHO84 gene coding for a high affinity phosphate transporter.
Incubation in Pi-depleted medium leads to a drastic decrease in the amount of Pho87 (the full-length protein becomes undetectable by immunoblotting), most probably due to activation of its degradation, as observed in S. cerevisiae7. Nevertheless, some amount of functional Pho87 should be retained in the cell, since it essentially influences vanadate resistance and PHO84 regulation. Remarkably, higher Pho87 level at high Pi concentration does not provide efficient vanadate uptake indicating that its transporter function is strongly inhibited.
Another identified gene is a homologue of S. cerevisiae MNN4 designated here as ABV1. Like its S. cerevisiae homolog, ABV1 is responsible for mannosylphosphate attachment to glycoside chains of proteins passing through the secretory pathway, since its inactivation abolishes Alcian blue binding to the cell surface. Although it was identified as a gene whose inactivation decreases vanadate resistance in the vanadate resistant species O. polymorpha, this mutation has the opposite effect in O. parapolymorpha (the vanadate sensitive species). The increased vanadate resistance in the O. parapolymorpha abv1-Δ mutant can be mediated by the decrease in Pho87 level, since Pho87 confers pronounced vanadate sensitivity in this yeast. Indeed, the effect of the abv1-Δ mutation on vanadate resistance in the O. parapolymorpha strain lacking PHO87 is almost indistinguishable. At the same time, ABV1 extra copies are able to increase vanadate resistance in O. parapolymorpha independently of the presence of PHO87. This effect in the pho87-Δ strain can be easily explained, since it correlates with much lower activity of the promoter of the PHO84 gene coding for the high affinity plasma membrane Pi transporter. However, in the strain bearing wild-type PHO87, the increase in vanadate resistance in response to ABV1 extra copies seems to be puzzling, since they do not lead to significant repression of the PHO84 promoter, but increase Pho87 level. This suggests that the Pho87 transporter function is essentially inhibited since otherwise vanadate resistance would be decreased.
O. polymorpha Pho87 seems to be less effective in conferring vanadate uptake compared to O. parapolymorpha Pho87. This, along with higher activity of Abv1, can determine the difference in vanadate resistance between these two yeast species. Indeed, O. polymorpha strain lacking ABV1 and expressing OpaPHO87 becomes almost as sensitive to vanadate as O. parapolymorpha wild type strain and vice versa, O. parapolymorpha expressing OpoPHO87 instead of its own gene and bearing OpoABV1 is as resistant as O. polymorpha (Supplementary Fig. S3). One can assume that high vanadate resistance is achieved when Pho87 is efficient as a sensor but not as a transporter, providing efficient repression of genes for the high affinity transporters, while high activity of Abv1 supports this repression via a Pho87 independent pathway. Inability of ABV1 inactivation to reduce vanadate resistance in O. parapolymorpha pho87-Δ strain could be due to its naturally lower activity, which is insufficient to affect this Pho87 independent regulatory pathway. However, the present study does not provide experimental data which could solidly prove this suggestion.
The third identified gene affecting vanadate resistance codes for the Ypt6 GTPase involved in vesicle transport between secretory organelles including endosomes. This gene is unlikely to determine the difference between O. polymorpha and O. parapolymorpha, since its inactivation noticeably affects viability of both species. One could suggest that it influences vanadate resistance via glycosylation since its inactivation decreases Alcian blue staining and affects vanadate resistance in different genetic backgrounds similar to the abv1-Δ mutation. However, its effect on the PHO84 promoter was opposite to the effect of ABV1, which contradicts this suggestion. Theoretically, the loss of Ypt6 may influence endocytosis of the plasma membrane phosphate transporters by affecting endosome dynamics.
Summarizing the obtained data, we can conclude the following: vanadate is absorbed via the Pi transport system. Pho87 has a dual role in resistance to vanadate: it is involved in vanadate uptake as a Pi transporter and can provide protection against vanadate by repressing genes for other Pi transporters as an external Pi sensor. The role of mannosylphosphate attachment to protein glycoside chains is also complex. On one hand, it increases Pho87 level, which in turn has a dual effect on vanadate resistance, since Pho87 confers vanadate uptake as a transporter and represses PHO84 expression as a Pi sensor. On the other hand, increased expression of ABV1 deepens repression of PHO84 via a Pho87-independent pathway.
Methods
Genetic nomenclature and genetic manipulation techniques
Standard genetic nomenclature was used. For convenience, where necessary, O. polymorpha and O. parapolymorpha gene names were precluded with “Opo” or “Opa”, respectively.
Mutagenesis by the random integration of linear DNA fragments35 and following identification of mutant loci were performed in O. polymorpha strains u23M25 and m14 strains as described previously36. Vanadate sensitive mutants were selected by replica plating of transformants onto YPD plates supplemented with 3 mM Na3VO4. O. parapolymorpha DL1-L strain37 was mutagenized by random integration of the PvuII-linearized pKAM554A plasmid38. After transformation cells were overnight incubated in liquid YPD medium containing G418 (100 mg/L) and then were spread onto YPD plates supplemented with 5 mM Na3VO4. Obtained colonies were confirmed for G418 resistance. One of obtained clones demonstrated stable increase in the vanadate resistance and was resistant to G418. Its DNA was digested with HincII (no sites in the vector) and NruI (unique site in the vector) and treated with T4 DNA ligase. The ligation mix was used as a template for PCR with primers flanking the NruI site in the vector to amplify the fragment possessing portion of the integration locus, which was then sequenced and compared with O. parapolymorpha genome database by the BLAST search.
Yeast strains and plasmid construction
The O. parapolymorpha DLdaduA strain (leu2 ade2-Δ ura3::ADE2) was obtained by sequential inactivation of ADE2 and then URA3 in the DL1-L strain37. Manifestations of alterations in the PHO87, ABV1, and YPT6 genes were studied in transformants of the DLdaduA strain, its derivatives DLdaduA-R and DLdaduA-Z bearing Renilla luciferase gene under control of OpaPHO84 promoter (PPHO84-Rluc) or E. coli lacZ gene under control of OpaPHO87 promoter (PPHO87-lacZ), respectively, and O. polymorpha 1B27 (leu2 ade2 ura3::ADE2) strain39. Genotypes of these transformants are presented in the Supplementary Table S1.
The integrative vector pCCUR1 was constructed by insertion of BamHI-BamHI fragment of the OpoURA3 locus into the BamHI site of the pBCKS(+) vector (Agilent, USA). This vector was then modified by deletion of BglII-XhoI fragment followed by removing EcoRI site using the Klenow enzyme to obtain pCAI24 plasmid. The OpoPHO87 and OpaPHO87 genes were obtained by PCR using primers 5′-CAGGC TGGAT GTGAT GTTG-3′ and 5′-GCTGC GACAT CTTCA TCAC-3′. The OpaPHO87 PCR product was inserted into the SmaI site of pKAM55638 to obtain the pKAM585 plasmid. The latter was modified by insertion of OpoURA3 between Asp718 and PstI sites outside PHO87 to obtain pKAM591 plasmid. To obtain the OpaPHO87 disruption cassette, BamHI-BamHI 3367 bp fragment of pKAM585 was self-ligated, digested with BsrGI and StuI, and inserted between BsrGI and PsiI sites of pCAI24. Linearization of resulting pCAM599 plasmid with BamHI created the disruption cassette whose integration into the genome via double crossover recombination led to replacement of the BsrGI-StuI fragment of PHO87 ORF for the pCAI24 vector sequence.
The DLdaduA-R and DLdaduA-Z strains possessed pKAZ15 or pKAM615 plasmids, respectively, which were integrated into unidentified loci. The pKAZ15 plasmid was obtained by sequential insertion into the pKAM554A vector polylinker the sequence coding for the Renilla luciferase recovered from the pDB688 plasmid40 and the sequence of the OpaPHO84 promoter region obtained by PCR using primers 5′-TTGAA TTCGG CCATA AGAAA GATG-3′ and 5′-TCGGA TCCTC TGGCT CACTC-3′. The pKAM615 plasmid was obtained by replacement of HindIII-EheI fragment of pKAM585 for HindIII-PsiI fragment of Yep356R bearing the E. coli lacZ coding sequence41. The DLdaduA strain was transformed either with Psp5II-linearized pKAZ15 or with Bsp119I-linearized pKAM615 to obtain integrative G418-resistant transformants, which were confirmed for expression of Renilla luciferase or β-galactosidase, respectively.
The ABV1 gene was inactivated using plasmid, which was recovered from the O. polymorpha abv1 mutant obtained by random integration of the pCAD24 plasmid possessing OpoLEU2 as a selectable marker36. To do this, chromosomal DNA of this mutant was digested with BsrGI, which does not cut within the pCAD24 sequence, and self-ligated. The plasmid obtained from this ligateion by E. coli transformation was designated as pCAM468. It possessed O. polymorpha LEU2 gene as a selectable marker and fragments of the ABV1 gene conferring integration of the BsrGI-digested plasmid into the corresponding chromosomal locus via double crossing over homologous recombination. If transformation with a LEU2-carrying plasmid was required after ABV1 inactivation, the disruption cassettes based on a vector, which can be self-excised by the Cre-loxP-mediated recombination42,43, was used. This cassette was constructed by insertion of the SalI-BalI 1.1 kb pCAM468 fragment between the SalI and EcoRV sites of the pAM619 vector42.
To obtain strains possessing extra copies of ABV1 three different plasmids were used. The pAM467 plasmid was isolated from a O. polymorpha genomic library based on the AMIpSL1 vector44 by complementation of vanadate sensitivity of the abv1 mutant obtained by random integration. The pKAB1 plasmid was constructed by insertion of PciI-MluI fragment of pAM467 between NcoI-MluI sites of the pBGX1 plasmid consisting of the pUK21 E. coli vector45 and XhoII-XhoII fragment of the O. polymorpha LEU2 locus cloned into the BglII site. The O. parapolymorpha ABV1 was obtained by PCR with primers 5′-AAACG CGTAC TCTGA CAGCG AC-3′ and 5′-ATTCT GGGCG GGTTC ACG-3′ and cloned between NdeI and MluI sites of the AMIpSL1 vector to obtain the pAZ16 plasmid. The AMIpSL1-based plasmids were used for yeast transformation in circular form. This led to obtaining transformants bearing autonomously replicating plasmids. Most rapidly growing sub-clones of such transformants, obtained after several round of selection, as a rule, possess the plasmid integrated into the genome in several copies44. The pKAB1 plasmid was linearized by PstI within the selectable marker O. polymorpha LEU2 to target the plasmid integration into this locus in a single copy.
To obtain prototrophic transformants, strains were transformed with empty vectors pCHLX37, pCCUR1 and/or AMIpSL1, when required.
Culture media
Apart from the regular yeast media YPD (1% yeast extract, 2% peptone, 2% glucose) and SC-D (0,67% Yeast Nitrogen Base, 2% glucose), complex medium depleted of phosphate was also used. The latter was prepared according previously reported approach46 with slight modifications as follows: 5 g of yeast extract and 10 g of peptone were dissolved in 170 ml of distilled water by autoclaving. Then 2.46 g of MgSO4 was dissolved in the obtained solution. The solution was supplemented with 7 ml of 5 M NaOH to obtain precipitate, which was removed first by centrifugation and then by filtration through a 0.2 µM filter. pH was adjusted to 7.2 by concentrated HCl, then water was added to a final volume of 200 ml to obtain 5X concentrate of the medium without carbon source. This concentrate was autoclaved, filtered again and used to prepare 1X media supplemented with 2% glucose and 2% agar if required. When necessary, this medium was supplemented with Na-Pi buffer pH7.2 to achieve a required phosphate concentration. Phosphate concentration in YPD calculated from the BD Biosciences technical data on phosphate content in yeast extract and peptone (https://www.bd.com/documents/guides/user-guides/DS_CM_Bionutrients-technical-manual_UG_EN.pdf) was approximately 4 mM. Vanadate was added to complex media as filter-sterilized 100 mM Na3VO4 water solution.
Immunoblotting
Prior to immunoblotting proteins were resolved by the SDS-electrophoresis in 10% acrylamide gels47. To analyze intracellular carboxypeptidase Y, samples were prepared using the alkaline extraction method48 from equal amount of cells, which was determined by assaying total cellular protein49. Previously described antiserum50 was used to detect CPY. Samples for Pho87 analysis were obtained using a special enrichment procedure51. Antibody from antisera obtained from rabbits immunized with the E. coli-expressed O. parapolymorpha Pho87 fragment were affinity purified by binding to the protein used for immunization. Prior the performing immunoblotting, samples were equalized according to density of major bands on Coomassie Blue-stained gels. The regular (New England BioLabs (NEB), cat. # P7702) and pre-stained protein markers (ThermoFisher Scientific, cat. #sm0441) were used in Coomassie Blue-stained gels and immunoblots, respectively.
Enzyme activity and 32Pi uptake assays
β-Galactosidase and acid phosphatase activities were assayed using chromogenic substrates o-nitrophenyl-β-D-galactopyranoside52 and p-nitrophenylphosphate53, respectively. Renilla luciferase was assayed using commercial kit (Biotium Inc., cat. #30004-1). The values were normalized to the total cellular protein.
The 32Pi uptake was assayed according to the previously described protocol33 as follows. Cells cultures grown overnight in YPD were 20-fold diluted with the same medium and grown additionally for 4 h. Cells from 1 ml of obtained cultures were harvested by centrifugation, washed with 3% glucose solution containing 25 mM Tris-succinate buffer pH4.5 or 25 mM Tris-succinate buffer pH8.0 and 10 mM NaCl, and re-suspended in 30 µL of the same buffer. The uptake was initiated by adding 1 µL of [32P] orthophosphate (2.4 Ci L−1, 50 Ci mmol−1) to the cell suspension. Phosphate uptake was terminated by addition of 1 mL of ice-cold Tris-succinate dilution buffer. The cell suspensions were immediately filtered through the Whatman GF/F filters (Whatman, UK) and washed with the same cold dilution buffers. The radioactivity retained on the filters was determined by liquid scintillation spectrometry.
Sources of reagents and media components
Reagents and media components were purchased from following suppliers: Difco (Yeast Nitrogen Base, Peptone, Yeast Extract), Sigma (various salts and buffer components). Enzymes and kits for in vitro DNA manipulations were supplied by NEB, Thermo Scientific, and Sibenzyme.
Data Availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information.
References
Samyn, D. R. & Persson, B. L. Inorganic Phosphate and Sulfate Transport in S. cerevisiae in Advances in Experimental Medicine and Biology. Yeast Membrane Transport (eds Ramos, J., Sychrová, H. & Kschischo, M.) 892, 253–269 (Springer, 2016).
Secco, D., Wang, C., Shou, H. & Whelan, J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 586, 289–295 (2012).
Martinez, P. & Persson, B. L. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258, 628–638 (1998).
Bun-Ya, M., Nishimura, M., Harashima, S. & Oshima, Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11, 3229–3238 (1991).
Wykoff, D. D. & O’Shea, E. K. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159, 1491–1499 (2001).
Auesukaree, C., Homma, T., Kaneko, Y. & Harashima, S. Transcriptional regulation of phosphate-responsive genes in low-affinity phosphate-transporter-defective mutants in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 306, 843–850 (2003).
Ghillebert, R., Swinnen, E., De Snijder, P., Smets, B. & Winderickx, J. Differential roles for the low-affinity phosphate transporters Pho87 and Pho90 in Saccharomyces cerevisiae. Biochem. J. 434, 243–251 (2011).
Oshima, Y. The phosphatase system in Saccharomyces cerevisiae. Genes Genet. Syst. 72, 323–334 (1997).
Yoshida, K., Kuromitsu, Z., Ogawa, N. & Oshima, Y. Mode of expression of the positive regulatory genes PHO2 and PHO4 of the phosphatase regulon in Saccharomyces cerevisiae. Mol. Gen. Genet. 217, 31–39 (1989).
Ogawa, N. et al. Functional domains of Pho81p, an inhibitor of Pho85p protein kinase, in the transduction pathway of Pi signals in Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 997–1004 (1995).
Schneider, K. R., Smith, R. L. & O’Shea, E. K. Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science 266, 122–126 (1994).
Kaffman, A., Herskowitz, I., Tjian, R. & O’Shea, E. K. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85. Science 263, 1153–1156 (1994).
O’Neill, E. M., Kaffman, A., Jolly, E. R. & O’Shea, E. K. Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex. Science 271, 209–212 (1996).
Kaffman, A., Rank, N. M., O’Neill, E. M., Huang, L. S. & O’Shea, E. K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396, 482–486 (1998).
Komeili, A. & O’Shea, E. K. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284, 977–980 (1999).
Pinson, B., Merle, M., Franconi, J. & Daignan-Fornier, B. Low affinity orthophosphate carriers regulate PHO gene expression independently of internal orthophosphate concentration in Saccharomyces cerevisiae. J. Biol. Chem. 279, 35273–35280 (2004).
Giots, F., Donaton, M. C. V. & Thevelein, J. M. Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 47, 1163–1181 (2003).
Stankiewicz, P. J., Tracey, A. S. & Crans, D. C. Inhibition of phosphate-metabolizing enzymes by oxovanadium(V) complexes. Met. Ions Biol. Syst. 31, 287–324 (1995).
Mahanty, S. K., Khaware, R., Ansari, S., Gupta, P. & Prasad, R. Vanadate-resistant mutants of Candida albicans show alterations in phosphate uptake. FEMS Microbiol. Lett. 68, 163–166 (1991).
Bowman, B. J. Vanadate uptake in Neurospora crassa occurs via phosphate transport system II. J. Bacteriol. 153, 286–291 (1983).
Bowman, B. J., Allen, K. E. & Slayman, C. W. Vanadate-resistant mutants of Neurospora crassa are deficient in a high-affinity phosphate transport system. J. Bacteriol. 153, 292–296 (1983).
Kanik-Ennulat, C., Montalvo, E. & Neff, N. Sodium orthovanadate-resistant mutants of Saccharomyces cerevisiae show defects in Golgi-mediated protein glycosylation, sporulation and detergent resistance. Genetics 140, 933–43 (1995).
Mannazzu, I. et al. Vanadium affects vacuolation and phosphate metabolism in Hansenula polymorpha. FEMS Microbiol. Lett. 147, 23–28 (1997).
Kurtzman, C. P. A new methanol assimilating yeast, Ogataea parapolymorpha, the ascosporic state of Candida parapolymorpha. Antonie Van Leeuwenhoek 100, 455–462 (2011).
Kim, M. W., Agaphonov, M. O., Kim, J.-Y., Rhee, S. K. & Kang, H. A. Sequencing and functional analysis of the Hansenula polymorpha genomic fragment containing the YPT1 and PMI40 genes. Yeast 19, 863–871 (2002).
Luo, Z. & Gallwitz, D. Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J. Biol. Chem. 278, 791–799 (2003).
Marešová, L., Vydarený, T. & Sychrová, H. Comparison of the influence of small GTPases Arl1 and Ypt6 on yeast cells’ tolerance to various stress factors. FEMS Yeast Res. 12, 332–340 (2012).
Odani, T., Shimma, Y., Tanaka, A. & Jigami, Y. Cloning and analysis of the MNN4 gene required for phosphorylation of N-linked oligosaccharides in Saccharomyces cerevisiae. Glycobiology 6, 805–810 (1996).
Ballou, C. E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 185, 440–470 (1990).
Riley, R. et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA 113, 9882–9887 (2016).
Ravin, N. V. et al. Genome sequence and analysis of methylotrophic yeast Hansenula polymorpha DL1. BMC Genomics 14, 837 (2013).
Hürlimann, H. C., Pinson, B., Stadler-Waibel, M., Zeeman, S. C. & Freimoser, F. M. The SPX domain of the yeast low-affinity phosphate transporter Pho90 regulates transport activity. EMBO Rep. 10, 1003–1008 (2009).
Zvyagilskaya, R. A. et al. Characterization of the Pho89 phosphate transporter by functional hyperexpression in Saccharomyces cerevisiae. FEMS Yeast Res. 8, 685–96 (2008).
Zhou, Y. et al. Core regulatory components of the PHO pathway are conserved in the methylotrophic yeast Hansenula polymorpha. Curr. Genet. 62, 595–605 (2016).
van Dijk, R. et al. Tagging Hansenula polymorpha genes by random integration of linear DNA fragments (RALF). Mol. Genet. Genomics 266, 646–56 (2001).
Agaphonov, M. et al. Defect of vacuolar protein sorting stimulates proteolytic processing of human urokinase-type plasminogen activator in the yeast Hansenula polymorpha. FEMS Yeast Res. 5, 1029–1035 (2005).
Sohn, J. H. et al. A novel autonomously replicating sequence (ARS) for multiple integration in the yeast Hansenula polymorpha DL-1. J. Bacteriol. 178, 4420–4428 (1996).
Agaphonov, M., Romanova, N., Choi, E.-S. & Ter-Avanesyan, M. A novel kanamycin/G418 resistance marker for direct selection of transformants in Escherichia coli and different yeast species. Yeast 27, 189–195 (2010).
Fokina, A. V. et al. Inactivation of Pmc1 vacuolar Ca2+ ATPase causes G2 cell cycle delay in Hansenula polymorpha. Cell Cycle 11, 778–784 (2012).
Keeling, K. et al. Leaky termination at premature stop codons antagonizes nonsense-mediated mRNA decay in S. cerevisiae. Rna 10, 691–703 (2004).
Myers, A. M., Tzagoloff, A., Kinney, D. M. & Lusty, C. J. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene 45, 299–310 (1986).
Agaphonov, M. & Alexandrov, A. Self-excising integrative yeast plasmid vectors containing an intronated recombinase gene. FEMS Yeast Res. 14, 1048–1054 (2014).
Agaphonov, M. O. Improvement of a yeast self-excising integrative vector by prevention of expression leakage of the intronated Cre recombinase gene during plasmid maintenance in Escherichia coli. FEMS Microbiol. Lett. 364, 22–25 (2017).
Agaphonov, M. O. et al. Vectors for rapid selection of integrants with different plasmid copy numbers in the yeast Hansenula polymorpha DL1. Yeast 15, 541–551 (1999).
Vieira, J. & Messing, J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100, 189–94 (1991).
Kaneko, Y., Toh-e, A. & Oshima, Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol. Cell. Biol. 2, 127–137 (1982).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Kushnirov, V. V. Rapid and reliable protein extraction from yeast. Yeast 16, 857–860 (2000).
Herbert, D., Phipps, P. J. & Strange, R. E. Chemical analysis of microbial cells. Methods Microbiol. 5, 209–344 (1971).
Agafonov, M. O. et al. A novel approach to isolation and functional characterization of genomic DNA from the methylotrophic yeast Hansenula polymorpha. Mol. Biol. (Mosk). 37, 81–87 (2003).
Karginov, A. & Agaphonov, M. A simple enrichment procedure improves detection of membrane proteins by immunoblotting. Biotechniques 61, 260–261 (2016).
Stansfield, I., Akhmaloka & Tuite, M. F. A mutant allele of the SUP45 (SAL4) gene of Saccharomyces cerevisiae shows temperature-dependent allosuppressor and omnipotent suppressor phenotypes. Curr. Genet. 27, 417–426 (1995).
To-E, A., Ueda, Y., Kakimoto, S. I. & Oshima, Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J. Bacteriol. 113, 727–738 (1973).
Acknowledgements
This work in part was supported by Russian Science Foundation (grant number 17-14-01092). Part of the work related to study of the ypt6-Δ mutation was funded by Russian Foundation for Basic Research (grant number 16-34-00211). We are grateful to Alexander Alexandrov for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
M.O.A. conceived and designed the project; A.V.K., M.O.A., A.V.F., T.S.K. and T.A.S. acquired the data; H.A.K. provided sequencing data; M.O.A. and A.V.K. analyzed and interpreted the data; M.O.A. and M.D.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karginov, A.V., Fokina, A.V., Kang, H.A. et al. Dissection of differential vanadate sensitivity in two Ogataea species links protein glycosylation and phosphate transport regulation. Sci Rep 8, 16428 (2018). https://doi.org/10.1038/s41598-018-34888-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34888-5
Keywords
This article is cited by
-
Multiple effects of the PHO91 gene knockout in Ogataea parapolymorpha
Folia Microbiologica (2023)
-
Insights into the structure and function of Est3 from the Hansenula polymorpha telomerase
Scientific Reports (2020)
-
Functional duplication of Rap1 in methylotrophic yeasts
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.