Abstract
The prognostic significance of various systemic inflammation‐based markers has been explored in different cancers after surgery. This study aimed to investigate whether these markers could predict outcomes in patients with early-stage hepatocellular carcinoma (HCC) undergoing radiofrequency ablation (RFA). One hundred eighteen patients with newly diagnosed HCC within the Milan criteria receiving RFA as initial therapy were retrospectively enrolled. Pretreatment inflammation-based markers including the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and prognostic nutritional index (PNI), together with other clinicopathologic parameters were collected. Cumulative overall survival (OS) and recurrence-free survival (RFS) were estimated by the Kaplan-Meier method and by multivariate analysis using Cox proportional hazard model. The 1-, 3-, and 5-year OS rates of patients were 90%, 67%, and 52%, respectively. Kaplan-Meier curves showed that baseline high NLR ≥ 2.5 (p = 0.006), low PNI < 40 (p = 0.005), history of end-stage renal disease (ESRD) (p = 0.005), non-Child-Pugh class A (p = 0.001) and elevated alpha-fetoprotein (AFP) ≥ 200 ng/mL (p = 0.005) significantly associated with the poor OS, whereas high PLR ≥ 100 did not. By multivariate analysis, high NLR ≥ 2.5 (hazard ratio (HR) 1.94; 95% confidence interval (CI), 1.05–3.59; p = 0.034), low PNI < 40 (HR 0.38; 95% CI, 0.20–0.72; p = 0.003), ESRD history (HR 3.60; 95% CI, 1.48–8.76; p = 0.005) and elevated AFP ≥ 200 ng/mL (HR 4.61; 95% CI, 1.75–12.13; p = 0.002) were independent factors. An elevated AFP level of ≥200 ng/mL was the significant factor associated with intrahepatic new RFS by univariate and multivariate analyses. In conclusion, pretreatment NLR and PNI are simple and useful predictors for OS in patients with early-stage HCC after RFA.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, being fifth in men and ninth in women1. It is the fourth most common cause of cancer-related death2. Male to female predominance is greater than 2:1 with HCC, and approximately 83% of the estimated 782,000 new HCC cases in 2012 occurred in less developed regions of the world, with Southeast Asia and sub-Saharan Africa being the high-incidence regions, while Southern Europe and North America are the intermediate regions, and Northern Europe and South Central Asia are the low-incidence regions3.
In Taiwan, the crude mortality rate of HCC is 30.21 per 100,000 person-years, which is the first and second leading cause of cancer-related mortality in males and females, respectively4. Hence, an active surveillance program has been implemented in this country. HCC is diagnosed at an early stage in 70% of patients and is amenable to potential curative treatments, such as surgical resection and liver transplantation. However, only 40% of HCC patients are candidates for resection due to poor liver function reserve secondary to underlying cirrhosis upon diagnosis5. Liver transplantation is another option for curative treatment, but it is not often feasible due to shortage of liver donors. Therefore, non-surgical treatments have been introduced. Among these, local ablative therapies have contributed as curative treatments for early stage HCC6.
Percutaneous radiofrequency ablation (RFA) is one of the most widely used local ablation therapies for HCC, with an effective complete ablation rate of 85% for solitary tumor <5 cm in diameter or up to 3 tumors with a maximum size of 3 cm6,7. Previous randomized controlled trials have demonstrated no significant differences on survival rates between RFA and surgical resection8,9.
In the past decade, studies on systemic inflammatory response are considered as enabling markers to cancer pathogenesis, including the proliferation, invasion, recurrence and metastasis of tumors. Valid indicators to predict the prognosis are important in the therapeutic management options of cancer. Published data revealed that these low cost inflammation-based markers including the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and prognostic nutritional index (PNI) are associated with prognostic significance in patients with HCC10,11,12,13. The elevated NLR was correlated with worse prognosis in HCC patients treated by RFA13, surgical resection14, transplantation15, and transcatheter arterial chemoembolization (TACE)16. A combination of the NLR and the PLR is a useful predictor for recurrence and prognosis in patients with HCC post RFA17.Another marker is the PNI, a combination of the serum albumin concentration and total lymphocyte count. This has been extensively introduced in clinical practice for predicting nutritional status and surgical risks of patients undergoing gastrointestinal cancer surgeries18.It has also been found that low PNI can be used to identify patients with increased risk of postoperative complications19. To the best of our knowledge, there has been no prior study on PNI in HCC patients undergoing RFA. Therefore, the present study will determine the prognostic impact and clinicopathological correlations of these inflammatory indices in patients with early HCC treated with RFA.
Methods
Patients
A retrospective analysis of 118 patients with newly diagnosed HCC within the Milan criteria (solitary nodule ≤ 5 cm; ≤3 nodule, none >3 cm; no macrovascular invasion) receiving RFA as initial therapy in Chiayi Chang Gung Memorial Hospital was conducted between January 2013 and August 2015. The diagnosis of HCC was according to the radiological and/or histological criteria as recommended by the American Association for the Study of the Liver Diseases guidelines20. Patients with positive history of chronic systemic inflammatory disease, active concomitant infection, uncontrolled diabetes, heart failure, coronary artery diseases, arrhythmia, and other diseases which might affect the inflammation-based markers were excluded.
Standard demographic and clinicopathological data were collected, including the following: age, sex, body mass index (BMI), routine blood count, liver and kidney functions, alpha-fetoprotein(AFP) and tumor characteristics. Clinically significant portal hypertension (CSPH) was defined as (1) a platelet count <100,000/mm3 associated with splenomegaly and/or (2) the presence of oesophageal/gastric varices by endoscopy21. The blood tests were routinely obtained in all patients before therapy. Pretreatment inflammation-based markers including the NLR, PLR and PNI were computed. The NLR was defined as the absolute neutrophil count divided by the lymphocyte count10,13; the PLR was estimated as platelet count divided by lymphocyte count;17 and the PNI was calculated as the sum of serum albumin(g/L) and 5 X absolute lymphocyte count (109/L)22. The formula of Fib-4 test was [age (years) x aspartate aminotransferase (AST) (U/L)]/[Platelets (109/L) × sqr alanine transaminase (ALT) (U/L)], and the albumin-bilirubin (ALBI) score was defined as −0.085 × (albumin g/L) + 0.66 × log (total bil irubin μmol/L)23,24. Written informed consent was obtained from patients prior to treatment. This study was approved by the Research Ethics Committee of Chang Gung Memorial Hospital and was conducted in accordance with the principles of Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
RFA procedure
RFA was performed on an in-patient basis using a Cool-tip RFA electrode (Radionics, Burlington, MA, USA) or Viva RF electrode system (STARmed, Korea). Procedures were done percutaneously, under local anesthesia, by qualified hepatologists with the guidance of real-time ultrasonography (US) using a 17-gauge, 2 or 3-cm needle. To avoid a rapid increase in intra-tumoral pressure, RFA was started at a low power (60 W), and the power was increased in increments of 10 watts/min. After each ablation in the tumor, the electrode was stepwise pulled out with ablation from the insertion site under the tip temperature more than 80 °C to reduce the risk of bleeding and tumor seeding. Creation of artificial ascites by intraperitoneal glucose water injection was used for lesions abutting the visceral organ and abdominal wall.
Follow-up
All patients were followed up in the out-patient clinic. Abdominal triphasic computed tomography (CT) or magnetic resonance imaging (MRI) was performed 4–6 weeks post-treatment. Treatment response to RFA was considered as complete ablation based on the absence of contrast enhancement or abnormal wash-out within or around the ablation zone. Follow-up included the physical examination; blood exams, including the liver function tests and AFP level assessment; and abdominal US every 3–6 months and triphasic CT scan every 6 months. Overall survival (OS) and recurrence-free survival (RFS) of local recurrence, intrahepatic new recurrence and extrahepatic recurrence were assessed. Local recurrence was defined as locally developing HCC besides the ablation lesion or a new one developing in the same segment. The OS time was defined as the time between the termination of RFA and the date of mortality or the last follow-up. The closing date of the study was 28, February, 2018. The RFS time was defined as the time between termination of RFA and the first recording of recurrence or the date of mortality of patients without evidence of disease recurrence. When recurrent tumors were diagnosed, patients received appropriate treatment, including repeated RFA, percutaneous ethanol injection therapy, TACE, surgical resection, liver transplantation, targeted therapy, chemotherapy, radiotherapy or supportive treatment.
Statistical analyses
The analysis software used was SPSS for Windows version 18 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the mean ± standard deviation (SD) and compared using Student’s t test. Categorical data were presented as frequency and were analyzed using the χ2 and Fisher’s exact test. Survival curves were estimated using the Kaplan-Meier analyses, and the differences of survival rates between groups were compared using the log-rank test. Univariate analysis was performed to assess significant differences in clinicopathologic characteristics that influenced the OS after RFA. Multivariate analysis was performed using Cox proportional hazards regression model for significant variables identified by univariate analysis. A time-dependent receiver operating characteristic (ROC) curve analysis was used to determine the cut-off values of AFP, NLR, PLR and PNI. All statistical tests were two-sided and differences were considered significant with a p < 0.05.
Results
Patient characteristics
The study included 118 patients with early stage HCC who had undergone RFA during the study period, for whom complete data were available. The study group consisted of 74 males (63%) and 44 females (37%) with a mean age of 69 ± 10 years (range 32–90). Fifty-one patients (45%) had concomitant type 2 diabetes mellitus. Eight patients (7%) had end-stage renal disease (ESRD) undergoing maintenance hemodialysis. In terms of etiologies, 88 (75%) patients were positive for anti-hepatitis C virus (HCV) antibody and 32 (27%) patients were positive for hepatitis B virus (HBV) surface antigen (HBsAg). Of them, 13 patients with HBV received NA (nucleos(t)ide analogue) therapy before RFA until the last follow-up and the additional 7 patients received NA after RFA. On the other hand, 24 patients with HCV received interferon (IFN) based therapies before RFA, and 26 patients received IFN (n = 7) or direct acting antivirals (DAA) therapy (n = 19) after RFA, of whom 6 had previous IFN treatment failure. The AFP, liver function tests, white blood cell, neutrophil, lymphocyte and platelet counts, as well as NLR, PLR and PNI, of the patients prior to RFA are shown in Table 1.
OS analyses
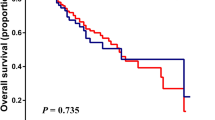
The median follow-up was 36 months (25–75th percentiles, 20.6–45.2 months). At the time of final analysis, 17 of 118 (14%) patients had died of HCC progression, 12 (10%) was related to liver failure, and 15 (13%) was unrelated to liver disease. Fifty-eight (49%) patients were still alive at their last visit and the remaining 16 (14%) patients were lost to follow-up before the closing date with the median follow-up of 27.5 months (25–75th percentiles, 14.7–38.8 months) after RFA. The 1-, 3-, and 5-year OS rates of patients were 90%, 67%, and 52%, respectively. Based on ROC curves, the cut-off values of NLR, PLR and PNI were 2.5, 100 and 40, respectively. The survival analyses confirmed that the inflammation-based indices were strong predictors of OS, as the median OS durations for patients with low-NLR was 38.1 months (95% CI: 4.1–62.1 months) vs. 33 months (95% CI: 1.2–58.1 months) for those with high-NLR (Fig. 1A). PLR was not a prognostic factor for OS (Fig. 1B). HCC patients with a low-PNI had a median OS of 33.2 months (95% CI: 1.2–58.2 months) vs. 38.2 months (95% CI: 8.1–62.1 months) for those with high-PNI (Fig. 1C). In addition, patients with combined low-NLR and high-PNI had the most favorable outcomes, with a median OS of 38.6 months (95% CI: 8.4–62.1 months) vs. 35.7 months (95% CI: 8.1–52.0 months) and 30.8 months (95% CI: 1.2–58.1 months) for those in the combined high-NLR/high-PNI or low NLR/low PNI and high-NLR/low-PNI groups, respectively. The 1-, 3-, and 5-year OS rates were 96%, 86%, and 83% in patients with combined low-NLR and high-PNI, 89%, 61%, and 36% in those with combined high-NLR/high-PNI or low NLR/low PNI, and 89%, 40%, and 24% in the high-NLR/low-PNI group, respectively (p < 0.001) (Fig. 2).
The univariate OS analysis showed significant associations of unfavourable OS with the following parameters: baseline high-NLR (p = 0.006), low-PNI (p = 0.005), history of ESRD (p = 0.005), antiviral therapy after RFA (p = 0.003), non-Child-Pugh class A (p = 0.001), ascites (p = 0.001), ALBI score (p = 0.02) and elevated AFP ≥ 200 ng/mL (p = 0.005). A high-PLR did not show significance. By multivariate Cox proportional hazards model, high-NLR (HR 1.94; 95% CI, 1.05–3.59; p = 0.034), low-PNI (HR 0.38; 95% CI, 0.20–0.72; p = 0.003), history of ESRD (HR 3.60; 95% CI, 1.48–8.76; p = 0.005) and elevated AFP ≥ 200 ng/mL (HR 4.61; 95% CI, 1.75-12.13; p = 0.002) were independent factors for OS (Table 2). In a subgroup analysis of 88 anti-HCV positive patients, multivariate Cox proportional hazards model showed that low-PNI (HR 0.44; 95% CI, 0.22–0.90; p = 0.024) and antiviral therapy after RFA (HR 0.05; 95% CI, 0.01–0.39; p = 0.004) were significant factors for OS.
Recurrence analyses
During the follow-up period, 52, 59 and 24 patients developed local recurrence, intrahepatic new recurrence and extrahepatic recurrence, respectively. The NLR, PLR and PNI did not carry any prognostic significance on the local recurrence, intrahepatic new recurrence and extrahepatic recurrence free survivals (Supplementary Table 1). The 1-, 3-, and 5-year intrahepatic new RFS rates were 74%, 52%, and 43% in patients with combined low-NLR and high-PNI, 67%, 41%, and 38% in those with combined high-NLR/high-PNI or low NLR/low PNI, and 88%, 68%, and 52% in the high-NLR/low-PNI group, respectively (p = 0.290).
An elevated AFP level of ≥200 ng/mL was the significant factor associated with intrahepatic new RFS by univariate and multivariate analyses (Table 3). In a subgroup analysis of anti-HCV positive patients, multivariate Cox proportional hazards model showed that antiviral therapy after RFA (HR 0.42; 95% CI, 0.21–0.84; p = 0.014) was an independent factor.
Association between the NLR/PNI with various clinicopathological characteristics
The association of inflammatory markers and clinicopathologic features of HCC patients are summarized in Table 4. As previously mentioned that patients were classified into two groups: low-NLR group (65%) and high-NLR group (35%). The high-NLR group were associated with larger tumor size (≥3 cm), lower serum AST level, ascites, higher white blood cell (WBC) count, higher neutrophil and lower lymphocyte counts compared with low-NLR group. There were 70 patients (59%) classified as high-PNI, and 48 patients (41%) as low-PNI. The low-PNI group had significantly worse hepatic function reserve (higher serum AST level, non-Child-Pugh class A and its constitutive variables: ascites, lower serum albumin, higher serum total bilirubin, prolonged prothrombin time and lower platelet count) compared to high-PNI group.
Discussion
Accumulated evidences have shown strong correlation between inflammation and cancer. A complex tumor microenvironment is one of the most important factors in a cancer prognosis. This has been confirmed that the interaction between the tumor itself and systemic inflammatory response will lead to tumor development25. The main features of a tumor-associated inflammatory response are the infiltration of leukocytes, the production of cytokines, the remodelling of tissue and angiogenesis26. Particularly in Taiwan, the majority of HCC cases developed from underlying chronic HBV or HCV infections. Both tumor inflammation and immunologic factors are known to enable cancer characteristics, and data support the involvement of both factors in cancer progression and metastasis26,27. Therefore, this has led to the development of various inflammatory indices in predicting clinical outcomes of HCC.
Our study showed that high-NLR and low-PNI were independent predictors of OS in early stage HCC patients after RFA. Multivariate analysis also identified other factors, including the serum AFP and ESRD on hemodialysis,which were similar in previous reports28,29. The finding of high-NLR concurred with the study of Chen et al.13 where high baseline NLR was associated with mortality in early HCC after RFA. Although the exact mechanism of high-NLR remains unknown, there are potential accepted explanations for this association. First, increasing neutrophils can promote tumor growth and invasion by releasing the vascular endothelial growth factor (VEGF), series of inflammatory mediators including angiogenesis circulating chemokines (CXCL8), matrix metalloproteinase-8/9 and the anti-apoptotic factor, nuclear factor-κB30,31. These inflammatory mediators further lead to oxidative damage, DNA mutation and altered microenvironment, which promote tumor cell growth and progression32. Secondly, lymphocytes play a vital role in an adaptive immune system that provide a cellular basis for cancer immunosurveillance and immuno-editing. Therefore, a reduced number of lymphocytes may weaken the ability to inhibit proliferation and metastatic activity of tumor cells despite the presence of cytotoxic cell death and cytokine production33,34. Wada et al.35 reported that increased infiltration of CD4+ T lymphocytes at the tumor margins among HCC patients was associated with a lower recurrence rate and better prognosis. These results suggest that lymphocytes have a role of anti-tumor effect.
The PNI was originally used to assess the immune-nutritional status and surgical risk in patients undergoing gastrointestinal surgery22. Subsequently, published data have correlated low-PNI index with tumor development and progression in various gastrointestinal malignancies19,36,37. In a meta-analysis by Man et al.38, preoperative PNI has been shown to be a prognostic predictive factor for OS, disease-free survival and recurrence in patients with HCC. Chan and colleagues39 confirmed that a low preoperative PNI was a significant prognostic factor for OS and disease-free survival in patients undergoing curative hepatic resection for HCC. In this study, we provided the first evidence that low PNI predicted OS for early stage HCC patients undergoing RFA. A low-PNI was found to be signficantly associated with poor hepatic function and portal hypertension, which was concordant with the findings reported by Chan et al.39 who also found poor tumor differentiation, large tumor size, high AFP and old age in addition to poor hepatic function and portal hypertension to be associated with a low-PNI. Valid reasons to explain this association are that low PNI in HCC patients has been associated with an increase in malnutrition, as well as concomitant underlying cirrhosis that might weaken anti-tumor and anti-metastasis response. Furthermore, hypoalbuminemia and lymphopenia have been demonstrated in liver cirrhosis40, suggesting that patients with worse hepatic function have lower PNI than those with a better hepatic function.
As to evaluate the prognostic value of combination of NLR and PNI, Okamura et al.41 presented that both high-NLR and low-PNI were poor predictors of OS in patients undergoing hepatectomy for HCC with curative intent.This was consistent with the findings of the present study which indicated that combined two markers could better reflect the systemic inflammatory response for patients with HCC after RFA, compared with either score alone.
We acknowledged the limitations of our study. First, this was a retrospective cohort in single center. Our predictive markers are needed to be validated in further studies with a multi-center, large sample size and prospective setting. Second, we did not evaluate for other inflammatory factors used in prognostication of HCC, namely C-reactive protein (CRP) and Glasgow Prognostic Score (GPS)42,43. The CRP is not routinely taken for examination in the treatment of HCC patients. Further studies are needed to determine which better reflect a poor inflammatory condition or whether combination of markers can improve prognostic performance.
In conclusion, the NLR and PNI serve as an effective inflammatory and immune-nutritional markers in daily clinical practice. Our data confirmed that pretreatment NLR and PNI are simple and useful in predicting the OS of early stage HCC patients undergoing RFA.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136, E359–386 (2015).
Wang, H. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388, 1459–1544 (2016).
Choo, S. P., Tan, W. L., Goh, B. K., Tai, W. M. & Zhu, A. X. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 122, 3430–3446 (2016).
Chiang, C. J. et al. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology. 61, 1154–1162 (2015).
Park, J. W. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 35, 2155–2166 (2015).
Lencioni, R. et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus ethanol injection. Radiology. 228, 235–240 (2003).
Shiina, S. et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 129, 122–130 (2005).
Chen, M. S. et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 243, 321–328 (2006).
Feng, K. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 57, 794–802 (2012).
Xue, T. C. et al. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One. 9, e96072 (2014).
Ke, M. et al. Prognostic nutritional index predicts short-term outcomes after liver resection for hepatocellular carcinoma within the Milan criteria. Oncotarget. 7, 81611–81620 (2016).
Pinato, D. J., North, B. V. & Sharma, R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index. Br J Cancer. 106, 1439–1445 (2012).
Chen, T. M., Lin, C. C., Huang, P. T. & Wen, C. F. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma after radiofrequency ablation. Journal of Gastroenterology and Hepatology. 27, 553–561 (2012).
Gomez, D. et al. Preoperative neutrophil to lymphocyte ratio as prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 32, 1757–1762 (2008).
Wang, G. Y. et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PloS One. 6, e25295 (2011).
Liu, C. et al. Neutrophil-lymphocyte ratio plus prognostic nutritional index predicts the outcomes of patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Scientific reports. 7, 13873 (2017).
Chen, K. et al. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma. Oncology Letters. 15, 315–323 (2018).
Kanda, M. et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 98, 268–274 (2011).
Jiang, N. et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 20, 10537–10544 (2014).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 67, 358–380 (2018).
Fang, K. C. et al. The impact of clinically significant portal hypertension on the prognosis of patients with hepatocellular carcinoma after radiofrequency ablation. Eur Radiol. 27, 2600–2609 (2017).
Onodera, T., Goseki, N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 85, 1001–1005 (1984).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-The ALBI grade. J Clin Oncol. 33, 550–558 (2015).
Ho, S. Y. et al. Prognostic performance of ten liver function models in patients with hepatocellular carcinoma undergoing radiofrequency ablation. Sci Rep 8, 843 (2018).
De Nardo, D. G., Johansson, M. & Coussens, L. M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 27, 11–18 (2008).
Colotta, F. et al. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 30, 1073–1081 (2009).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell. 144, 646–674 (2011).
Ji, F. et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio(NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. 16, 137 (2016).
Lee, C. H. et al. Hepatocellular carcinoma in hemodialysis patients. Oncotarget. 8, 73154–73161 (2017).
Kusumanto, Y. H., Dam, W. A., Hospers, G. A., Mejer, C. & Mulder, N. H. Platelets and granulocytes in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 6, 283–287 (2003).
Kuang, D. M. et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 54, 948–955 (2011).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature. 454, 436–444 (2008).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immmunobiology of cancer immunosurveillance and immunoediting. Immunity. 21, 137–148 (2004).
Kitayama, J., Yasuda, K., Kawai, K., Sunami, E. & Nagawa, H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 5, 47 (2010).
Wada, Y., Nakashima, O., Kutami, R., Yamamoto, O. & Kojiro, M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 27, 407–414 (1998).
Mohri, Y. et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 37, 2688–2692 (2013).
Geng, Y. et al. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 41, 1508–1514 (2015).
Man, Z. et al. Prognostic significance of preoperative nutritional index in hepatocellular carcinoma: a meta-analysis. HPB(Oxford). 18, 30796–2 (2018).
Chan, A. W. et al. Prognostic nutritional index predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 22, 4138–4148 (2015).
O’Keefe, S. J. et al. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 20, 615–617 (1980).
Okamura, Y. et al. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 39, 1501–1509 (2015).
Yamamura, K. et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 21, 682–688 (2014).
Kinoshita, A. et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 107, 988–993 (2012).
Acknowledgements
The authors would like to thank the Biostatistics Center of Kaohsiung Chang Gung Memorial Hospital for the helpful contribution.
Author information
Authors and Affiliations
Contributions
Conception and design: C.H. Hung. Analysis and interpretation of data: M. Chu, C.H. Hung. Drafting of the article: M. Chu, C.H. Hung. Critical revision of the article for important intellectual content: M. Chu, S.N. Lu, C.H. Hung. Final approval of the article: M. Chu, C.H. Shen, T.S. Chang, H.W. Xu, C.W. Yen, S.N. Lu, C.H. Hung. Provision of study materials or patients: C.H. Shen, T.S. Chang, H.W. Xu, C.W. Yen, S.N. Lu. Statistical expertise: C.H. Hung. Collection and assembly of data: CH. Hung.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, M.O., Shen, CH., Chang, TS. et al. Pretreatment Inflammation-Based Markers Predict Survival Outcomes in Patients with Early Stage Hepatocellular Carcinoma After Radiofrequency Ablation. Sci Rep 8, 16611 (2018). https://doi.org/10.1038/s41598-018-34543-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34543-z
Keywords
This article is cited by
-
Prognostic value of a nomogram based on peripheral blood immune parameters in unresectable hepatocellular carcinoma after intensity-modulated radiotherapy
BMC Gastroenterology (2022)
-
Value of Preoperative Hematological Parameters in the Prognosis of Gastric Cancer Patients Undergoing a Total Gastrectomy
Current Medical Science (2022)
-
Prediction Model for Intrahepatic Distant Recurrence After Radiofrequency Ablation for Primary Hepatocellular Carcinoma 2 cm or Smaller
Digestive Diseases and Sciences (2022)
-
Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.