Abstract
The aim of this study was to investigate the effect of early calf-hood nutrition on the transcriptomic profile of the arcuate nucleus of the hypothalamus, anterior pituitary and testes in Holstein-Friesian bulls. Holstein-Friesian bull calves with a mean (±S.D.) age and bodyweight of 19 (±8.2) days and 47.5 (±5.3) kg, respectively, were offered a high (n = 10) or low (n = 10) plane of nutrition in order to achieve an overall growth rate of 1.2 and 0.5 kg/day. At 126 (±3) days of age, calves were euthanized, hypothalamus (arcuate region), anterior pituitary and testicular parenchyma samples were harvested and RNAseq analysis was performed. There were 0, 49 and 1,346 genes differentially expressed in the arcuate nucleus, anterior pituitary and testicular tissue of bull calves on the low relative to the high plane of nutrition, respectively (P < 0.05; False Discovery Rate <0.05). Cell cycle processes in the anterior pituitary were down regulated in the low relative to the high plane of nutrition; there was no differential expression of genes related to reproductive processes. Gene expression involved in cholesterol and androgen biosynthesis in the testes were down regulated in animals on the low plane of nutrition. This study provides insight into the effect of early life plane of nutrition on the regulation of the HPT axis.
Similar content being viewed by others
Introduction
Dairy bulls are now selected as potential artificial insemination (AI) sires soon after birth using genomic selection1. There is increasing evidence for a positive effect of the plane of nutrition during calfhood on the early onset of puberty in the bull2,3,4. It is desirable not only for such bulls to reach puberty earlier but also that they have the capability of producing high volumes of good quality semen early in life, particularly within the context of seasonal dairy production systems. It is critically important, therefore, to gain a better understanding of the effect of early calf-hood nutrition on the biochemical pathways and key factors affecting sperm production to facilitate the design of improved rearing protocols for such genetically elite and valuable bulls.
Enhanced nutrition in the early calf-hood period has been shown to positively impact the hypothalamic gonadotropin releasing hormone (GnRH) pulse generator and its action on the anterior pituitary gland; thereby advancing the age at onset of puberty in bulls5. The hypothalamus is widely acknowledged as the homeostatic regulator of the body6. Metabolic signals are sent from organs such as the liver (IGF-1), pancreas (insulin) and adipose tissue (leptin, adiponectin) and received by metabolic sensing neurons involved in satiety and energy homeostasis within the arcuate (ARC) nucleus. Such biochemical messages are mediated by proteins including neuropeptide Y (NPY), agouti-related protein (AgRP)7,8 as well as kisspeptin (Kiss)9,10. These metabolic sensing neurons stimulate GnRH release thereby affecting reproductive function.
The anterior pituitary is the principal regulator for growth, metabolism and reproduction via the synthesis and/or release of an array of hormones that control these functions in multiple peripheral organs11. The gonadotrophic cells in the anterior pituitary are characterised by the expression of GnRH receptors; which are responsible for the regulation of testicular function through secretion of gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH)12. The age at which bulls of dairy breeds attain puberty can range from 8–11 months13, although this is influenced by management. There is a transient rise of LH which occurs between 8 and 20 weeks of age, with a peak at 12–15 weeks, declines between 20 and 24 weeks of age14. Restricted nutrition during calfhood has been reported to affect steroidogenesis in the testes via inhibition of the magnitude of the hypothalamic GnRH pulse and therefore, the response of the anterior pituitary5.
The effect of level of nutrition offered to calves’ during the first six months of life on the early gonadotropin rise and the age at which puberty is reached cannot be rectified by the level of nutrition received thereafter2,4,5. We have recently demonstrated that Holstein-Friesian bulls fed a high plane of nutrition for the first six months of life reached puberty approximately one month earlier than bulls on a lower plane irrespective of their plane of nutrition during the subsequent months2.
Studies have been carried out in heifers using microarray, in situ hybridisation and immunohistochemical technologies to investigate the effect of early life nutrition on the molecular control of the hypothalamus and its knock on effects on age at onset of puberty but there is a lack of information on the nutritional influence on the molecular control of the HPT axis of the bull calf15,16,17. Recent advances in deep-sequencing technology provides the opportunity for in-depth insight into the global transcriptome of key biologically important tissues. The hypothesis under investigation in this study was that the global transcriptomic profiles of hypothalamic-pituitary-testicular tissues would be affected by plane of nutrition during the early calf-hood period in Holstein-Friesian bulls.
Material and Methods
All procedures involving animals were approved by the Teagasc Animal Ethics Committee (TAEC30/2013) licensed by the Health Products Regulatory Authority (licence number AE19132/P013) in accordance with the European Union Directive 2010/36/EU.
Animal Model
This experiment was conducted as part of a larger study designed to examine the effect of early calfhood nutrition on the molecular control of the HPT axis. The animal model and management has previously been described by Byrne et al.18. Briefly, twenty Holstein-Friesian bull calves with a mean (±S.D.) age and bodyweight of 19 (±8.2) days and 47.5 (±5.3) kg, respectively, were sourced from commercial dairy farms blocked on age, sire, liveweight and farm of origin. After five days acclimatisation, calves were assigned to either high or low plane of nutrition. Calves were individually fed milk replacer and concentrates (Tables 1 and 2) using an electronic feeding system (Forster-Tecknik Vario; Engen, Germany). Calves on the high plane of nutrition received 1200 g of milk replacer reconstituted to 8 L daily, together with concentrate ad libitum. Calves on the low plane of nutrition were allocated 500 g of milk replacer reconstituted to 4 L plus a maximum of 1 kg of concentrate daily. Diets were designed based on National Research Council guidelines (NRC 2001). Calves on both treatments were weaned when consuming a minimum of 1 kg of concentrate for three consecutive days, at a mean age of 82 (±3.9) days. There was no difference between the two groups in mean age at weaning. Following weaning, the high plane of nutrition group was offered concentrate feed ad libitum, while the low plane of nutrition group was offered 1 kg of concentrate, daily. All calves had daily access to approximately 0.5 kg of straw each and a constant supply of fresh water.
Tissue collection
The calves were euthanized at a mean age of 126 (±1.1) days of age, using an intravenous overdose of sodium pentobarbitone. The timing of slaughter was chosen as all calves at this stage would have been expected to have experienced an endogenous transient LH rise13 and the timing and magnitude of this rise affects would have affected testicular testosterone synthesis. Blood samples were collected on a fortnightly basis to determine systemic concentrations of LH and was quantified by RIA as previously described by Byrne et al.18. Death was confirmed by exsanguination followed by decapitation. The skullcap was opened and the brain was removed from the skull by severing the infundibulum, optic nerves and brain stem. Tissue enclosing the ARC nucleus region of the hypothalamus was dissected according to Komatsu et al.19. Two small triangular sections containing the arcuate nucleus were taken from either side of the bottom of the third ventricle. The pituitary gland was removed from the sella turcica and anterior and posterior sections of the pituitary gland were separated. The testes were excised and the tunica albuginea, epididymides and any excess connective tissue removed. Two sections of the parenchyma were dissected from the middle region of each testis. All samples were washed in sterile Dulbecco’s phosphate-buffered saline (DPBS), snap-frozen in liquid nitrogen, and subsequently stored at −80 °C pending further processing.
RNA isolation and purification
Total RNA was extracted from each tissue sample using RNeasy Universal plus Kit (Qiagen, Manchester, UK). The quantity and quality of the RNA isolated was determined using the same procedure as described by English et al.20. RNA integrity number (RIN) for all samples was greater than 8. Further RNA library preparation, sequencing and analysis were carried out as described by English et al.20.
Sixty (20 calves × 3 tissues) cDNA libraries were prepared from high quality RNA using an Illumina TruSeq RNA Sample Preparation kit v2 following the manufacturer’s instructions (Illumina, San Diego, CA, USA).
Following sequencing two testes samples from the low plane of nutrition and one anterior pituitary sample from the high plane of nutrition were removed from the study due to low sequence reads.
Results
Animal performance
Weight gain and other indicators of animal performance were outlined previously21. In brief, calves on a high plane of nutrition had larger testes, greater seminiferous tubule diameter, more mature spermatogenic cells and more Sertoli cells (Supplementary Table 4). The effect of a high plane of nutrition on the testicular development was validated at a transcriptional level in this study. There was no effect of nutritional treatment on FSH, LH or testosterone (P > 0.05) but there was an effect of week of sampling on all three hormones (P < 0.0001; Supplementary Fig. 1).
Differential gene expression
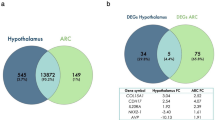
The RNAseq data have been deposited in NCBI’s Gene Expression Omnibus and are accessible via GEO series accession number GSE97673. The average number of raw reads for arcuate, anterior pituitary and testes samples were 16.3 million (mean ± SD; 16,319,326 ± 7,160,418), 16.3 million (16,346,430 ± 5,094,647) and 18.3 million (18,324,101 ± 7,135,544), respectively. There were 0, 49 and 1,346 DEG identified in ARC, anterior pituitary and testes tissues between animals on the two divergent planes of nutrition (P < 0.05; False Discovery Rate < 0.05), respectively. A multi-dimensional scaling (MDS) plot was created in Edge-R; the plot showed a lack of separation between samples from the two planes of nutrition with regard to the arcuate nucleus and anterior pituitary tissue but generally good separation between samples from animals on the high compared with the low planes of nutrition in the testes tissue (Fig. 1).
Multidimensional scaling plot which shows the measured similarity of the samples in 2-dimensions. The samples labelled in yellow are the Holstein-Friesian dairy bulls fed on a low plane of nutrition and slaughtered at 18 weeks of age and those labelled in red are the Holstein-Friesian dairy bulls fed on a high plane of nutrition and slaughtered at 18 weeks of age. Arc = Arcuate nucleus, Pit = Anterior Pituitary, Tes = Testes.
Pathway analysis
There were 45 DEG out of the original 49 anterior pituitary genes (seven genes with increased expression and 38 genes with decreased expression in the low compared to the high plane of nutrition groups; Supplementary Table 1) and 1,315 DEGs out of the original 1,346 testes genes (1,049 genes with increased expression and 266 genes with decreased expression in the low compared to the high plane of nutrition; Supplementary Table 2) that mapped successfully to a molecular/biological pathway using IPA. Mapped DEG were analysed and allocated to a biological function within IPA. The pathways and processes most affected within anterior pituitary tissue of the calves on the low, compared to the high plane of nutrition were all related to cycle cell processes such as mitotic roles of polo-like kinase (P < 0.0001) and cell cycle: G2/M DNA damage checkpoint regulation (P < 0.0001). Those pathways and processes identified as most differentially expressed within testicular tissue were super pathway of cholesterol biosynthesis (P < 0.0001) and androgen biosynthesis (P < 0.0001). Information on the effect of plane of nutrition on the molecular and cellular functions and on the biochemical pathways of the anterior pituitary and testes are presented in Figs 2–5, respectively. Gametogenesis was predicted to be increased (z-score: 2.279) in testicular tissue of the low compared to the high plane of nutrition calves (Supplementary Table 3). Male infertility function was predicted to be decreased (z-score: −2.236) in the low compared to the high plane of nutrition group.
Biochemical pathways statistically significantly enriched in the anterior pituitary of Holstein-Friesian bull calves offered either a low or a high plane of nutrition and, slaughtered at 18 weeks of age. Green and red bars represent genes down and up regulated, respectively, as a percentage of the overall number of genes in each pathway. The significance of each pathway is represented by the yellow line describing −log(p-value). The P-value was calculated by the number of genes from our dataset of differentially expressed genes represented in a particular pathway, divided by the total number of genes in the Canonical Pathway in IPA analysis. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)51.
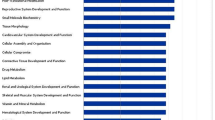
Molecular and cellular function of differentially expressed genes in anterior pituitary tissue of Holstein-Friesian bull calves offered either a low or a high plane of nutrition and and slaughtered at 18 weeks of age. The bars indicate the likelihood [−log (P-value)] that the specific molecular and cellular function was affected by a high plane of nutrition. The threshold line in the bar chart represents a p-value of 0.05. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)51.
Biochemical pathways statistically significantly enriched in the testes of Holstein-Friesian bull calves offered either a fed on low or a high plane of nutrition and, slaughtered at 18 weeks of age. Green and red bars represent genes down and up regulated, respectively, as a percentage of the overall number of genes in each pathway. The significance of each pathway is represented by the yellow line describing −log(p-value). The P-value is calculated by the number of genes from our dataset of differentially expressed genes represented in a particular pathway, divided by the total number of genes in the Canonical Pathway in IPA analysis. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)51.
Molecular and cellular function of differentially expressed genes of the testes of Holstein-Friesian bull calves offered either a fed on low or a high plane of nutrition and and slaughtered at 18 weeks of age. The bars indicate the likelihood [−log (P-value)] that the specific molecular and cellular function was affected by a high plane of nutrition. The threshold line in the bar chart represents a p-value of 0.05. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)51,52,53,54.
Discussion
This is the first study to investigate the effect of plane of nutrition during the early calf-hood period on the transcriptomic profile of the HPT axis in young bulls. Despite large differences in animal growth rate, metabolic status and testicular development between the nutritional treatments groups employed in the study, no DEG were detectable within tissue from the arcuate nucleus region of the hypothalamus. Offering calves a low plane of nutrition caused down regulation of processes involved in cell cycle in the anterior pituitary. The high plane of nutrition induced an increase in DEG in the testes, affecting processes involved in hormone production including androgen and cholesterol biosynthesis in comparison to the low plane of nutrition. Surprisingly, the function of ‘gametogenesis’ was predicted to be upregulated in testicular tissue of calves on the low compared with the high plane of nutrition.
Cholesterol biosynthesis
Cholesterol is the precursor for all steroid hormones and its availability is vital for their optimal production22. Cholesterol can be synthesised de novo from acetate, cholesterol ester stores in intracellular lipid droplets or the uptake of cholesterol from low density lipoprotein receptors (LDLR)23. There are five major stages in the process of cholesterol synthesis. Firstly, two acetyl CoA molecules are converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) and HMG-CoA is converted to mevalonate. Mevalonate is converted to isopentenyl pyrophosphate (IPP), which is then converted to squalene and the final step is the transformation of squalene to cholesterol. Genes encoding for the enzymes catalysing the biosynthesis of cholesterol including mevalonate kinase (MVK), phosphomevalonate (PMVK), disphosphomevalonate decarboxylase (MVD), dimethylallytranstransferase (FDPS), lanosterol synthase (LSS), D24 sterol reductase (DHCR24), D14 sterol reductase (TM7SF2), cholesterol D-isomerase (EBP) and 7- dehydrocholesterol reductase (DHCR7) were all found to be down regulated in the low compared to the high plane of nutrition group in testicular tissue. This is of interest as under normal physiological conditions, de novo cholesterol synthesis replenishes cholesterol stores in Leydig cells22.
The low plane of nutrition had a negative effect on genes that mediate the transport of cholesterol. Interestingly, the uptake of high density lipoprotein (HDL) cholesteryl esters via binding Apolipoprotein E (HDL-ApoE)24, the selective uptake pathway of HDL cholesterol to the testes via SCARB125 and the LDLR pathway23 were downregulated in calves on the low plane of nutrition. Liver X receptor (LXR) has been reported to be crucial for maintaining cholesterol homeostasis in murine Sertoli cells26. In our study plane of nutrition had no effect on LXR gene expression; however, the low plane of nutrition did affect its target genes such as Apolipoprotein E (APOE; log fold expression: −2.274) which is expressed in both Sertoli cells and germ cells of sexually immature rats and the Leydig cells of sexually mature rats27 and Scavenger receptor B1 (SCARB1; log fold expression: −1.816) which is expressed in murine Leydig cells28. The expression of LDLR was down regulated (log fold expression: −1.789) in calves on the low compared to the high plane of nutrition, indicating that this source of cholesterol inhibited testosterone synthesis as testicular Leydig cells under certain conditions obtain plasma lipoprotein-derived cholesterol for steroid synthesis29. In an associated study we analysed blood from the animals employed here and found greater serum testosterone concentrations in the calves on the high compared to the low plane of nutrition (Supplementary Fig. 1). Therefore, down regulation of cholesterol biosynthesis will likely negatively affect androgen biosynthesis.
Androgen biosynthesis
Androgens are crucial for optimal male reproductive function, playing important roles in maintaining spermatogenesis and sexual function. The rate of testosterone and estradiol production is controlled by gonadotropins, LH and FSH22. In our study we observed that FSHR (follicle stimulating hormone receptor) was down regulated in the high compared to the low plane of nutrition in the testes (log fold expression: −1.021). In the male, FSH stimulates Sertoli cell proliferation, as well as the induction and maintenance of normal spermatogenesis30. Expression of FSHR mRNA and number of FSH receptors has been found to decrease with testes development as bulls mature31. It has been reported that steroidogenic acute regulatory protein (StAR) is the primary determinant in the process of steroid synthesis32. The expression of StAR mRNA has previously been shown to be expressed in bull testes33 and it regulates the transport of cholesterol from the outer into the inner mitochondrial membrane. Treating bulls with GnRH agonist caused increased testosterone biosynthesis and StAR protein in the anterior pituitary34. In our study, calves offered the low plane of nutrition had a lower expression of StAR (log fold change: −1.914) compared to their contemporaries on the high plane of nutrition. The expression of StAR has been reported to be stimulated by IGF-1 in fetal and adult mice Leydig cells35,36. This is consistent with previous reports from our group showing greater plasma IGF-1 concentrations when the same animals in this study were offered a high compared with a low plane of nutrition (Supplementary Fig. 1).
There are two pathways involved in the conversion of pregnenolone to testosterone in the Leydig cells, Δ4 or Δ5. In the Δ5 pathway, pregnenolone is converted to 17α-hydroxypregnenolone then to dehydroepiandrosterone and finally to testosterone through either androstenediol or androstenedione. Our data show that this pathway was negatively affected by the low plane of nutrition. The conversion of cholesterol to pregnenolone is catalysed by cytochrome P450 side chain cleavage enzyme (P450scc) located in the inner mitochondrial membrane37. The transcription of CYP11A1 gene encoding P450scc regulates the quantity of P450scc and therefore, the steroidogenic function23. In the current study, CYP11A1 was down regulated in the low compared to the high plane of nutrition. Genes encoding for the enzymes catalysing the formation of testosterone including 3-β-hydroxysteroid dehydrogenase (HSD3B7 and HSD3B2) and steroid D-isomerase (HSD3B2 and EBP) were down regulated in the low compared to the high plane of nutrition, which led to decreased production of testosterone. However, reducing dietary nutrient intake did not down regulate all genes related to testosterone production as 17β-HSD, which codes for the enzyme responsible for the conversion of androstenedione to testosterone, was not altered by plane of nutrition.
Gametogenesis
There were 11 of 87 genes displaying upregulated expression in the low plane of nutrition animals consistent with a predicted increase in gametogenesis (spermatogenesis). These genes included SIAH1, RNF2, MCM9, CTCFL, FSHR, AGFG1, DDX25, MCM8 and KDM3A, which have all been reported to be involved in aiding gametogenesis38,39,40. This was surprising and may be due to the low plane of nutrition calves experiencing a late spurt of gametogenesis. Male infertility function was predicted to be decreased in the low plane of nutrition compared to the high. There were five genes out of 11 genes which had an expression consistent with an increase in male infertility. These genes including KMT2E (MLL5), SETX, GMCL1, SIRT1 and TDRD5; which have all been reported to be involved in aiding spermatogenesis in adult mice and humans41,42,43,44,45. Gonadotropin regulated testicular RNA helicase (GRTH/DDX25) is regulated by gonadotropin in Leydig cells and germ cells and is necessary for completion of spermatogenesis46. In Leydig cells, GRTH is a negative regulator of the expression of genes involved in cholesterol synthesis and transfer (SREBP2, HMG-CoA and StAR) and therefore, exhibiting control over androgen synthesis47. However, we found that testosterone concentrations were significantly higher for the calves on the high compared with the low plane of nutrition from 10 weeks of age until their slaughter at 18 weeks of age (Supplementary Fig. 1) and the high calves had larger testes48, a greater seminiferous tubule diameter, a greater number of more spermatogenic cells and a greater number of Sertoli cells (Supplementary Table 4). Our research group has also reported on the effect of plane of nutrition on systemic concentrations of testosterone in Holstein Friesian bulls calves at 16 and 32 weeks of age, following an exogenous GnRH challenge2. Additionally we have shown Holstein Friesian bulls calves offered a high plane of nutrition pre six months to reach puberty approximately 30 days earlier that their counterparts on a lower dietary allowance2. Although both functions were predicted to be upregulated in the calves on the low compared to the high treatment, the testes from the high plane of nutrition calves were at a more mature stage with regard to testes weight, seminiferous tubule diameter, stage of spermatogenesis and Sertoli cell number and lumen development at slaughter.
Differences in metabolic function in the ARC using microarray technologies were reported in heifer calves fed on a high concentrate diet to achieve rapid bodyweight gain, compared to contemporaries offered a high forage diet, from three-seven months of age15. This study found that key genes involved in satiety such as NPY and AGRP were down regulated and POMC and α-MSH were upregulated in the high concentrate heifers. The genes NPY/AGRP are well documented in have opposing roles to that of POMC/α-MSH in the control of feeding and energy expenditure8. It has been reported that NPY acts directly on GnRH neurons and that this may also be the case for AGRP as melanocortin agonists have direct action on GnRH neurons49. A study also carried out by the same research group using in-situ hybridisation and immunohistochemical technologies reported that increased rates of growth during the juvenile period in heifers alter the methylation pattern of genomic DNA from the ARC and such alterations may be linked to advanced age at puberty16. Our study however, found no DEG within ARC tissue between treatments. However, the studies reported above involved animals of a different sex, age, nutritional treatment and analytical technologies compared to our study which may have contributed to the difference in findings between studies. Our study is the first to date to utilize next generation sequencing technology to examine the effect of early calf hood nutrition on the global transcriptome of HPT tissue in Holstein-Friesian bull calves. The transcriptional profile of the ARC nucleus and anterior pituitary found in this study does not reflect the phenotype, in that the only DEG found in the anterior pituitary related to cell cycle and not specifically to metabolism or reproductive processes, as might be expected. This may be due to the transitory nature of gene transcripts in the brain50. However, while we did not detect DEG in either ARC or the anterior pituitary tissues which were specific to reproductive processes, gene expression profiles for testicular tissue were indicative of prior influence of plane of nutrition on the functionality of the hypothalamic-anterior pituitary axis, upon which testicular development depends.
References
Schefers, J. M. & Weigel, K. A. Genomic selection in dairy cattle: Integration of DNA testing into breeding programs. Animal Frontiers 2, 4–9, https://doi.org/10.2527/af.2011-0032 (2012).
Byrne, C. J., Fair, S., English, A. M., Cirot, M., Staub, C., Lonergan, P. & Kenny, D. A. Plane of nutrition pre and post-six months of age in Holstein-Friesian bulls: I. Effects on performance, body composition, age at puberty and post-pubertal semen production. J Dairy Sci 101, 3447–3459 (2018).
Brito, L. F. et al. Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls. Domest Anim Endocrin 33, 1–18, https://doi.org/10.1016/j.domaniend.2006.04.001 (2007).
Dance, A., Thundathil, J., Wilde, R., Blondin, P. & Kastelic, J. Enhanced early-life nutrition promotes hormone production and reproductive development in Holstein bulls. J Dairy Sci 98, 987–998, https://doi.org/10.3168/jds.2014-8564 (2015).
Brito, L. F. et al. Effect of improved nutrition during calfhood on serum metabolic hormones, gonadotropins, and testosterone concentrations, and on testicular development in bulls. Domest Anim Endocrin 33, 460–469, https://doi.org/10.1016/j.domaniend.2006.09.004 (2007).
Coll, A. P. & Yeo, G. S. H. The hypothalamus and metabolism: integrating signals to control energy and glucose homeostasis. Curr Opin Pharmacol 13, 970–976, https://doi.org/10.1016/j.coph.2013.09.010 (2013).
McShane, T. M., Petersen, S. L., McCrone, S. & Keisler, D. H. Influence of food restriction on neuropeptide-Y, proopiomelanocortin, and luteinizing hormone-releasing hormone gene expression in sheep hypothalami. Biology of reproduction 49, 831–839 (1993).
Stanley, S., Wynne, K., McGowan, B. & Bloom, S. Hormonal Regulation of Food Intake. Physiol Rev 85, 1131 (2005).
Gottsch, M. L. et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077, https://doi.org/10.1210/en.2004-0431 (2004).
Estrada, K. M., Clay, C. M., Pompolo, S., Smith, J. T. & Clarke, I. J. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. Journal of neuroendocrinology 18, 806–809, https://doi.org/10.1111/j.1365-2826.2006.01485.x (2006).
Musumeci, G. et al. A journey through the pituitary gland: Development, structure and function, with emphasis on embryo-foetal and later development. Acta histochemica 117, 355–366, https://doi.org/10.1016/j.acthis.2015.02.008 (2015).
Schanbacher, B. D. Hormonal interrelationships between hypothalamus, pituitary and testis of rams and bulls. Journal of animal science 55(Suppl 2), 56–67 (1982).
Rawlings, N., Evans, A. C., Chandolia, R. K. & Bagu, E. T. Sexual maturation in the bull. Reprod Domest Anim, 295–301, https://doi.org/10.1111/j.1439-0531.2008.01177.x (2008).
Evans, A. C. et al. Changes in circulating hormone concentrations, testes histology and testes ultrasonography during sexual maturation in beef bulls. Theriogenology 46, 345–357 (1996).
Allen, C. C. et al. Gene expression in the arcuate nucleus of heifers is affected by controlled intake of high- and low-concentrate diets. J Anim Sci 90, 2222–2232, https://doi.org/10.2527/jas.2011-4684 (2012).
Alves, B. R. C. et al. Nutritional programming of accelerated puberty in heifers: alterations in DNA methylation in the arcuate nucleus. Biology of reproduction 96, 174–184, https://doi.org/10.1095/biolreprod.116.144741 (2017).
Cardoso, R. C., Alves, B. R. C., Sharpton, S. M., Williams, G. L. & Amstalden, M. Nutritional Programming of Accelerated Puberty in Heifers: Involvement of Pro-Opiomelanocortin Neurones in the Arcuate Nucleus. Journal of neuroendocrinology 27, 647–657, https://doi.org/10.1111/jne.12291 (2015).
Byrne, C. J. et al. Plane of nutrition before and after 6 months of age in Holstein-Friesian bulls: II. Effects on metabolic and reproductive endocrinology and identification of physiological markers of puberty and sexual maturation. J Dairy Sci, https://doi.org/10.3168/jds.2017-13720 (2018).
Komatsu, M. et al. Age-related changes in gene expression of the growth hormone secretagogue and growth hormone-releasing hormone receptors in Holstein-Friesian cattle. Domestic animal endocrinology 42, 83–93, https://doi.org/10.1016/j.domaniend.2011.09.006 (2012).
English, A. M. et al. Effect of early calf-hood nutrition on the transcriptomic profile of subcutaneous adipose tissue in Holstein-Friesian bulls. BMC genomics 19, 281–293, https://doi.org/10.1186/s12864-018-4681-2 (2018).
English, A. M. et al. Role of early life nutrition on the hypothalamic-pituitary-testicular axis of the bull. Reproduction 156, 283–297 https://doi.org/10.1530/REP-17-0671 (2018).
Hu, J., Zhang, Z., Shen, W.-J. & Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutrition & Metabolism 7, 47–47, https://doi.org/10.1186/1743-7075-7-47 (2010).
Miller, W. L. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1771, 663–676, https://doi.org/10.1016/j.bbalip.2007.02.012 (2007).
Fofana, M., Travert, C., Carreau, S. & Le Goff, D. Evaluation of cholesteryl ester transfer in the seminiferous tubule cells of immature rats in vivo and in vitro. Journal of reproduction and fertility 118, 79–83 (2000).
Acton, S. et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520 (1996).
Robertson, K. M. et al. TheLiver X Receptor-β Is Essential for Maintaining Cholesterol Homeostasis in the Testis. Endocrinology 146, 2519–2530, https://doi.org/10.1210/en.2004-1413 (2005).
Olson, L. M., Zhou, X. & Schreiber, J. R. Immunolocalization of Apolipoprotein E in the Testis and Epididymis of the Rat1. Biology of reproduction 50, 535–542, https://doi.org/10.1095/biolreprod50.3.535 (1994).
Casado, M. E. et al. HSL-knockout mouse testis exhibits class B scavenger receptor upregulation and disrupted lipid raft microdomains. Journal of Lipid Research 53, 2586–2597, https://doi.org/10.1194/jlr.M028076 (2012).
Azhar, S. & Reaven, E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Molecular and cellular endocrinology 195, 1–26 (2002).
Simoni, M., Weinbauer, G. F., Gromoll, J. & Nieschlag, E. Role of FSH in male gonadal function. Annales d’endocrinologie (Paris) 60, 102–106 (1999).
Dias, J. A. & Reeves, J. J. Testicular FSH receptor numbers and affinity in bulls of various ages. Journal of reproduction and fertility 66, 39–45 (1982).
Christenson, L. K. & Strauss, J. F. 3rd Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Archives of medical research 32, 576–586 (2001).
Pilon, N. et al. Porcine and bovine steroidogenic acute regulatory protein (StAR) gene expression during gestation. Endocrinology 138, 1085–1091, https://doi.org/10.1210/endo.138.3.5003 (1997).
Aspden, W. J. et al. Changes in testicular steroidogenic acute regulatory (STAR) protein, steroidogenic enzymes and testicular morphology associated with increased testosterone secretion in bulls receiving the luteinizing hormone releasing hormone agonist deslorelin. Domestic animal endocrinology 15, 227–238 (1998).
Manna, P. R. et al. Molecular Mechanisms of Insulin-like Growth Factor-I Mediated Regulation of the Steroidogenic Acute Regulatory Protein in Mouse Leydig Cells. Molecular Endocrinology 20, 362–378, https://doi.org/10.1210/me.2004-0526 (2006).
Villalpando, I. & Lopez-Olmos, V. Insulin-like growth factor I (IGF-I) regulates endocrine activity of the embryonic testis in the mouse. The Journal of steroid biochemistry and molecular biology 86, 151–158 (2003).
Stocco, D. M. StAR protein and the regulation of steroid hormone biosynthesis. Annual review of physiology 63, 193–213, https://doi.org/10.1146/annurev.physiol.63.1.193 (2001).
Garikapati, K. R. et al. Down-regulation of BORIS/CTCFL efficiently regulates cancer stemness and metastasis in MYCN amplified neuroblastoma cell line by modulating Wnt/β-catenin signaling pathway. Biochemical and Biophysical Research Communications 484, 93–99, https://doi.org/10.1016/j.bbrc.2017.01.066 (2017).
Lindgren, I., Giwercman, A., Axelsson, J. & Lundberg Giwercman, Y. Association between follicle-stimulating hormone receptor polymorphisms and reproductive parameters in young men from the general population. Pharmacogenetics and Genomics 22, 667–672, https://doi.org/10.1097/FPC.0b013e3283566c42 (2012).
Kang-Decker, N., Mantchev, G. T., Juneja, S. C., McNiven, M. A. & van Deursen, J. M. Lack of acrosome formation in Hrb-deficient mice. Science 294, 1531–1533 (2001).
Yap, D. B. et al. Mll5 Is Required for Normal Spermatogenesis. Plos One 6, e27127, https://doi.org/10.1371/journal.pone.0027127 (2011).
Becherel, O. J. et al. Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing. Plos Genetics 9, e1003435, https://doi.org/10.1371/journal.pgen.1003435 (2013).
Kleiman, S. E. et al. Reduced human germ cell-less (HGCL) expression in azoospermic men with severe germinal cell impairment. Journal of andrology 24, 670–675 (2003).
Bell, E. L. et al. SirT1 is required in the male germ cell for differentiation and fecundity in mice. Development 141, 3495–3504, https://doi.org/10.1242/dev.110627 (2014).
Yabuta, Y. et al. TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. The Journal of Cell Biology 192, 781–795, https://doi.org/10.1083/jcb.201009043 (2011).
Tsai-Morris, C.-H., Sato, H., Gutti, R. & Dufau, M. L. Role of Gonadotropin Regulated Testicular RNA Helicase (GRTH/DDX25) on Polysomal Associated mRNAs in Mouse Testis. Plos One 7, e32470, https://doi.org/10.1371/journal.pone.0032470 (2012).
Fukushima, M., Villar, J., Tsai-Morris, C.-H. & Dufau, M. L. Gonadotropin-regulated Testicular RNA Helicase (GRTH/DDX25), a Negative Regulator of Luteinizing/Chorionic Gonadotropin Hormone-induced Steroidogenesis in Leydig Cells Central Role of Steroidogenic Acute Regulatory Protein (StAR). Journal of Biological Chemistry 286, 29932–29940 (2011).
Brito, L. F. C. In Bovine Reproduction (John Wiley & Sons, Inc, 2014).
Roa, J. & Herbison, A. E. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 153, 5587–5599, https://doi.org/10.1210/en.2012-1470 (2012).
Bondy, C. A. & Lee, W.-H. Patterns of Insulin-like Growth Factor and IGF Receptor Gene Expression in the Brain: Functional Implications. Ann NY Acad Sci 692, 33–43, https://doi.org/10.1111/j.1749-6632.1993.tb26203.x (1993).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 30, 523–30 (2014).
Amann, R. P., Wise, M. E., Glass, J. D. & Nett, T. M. Prepubertal changes in the hypothalamic-pituitary axis of Holstein bulls. Biology of reproduction 34, 71–80 (1986).
Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11, R14, https://doi.org/10.1186/gb-2010-11-2-r14 (2010).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28, 27–30 (2000).
Acknowledgements
The contribution of Dr. M. McCabe in the preparation of the RNAseq libraries, Dr. K. Keogh in interpreting the data as well as that of the farm staff at Teagasc, Grange Beef Research Centre, for care and management of the animals is gratefully acknowledged. This project was jointly funded by the Irish Department of Agriculture, Food and the Marine under the Research Stimulus Fund (Grant Number: 11/S/116) and the Irish Research Council (Grant Number GOIPG/2013/1391).
Author information
Authors and Affiliations
Contributions
A.M.E., S.M.W., S.F. and D.A.K. conception and design of the study; A.M.E. and C.J.B. performed animal experiment; A.M.E. prepared the cDNA libraries. A.M.E. and P.C. analysed the data; A.M.E. and C.J.B. prepared the figures and drafted the manuscript; A.M.E., C.J.B., P.C., S.M.W., S.F. and D.A.K. edited,revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
English, A.M., Byrne, C.J., Cormican, P. et al. Effect of Early Calf-Hood Nutrition on the Transcriptional Regulation of the Hypothalamic-Pituitary-Testicular axis in Holstein-Friesian Bull Calves. Sci Rep 8, 16577 (2018). https://doi.org/10.1038/s41598-018-34611-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34611-4
Keywords
This article is cited by
-
Early life nutrition affects the molecular ontogeny of testicular development in the young bull calf
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.