Abstract
Hypocotyl elongation is influenced by light and hormones, but the molecular mechanisms underlying this process are not yet fully elucidated. We had previously suggested that the Arabidopsis DOF transcription factor DAG1 may be a negative component of the mechanism of light-mediated inhibition of hypocotyl elongation, as light-grown dag1 knock-out mutant seedlings show significant shorter hypocotyls than the wild type. By using high-throughput RNA-seq, we compared the transcriptome profile of dag1 and wild type hypocotyls and seedlings. We identified more than 250 genes differentially expressed in dag1 hypocotyls, and their analysis suggests that DAG1 is involved in the promotion of hypocotyl elongation through the control of ABA, ethylene and auxin signaling. Consistently, ChIP-qPCR results show that DAG1 directly binds to the promoters of WRKY18 encoding a transcription factor involved in ABA signaling, of the ethylene- induced gene ETHYLENE RESPONSE FACTOR (ERF2), and of the SMALL AUXIN UP RNA 67 (SAUR67), an auxin-responding gene encoding a protein promoting hypocotyl cell expansion.
Similar content being viewed by others
Introduction
Once germination is completed, the seedling undergoes photomorphogenesis or skotomorphogenesis, depending on the presence or absence of light1.
Photomorphogenic development is characterised by open and expanded cotyledons, short hypocotyls and functional chloroplasts, whereas skotomorphogenesis causes long hypocotyls, closed and unexpanded cotyledons with apical hooks, and undifferentiated chloroplasts (etioplasts).
Hypocotyl elongation is influenced by both environmental (primarily light and gravity) and hormonal cues, and it has been extensively studied as a model for cell elongation. Among phytohormones, auxin plays a pivotal role in promoting cell elongation, and its effect is mediated by the TRANSPORT INHIBITOR RESPONSE1 (TIR1)/AUXIN SIGNALING F-BOX (AFB)-Aux/IAA nuclear auxin receptor and the activation of SMALL AUXIN UP RNA (SAUR) genes2,3. The role of ethylene in hypocotyl development is strictly dependent on light conditions; indeed, in the dark ethylene represses, whereas in red light it promotes hypocotyl elongation4,5. Ethylene regulates seedling emergence from the soil through the master positive regulator of ethylene signaling ETHYLENE INSENSITIVE3 (EIN3), which is stabilised through COP1-mediated degradation of the F-box proteins EIN3-BINDING F BOX PROTEIN 1 and 2 (EBF1 and 2)6,7. EIN3 induces two downstream signaling pathways, respectively mediated by PHYTOCHROME INTERACTING FACTOR3 (PIF3) and ETHYLENE RESPONSE FACTOR 1 (ERF1). Once the seedling perceives the light, ethylene signaling is switched off by phyB-mediated EIN3 degradation7.
Abscissic acid (ABA) is known as a growth-inhibitory hormone, although some reports described a stimulatory effect of this hormone in maize, wheat, rice and Arabidopsis8,9,10. Recently, it has been proposed that the stimulatory or inhibitory response to exogenous ABA depend on both doses of, and tissue sensitivity to this hormone11. Hayashi and collaborators12 have shown that in dark-grown Arabidopsis seedlings ABA reduces the phosphorylation levels and consequently the activity of the plasma membrane H+-ATPase that triggers growth of the hypocotyl.
Although hypocotyl elongation has been extensively described, the molecular mechanisms underlying the hormonal regulation of this process are not yet fully elucidated.
The Arabidopsis Dof transcription factor Dof AFFECTING GERMINATION 1 (DAG1) is a repressor of light-mediated seed germination acting downstream of the master repressor PIF113,14; accordingly, dag1 knock-out mutant seeds require less GAs and lower red light fluence rates than wild type seeds to germinate15,16.
More recently, we demonstrated that DAG1 plays a key role in the control of the developmental switch between seed dormancy and germination17, acting on ABA and GA levels to establish (and maintain) seed dormancy and repress germination. DAG1 negatively controls the ABA catabolic gene CYP707A2 and the GA biosynthetic gene GA3ox1 through direct binding to their promoters. Consistently, in dag1 mutant seeds the ABA level is reduced while the GA level is increased compared to the wild type17. We had also shown that light-grown dag1 mutant seedlings have hypocotyls significantly shorter than the wild type, suggesting that DAG1 is a negative regulator in the light-mediated inhibition of hypocotyl elongation14.
Here, we investigated the role of DAG1 in the light-mediated inhibition of hypocotyl elongation by analyzing the transcriptome profile of 4 days-old dag1 and wild type hypocotyls and whole seedlings by means of high-throughput RNA-sequencing.
Results
Inactivation of DAG1 reduces hypocotyl cell elongation
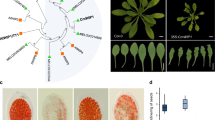
We have previously shown that dag1 mutant seedlings grown under continuous red light have significantly shorter hypocotyls compared to wild type14. To further corroborate this result we measured hypocotyl length of an Arabidopsis line overexpressing the DAG1-HA chimeric protein in a dag1 mutant background (dag1DAG1-HA)14. Five days-old dag1DAG1-HA seedlings grown under red light showed hypocotyls of the same length of wild type ones, suggesting that the chimeric protein DAG1-HA is functional and complements the hypocotyl phenotype of the dag1 mutant (Fig. 1a).
DAG1 inactivation affects hypocotyl cell expansion. (a) Hypocotyl length of dag1 (black bar), dag1DAG1-HA (grey bars) and wild type (white bar) five days-old seedlings, grown under under continuous monochromatic red light (40 μmolm−2s−1). (b) Hypocotyl growth of dag1 and wild type seedlings. Hypocotyl length was measured every day up to five days, using IMAGEJ software. Stratified seeds were induced to germinate under white light for 24 h, then grown for 5 days under continuous monochromatic red light (40 μmolm−2s−1). Three independent biological replicates were performed with SD values (n > 30). Significant differences were determined using two-way ANOVA followed by Tukey post-hoc test; significantly different groups are indicated by the letters. (c) Epidermal cell number of dag1 (black bar), dag1DAG1-HA (grey bars) and wild type (white bar) hypocotyls of four days-old seedlings grown on horizontal plates under continuous red light (40 μmolm−2s−1). For each sample, the number of cells in an epidermal cell file without stomata was counted. The values are the mean of three biological replicates, presented with SD values. Significant differences were analyzed by t-test. (d) Epidermal cells of wild type, dag1, and dag1DAG1-HA hypocotyls (top to bottom) of four days-old seedlings. The picture is referred to the third cell of the hypocotyl from the apex. Seedlings were grown as in (c).
Daily measurements of hypocotyl length for five days under red light revealed that at two days dag1 hypocotyls were slightly longer than wild type, possibly due to their faster germination rate15. At three days, hypocotyl length of mutant and wild type seedlings were comparable; at four and five days dag1 hypocotyls were significantly shorter than wild type ones (Fig. 1b).
Most of the hypocotyl cells derive from the embryo, and hypocotyl growth is mainly due to longitudinal expansion18. To assess whether the dag1 short-hypocotyl phenotype was due to a reduced number of cells or to decreased cell elongation, the number of hypocotyl epidermal cells was counted in four days-old dag1, dag1DAG1-HA and wild type seedlings grown under red light. This analysis revealed that dag1, dag1DAG1-HA and wild type hypocotyls do not show a significantly different number of epidermal cells (Fig. 1c). However, while dag1DAG1-HA cells are of the same size of wild type ones, dag1 epidermal cells are significantly shorter (Fig. 1d).
Inactivation of DAG1 affects several classes of (hormone-related) genes in hypocotyls
To elucidate the role of DAG1 in the control of hypocotyl growth, we performed RNA-seq analysis of 4 days-old dag1 and wild type hypocotyls and whole seedlings grown under continuous red light. Three biological replicates of each sample were sequenced using the Illumina Hi-seq platform. For each sample, more then 90% of reads successfully mapped to unique regions of the Arabidopsis genome (TAIR10) (Supplementary Table S1). To evaluate reproducibility among biological replicates, we performed a correlation analysis on normalized gene expression values (CPM, counts per million, see Methods). High positive correlation (Spearman’s correlation coefficient >0.95) was observed between the replicates of each sample (Supplementary Figs S1–S4). Clustering analysis of expression values led to a clear separation of samples according to tissue of origin (hypocotyls vs whole seedlings) as well as to sample condition (dag1 vs WT) (Supplementary Fig. S5a). However, the latter separation is less apparent in the case of hypocotyls, probably due to the higher gene expression variability observed in dag1 hypocotyls, as evidenced by Principal Component Analysis (Supplementary Fig. S5b).
Two comparison groups were constructed and differential expression analysis performed within each group: 1) dag1 vs wild type hypocotyls (hp dag1/WT) and 2) dag1 vs wild type whole seedlings (ws dag1/WT). The overall results of the differential expression analysis are shown in Fig. 2a, where all genes showing a significant expression change (False Discovery Rate, FDR < 0.05) in at least one comparison group are reported, for a total of 388 DE genes (Additional File 1).
Differentially expressed genes in dag1 hypocotyls and whole seedlings vs wild type. (a) Heatmap of differentially expressed genes. The colour scale indicates higher (red) to lower (green) gene expression levels. Gene expression values are expressed as log2 fold change. (b) Fraction of up- and down-regulated genes.
The comparison of dag1 and wild type hypocotyls (hp dag1/WT) revealed 257 differentially expressed (DE) genes. Of these, the majority (225) show up-regulated expression in dag1 (Fig. 2b).
Results of the functional enrichment analysis of DE up-regulated genes are shown in Fig. 3, where representative enriched Gene Ontology (GO) terms are reported for the Biological Process and Molecular Function categories according to REVIGO. Among the most significantly enriched processes we found different representative terms related to plant response to organic substances (“response to chitin”, “response to carbohydrate”, “response to organic substance”), stimuli (“response to stimulus”, “response to endogenous stimulus”) and immune response (Fig. 3a). A further inspection of these clusters revealed a subgroup of phytohormones-mediated responses, i.e. “response to hormone”, “hormone-mediate signalling pathway”, “response to auxin”, clustering together in the broader category “response to carbohydrate”. Instead, “response to abscisic acid”, “ethylene biosynthetic process” as well as “response to ethylene” form separate clusters and are also significantly enriched (Additional File 2). Notably, ABA, ethylene and auxin are known to be involved in hypocotyl elongation12,19,20. Other significantly enriched processes are “respiratory burst” and “immune system process”, possibly suggesting the involvement of DAG1 in the response to environmental stress (Fig. 3a). Finally, as shown in Fig. 3b, enrichment analysis in the Molecular Function category revealed the following significantly enriched GO terms: “transcription factor activity” and “transcription regulator activity” (Fig. 3b; Additional File 2).
Functional enrichment analysis of up-regulated DE genes in dag1 vs wild type hypocotyls. (a,b) Scatterplot view of enriched GO terms for Biological Process (a) and Molecular Function categories (b) according to REVIGO. Each bubble represents a representative GO term based on semantic similarity clustering. Bubbles are coloured according to p-values and their size indicates the absolute log10 (p-value). Bubbles x and y coordinates are derived by multidimensional scaling of GO term semantic similarity matrices so that more semantically similar GO terms are also closer in the two-dimensional plot.
The analysis of the down-regulated genes (32 out of 257 DE genes) revealed two enriched functional categories related to hormone response, i.e. “response to hormone” and “response to endogenous stimulus”, however the results are not statistically significant after correction for multiple testing (Additional File 2).
The analysis on whole seedlings (ws dag1/WT) identified 149 DE genes, most of them down-regulated in the dag1 mutant (105 out of 149) (Fig. 2b) (Additional File 1). The functional enrichment analysis on the latter genes revealed few enriched biological processes (FDR<0.05), where the most significantly enriched terms are “response to salicylic acid stimulus”, “defence response” as well as those related to immune response processes, again suggesting a possible involvement of DAG1 in the response to environmental stress (Additional File 2). No significant enrichment was observed in the Molecular Function category, while “extracellular region” resulted to be significantly enriched in the Cellular Component category. Finally, functional enrichment analysis of up-regulated genes revealed significantly enriched biological processes related to plant response to external/extracellular stimuli, nutrient levels, starvation and chemical homeostasis.
In summary, the results of our differential expression analysis between dag1 and wild type are consistent with the role of DAG1 as a transcription factor and suggest its involvement in the control of hormone-related genes in hypocotyls as well as in response to stress in the whole seedling. Moreover, a differential effect is observed on gene expression levels between hypocotyls and whole seedlings, resulting in a more prominent alteration of biological processes in hypocotyls.
Hormone-related genes
The RNA-seq data suggest that DAG1 is involved in the control of ABA, ethylene and auxin-related genes in hypocotyls. We validated the expression of a number of these hormone-related genes by RT-qPCR on dag1 and wild type hypocotyls.
Of the ABA-related genes, we analysed the expression of RAB GTPASE HOMOLOG B18 (RAB18), a stress-responsive gene involved in ABA and drought response21,22, ABA REPRESSOR1 (ABR1) encoding an APETALA2 (AP2) domain transcription factor known as a repressor of ABA23 and the ABA-responsive WRKY40 transcription factor encoding gene24. Among the genes enriching the “response to ethylene” biological process, we validated the expression of five ETHYLENE RESPONSE FACTORS (ERF)-encoding genes (ERF2, ERF5, ERF11, ERF105, ERF109, Additional File 2). This large family of transcription factors includes proteins with very diverse functions, involved in ethylene, ABA and gibberellins signaling25,26.
Four SAUR genes - auxin-induced genes highly expressed in hypocotyls2 where they promote hypocotyl elongation - namely SAUR50, SAUR63, SAUR65, and SAUR67 were among the DE genes belonging to the “response to auxin stimulus” enriched process (Additional File 2).
The results of this RT-qPCR analysis confirmed the RNA-seq data: the transcript levels of the ABA- and ethylene-related genes were higher and those of the SAUR genes were lower, respectively, in dag1 than in wild type hypocotyls (Fig. 4a–c), thus supporting the suggestion that the transcription factor DAG1 is involved in the hormonal regulation - specifically ABA, ethylene and auxin - of hypocotyl elongation.
DAG1 inactivation affects expression of ABA, ethylene and IAA-related genes. (a–c) Relative expression level of: RAB18, ABR1 and WRKY40 (a) ERF2, ERF5, ERF11, ERF105, and ERF109 (b) SAUR50, SAUR63, and SAUR65 and SAUR67 (c) in dag1 and wild type hypocotyls from four days-old seedlings grown under continuous monochromatic red light (40 μmolm−2s−1). RT-qPCR assays were performed with 1 μl of the diluted cDNA, along with the specific primers, listed in Table S3. Relative expression levels were normalized with UBQ10 (At4g05320) reference gene. The values of relative expression levels are means of three biological replicates, presented with SD values. Significant differences were analyzed by t-test (*P ≤ 0,05).
WRKY genes
Among the DE genes in dag1 hypocotyls, we also found the WRKY transcription factor family, encoding key regulators of many plant processes including the responses to abiotic stresses and to ABA, and seed dormancy/germination27. Since DAG1 plays a pivotal role in establishing seed dormancy and repressing seed germination by modulating both ABA and GA level, we set to validate these results by analysing the expression of WRKY6, WRKY18, WRKY28, WRKY33, WRKY46 and WRKY70 in dag1 and wild type hypocotyls by RT-qPCR. This analysis confirmed that inactivation of DAG1 results in a significantly increased expression of the WRKY encoding genes (Fig. 5).
DAG1 inactivation affects expression of WRKY genes in hypocotyls. Relative expression level of WRKY6, WRKY18, WRKY28, WRKY33, WRKY46 and WRKY70 in dag1 and WT hypocotyls from 4 days-old seedlings grown under monochromatic red light. RT-qPCR assays were performed with 1 μl of the diluted cDNA, along with the specific primers, listed in Supplementary Table S3. Relative expression levels were normalized with UBQ10 (At4g05320) reference gene. The values of relative expression levels are means of three biological replicates, presented with SD values. Significant differences were analyzed by t-test (*P ≤ 0,05).
DAG1 directly binds the promoters of ERF, SAUR and WRKY genes
DAG1 is known to bind, as all the Dof transcription factors, the CTTT sequence on the promoter of target genes14,17,28. Therefore, the validated set of DE genes was analysed for the presence of the CTTT binding site (BS) in their promoter by means of Promomer29. Significant matches were found for the ERF2, SAUR67 and WRKY18 promoters, suggesting that they could be direct targets of DAG1.
We performed ChIP assays, using dag1DAG1-HA plants14,17,30. Protein–DNA complexes were precipitated with anti-HA antibodies, or without antibodies as a negative control. As additional negative control, we performed the same assays on untransformed dag1 seedlings. Three regions of the ERF2, SAUR67 and WRKY18 promoters were amplified by qPCR (Fig. 6a–c).
DAG1 directly binds the promoters of ERF2, SAUR67 and WRKY18.(a–c) Top: graphic representation of the ERF2 (a) SAUR67 (b) and WRKY18 (c) promoters. Underlying thick lines marked by letters (a–c) are referred to different promoter fragments used for qPCR, containing 4, 1, 2 (ERF2), 1, 9, 5 (SAUR67) and 0, 4, 7 (WRKY18) Dof binding sites respectively. Bottom: chromatin from dag1DAG1-HA (left) and from dag1 (right) seedlings, as a negative control, was immunoprecipitated with anti-HA antibodies, and the amount of DNA was measured by qPCR for the ERF2 (a) SAUR67 (b) and WRKY18 (c) promoter fragments. Similar results were obtained from two independent biological replicates, The values are the average of two biological replicates presented with SD values. Significant fold enrichments were analyzed by t-test (*P ≤ 0,05).
As for SAUR67 and WRKY18, the relative amount of promoter fragments b and c (9, 5 and 4, 7 Dof BS in SAUR67 and WRKY18, respectively) precipitated by DAG1-HA were significantly higher than the negative controls, whereas the enrichment of precipitated promoter fragment a (1 and 0 Dof BS in SAUR67 and WRKY18, respectively) was very low in DAG1-HA and in the negative controls (Fig. 6b,c).
Of the three ERF2 promoter fragments (with 4, 1 and 2 Dof BS, respectively), amplification of fragments a and b was the most efficient, compared to the negative controls (Fig. 6a). Although only a single Dof site is present in fragment b, it is located within an optimal sequence context - it has been reported that the sequences flanking the Dof BS may influence DNA binding of the Dof proteins31.
This analysis indicates that DAG1 directly binds to the ERF2, SAUR67 and WRKY18 promoters in seedlings.
Upstream regions (500 bp) of the 257 DE genes found in the hp dag1/WT comparison group were used as input for the MEME discriminative motif discovery tool32 to identify patterns shared among, and/or repeated within these sequences.
Two enriched motifs were identified in some of the promoter sequences, i.e. CACGTG (E-value = 4.6e−013) and CTCTCTCT (E-value = 5.5e−004) (Supplementary Fig. S6). The former corresponds to a known bHLH-binding motif, i.e. the G-box that is bound by the PIF proteins, a family of transcription factors involved in light-mediated developmental processes33,34. Interestingly, DAG1 has been shown to be positively regulated by PIF1, the master repressor of seed germination13,14. PIF1, as well as PIF3, PIF4, PIF5 and PIF7, is also involved in the promotion of hypocotyl elongation35,36. Among the genes carrying the G-box motif in their promoters we identified seven LEA genes, five ERF genes and four SAUR genes, which we have previously validated by RT-qPCR. Interestingly, ERF2 and SAUR67 show both the PIF and the Dof binding sites (Additional File 3).
ABA response is altered in dag1 seedlings
The work described in the previous paragraphs suggests that DAG1 promotes hypocotyl cell elongation and represses the expression of ABA-responsive genes in hypocotyls.
Thus, we wondered whether DAG1 promotes hypocotyl elongation by repressing ABA-mediated inhibition. To substantiate this hypothesis, we measured hypocotyl length in dag1 and wild type seedling grown under red light in the presence of increasing ABA concentrations (0, 1, 10, 100, 150 µM). As shown in Fig. 7, while at 0 and 1 µM ABA dag1 hypocotyls are significantly shorter than the wild type, the difference becomes not significant at higher concentrations - thus ABA compensates the lack of DAG1 activity - suggesting that indeed the short-hypocotyl phenotype of dag1 is amenable to the inhibition of ABA-responsive genes (Figs 7 and S7).
ABA response in dag1 mutant seedlings. Hypocotyl length of dag1 and wild type seedlings in the presence of increasing concentration of ABA. Seeds were sown on MS agar with one layer of filter paper 595 (Schleicher & Schull, Dassel, Germany), and 48 h after stratification, seedlings were transferred to plates containing different ABA concentrations (0, 1, 10, 100 and 150 µM). Hypocotyl length was measured after 5 days. Three independent biological replicates were performed, with SD values (n > 30). Significant differences were determined using two-way ANOVA followed by Tukey post-hoc test; significantly different groups are indicated by the letters.
Analysis of overlapping DE genes between whole seedlings and hypocotyls comparison groups
Our transcriptome analysis revealed an overlapping set of differentially expressed genes showing opposite expression change between the two comparison groups (Fig. 8a).
Venn diagram for the up- and down-regulated genes. (a) Venn diagram of the hp dag1/WT and ws dag1/WT up- and down-regulated DE genes. (b) Expression analysis of the M17, CRA1, EM6, LEA18, M10, At2g23110, At3g17520, and At3g53040 DE genes in dag1 and wild type hypocotyls under continuous monochromatic red light (40 μmolm−2s−1). RT-qPCR assays were performed with 1 μl of the diluted cDNA, along with the specific primers, listed in Supplementary Table S3. Relative expression levels were normalized with UBQ10 (At4g05320) reference gene. The values of relative expression levels are means of three biological replicates, presented with SD values. Significant differences were analyzed by t-test (*P ≤ 0,05).
More specifically, the gene encoding the microRNA167D (miR167D), is down-regulated in hp dag1/WT and up-regulated in the ws dag1/WT group; the reads corresponding to miR167D map on a region corresponding to the stem–loop structure containing miRNA and complementary miRNA sequences (pre-miRNA) (Supplementary Fig. S8), suggesting that lack of DAG1 affects miR167D at the transcriptional and/or processing level.
Viceversa, 7 DE genes are up-regulated in hp dag1/WT and down-regulated in ws dag1/WT. The two seed-specific genes Late Embryogenesis Abundant Protein (LEA) gene M17 and Cruciferin 1 (CRA1) were among these genes (Fig. 8a). By means of RT-qPCR on dag1 and wild type hypocotyls, we validated the expression of these genes.
Since DAG1 has been previously shown to play a pivotal role during seed development17,37, we validated also the expression of other six LEA genes up-regulated in the hp dag1/WT group (Additional File 1; Supplementary Table S2). The expression analysis corroborated the RNA-seq data as in dag1 mutant hypocotyls the expression of these genes was sharply increased compared to wild type, thus suggesting that DAG1 is required to repress these seed-specific genes during hypocotyl development (Fig. 8b).
Discussion
We have previously demonstrated that DAG1 is involved in the repression of light-mediated inhibition of hypocotyl elongation14. Here, we show that DAG1 promotes hypocotyl cell expansion; based on the transcriptome analysis of dag1 hypocotyls, we suggest it does so through the control of ABA, ethylene and auxin signaling.
Indeed, the functional enrichment analysis of the DE up-regulated genes of the dag1/WT hypocotyl comparison, revealed that “response to abscisic acid”, “response to ethylene” and “response to auxin” are among the significantly enriched processes.
ABA, a growth-limiting hormone, has been shown to suppress hypocotyl elongation in etiolated squash hypocotyl segments38, as well as in etiolated Arabidopsis seedlings12. In addition, it has been recently demonstrated that ABA-responsive genes are repressed in shade avoidance-driven hypocotyl elongation39.
Among the up-regulated genes of the dag1/WT hypocotyl comparison group falling in the “response to abscisic acid” biological process and whose expression we validated by RT-qPCR were RAB18 and ABR1, encoding a GTPase and a transcription factor respectively, involved in ABA signaling21,22,23. Our results suggest that DAG1 is required to repress the expression of these genes in hypocotyls, consistent with the dag1 short-hypocotyl phenotype. In addition, our RNA-seq analysis identified seven WRKY transcription factors-encoding genes that are up-regulated in dag1 hypocotyls. The WRKY transcription factors are known as key components of ABA signaling: WRKY18, and the two closely related WRKY60 and WRKY40 proteins, have been shown to cooperate in plant response to biotic stress with both overlapping and distinct functions40,41. As for the response to abiotic stress, WRKY18 and WRKY60 have been shown to positively control the response to salt and osmotic stress, as well as ABA sensitivity; this function is counteracted by WRKY4042. It has been proposed a complex molecular model where these three WRKY factors may alternatively cooperate or play antagonistic roles to control the expression of the ABA INSENSITIVE 4 and 5 (ABI4 and ABI5) genes43. More recently, it has been demonstrated that both WRKY18 and WRKY40 are localised in nuclear bodies (NBs), discrete structures where the photoreceptor phyB, mainly in its active form Pfr44, co-localise, and physically interact, with PIF3 and PIF424. Here, we provide evidence that DAG1 negatively controls the expression of both WRKY18 and WRKY40, and that it directly binds the WRKY18 promoter. Recently, it has been shown that WRKY6 is repressed during seed germination and early seedling development, and is induced by exogenous ABA and involved in ABA signaling in these developmental processes45. We show that WRKY6 is up-regulated in dag1 hypocotyls, suggesting that DAG1 represses the expression of WRKY6 to promote hypocotyl elongation by negatively controlling ABA signaling. Consistently, we show here that the difference between dag1 and wild type hypocotyl length becomes not significant at higher concentration of ABA, indicating that ABA compensates the lack of DAG1 activity in the dag1 mutant.
Ethylene has opposite effects on hypocotyl elongation depending on light conditions19,46, promoting elongation in the light while suppressing it in the dark4,47. In promoting hypocotyl elongation, ethylene functions through PIF proteins positively controlling PIF3 transcription in the light6,48.
Among the genes up-regulated in dag1 hypocotyls are also seven members of the large ERF family encoding transcription factors with very diverse functions including ethylene and ABA signaling24,25. Among the ERF genes up-regulated in dag1 hypocotyls, ERF11 is the best characterised and represses the ACS2/5 ethylene biosynthetic genes49. Interestingly, ABA induces ERF11 thus repressing ethylene production.
We therefore suggest that DAG1 negatively controls ABA signaling, thus repressing ERF11 and increasing ethylene biosynthesis, ultimately promoting hypocotyl growth in the light.
Ethylene affects hypocotyl growth via different hormones including auxin, as inhibition of auxin transport, biosynthesis or perception suppresses ethylene-promoted hypocotyl elongation50. Consistently, our genome-wide analysis also indicates that four auxin-responsive SAUR genes - SAUR50, SAUR63, SAUR65 and SAUR67 - are down-regulated in dag1 hypocotyls; three of these, SAUR50, SAUR63 and SAUR67 are induced by ethylene50. In Arabidopsis there are 79 SAUR genes, originally identified because of their rapid induction by auxin51. More recently, an organ-specific genome-wide analysis characterised 32 SAUR genes whose expression is light-repressed in hypocotyls52 suggesting a positive role of these genes in hypocotyl elongation. Four of these SAUR genes are the ones down-regulated in dag1.
It was also shown that SAUR50 and SAUR65 are positively controlled by PIF3 and PIF4, which directly bind their promoters52. Consistently, the SAUR genes downregulated in dag1 are among the DE genes in hypocotyls of the dag1/WT comparison whose promoters are significantly enriched in the PIF-binding G-box motif.
Among these SAUR genes, the promoter of SAUR67 also contains a multiplicity (26) of DOF binding sites: by ChIP analysis we show here that DAG1 directly binds to this promoter. This suggests a cooperative action of DAG1 with PIF proteins in the regulation of SAUR67 and in promoting hypocotyl elongation.
It has been shown that PIF4 cooperates with the AUXIN RESPONSE FACTORS (ARFs) ARF6 and ARF8 in promoting hypocotyl elongation53. The ARF6 and ARF8 transcripts are targets of the MIR167 family of microRNAs54. In Arabidopsis, there are four MIR167 precursor genes (MIR167A-D). Expression profiling of these miRNAs showed that MIR167D, which has been proposed to be a pseudogene54, is primarily expressed in hypocotyls and cotyledons55. We show here that the expression of MIR167D is affected by inactivation of DAG1 in opposite ways in hypocotyls and whole seedlings. This suggest that MIR167D, rather than a pseudogene, may be a tissue-specific miRNA involved in hypocotyl elongation.
Finally, we have previously shown that DAG1 is also a key component of the molecular network controlling the seed-to-seedling transition in Arabidopsis17. Consistently, quite a high number of DE genes in the hp dag1/WT comparison group are related to seed-specific functions; in particular, seven LEA genes are up-regulated in dag1 hypocotyls. LEA proteins were first described to accumulate during plant seed dehydration at late stages of embryogenesis56. Indeed, the LEA genes that are up-regulated in dag1 hypocotyls are mainly expressed in seeds57, suggesting that DAG1 is required to repress the expression of these genes during seed-to-seedling transition.
In conclusion, the resources resulting from our genome-wide analysis substantiate the role of DAG1 in promoting hypocotyl elongation, and provide clues pointing to a role of this transcription factor in regulating and coupling ABA, ethylene and auxin signaling in this developmental process.
Methods
Plant material and growth conditions
All Arabidopsis thaliana lines used in this work were grown in a growth chamber at 22 °C with 16/8-h day/night cycles and light intensity of 300 μmol/m−2 s−1 as previously described15. dag1 is the allele described in Papi et al.15, in Ws-4 ecotype, dag1DAG1-HA is the transgenic line described in Gabriele et al.14. Seeds were surface sterilized and plated on MS agar (halfstrength MS, 0.8% agar, pH 5.7) and stratified at 4 °C for 3 days in the dark.
Phenotypic analysis
For hypocotyl elongation analysis, all seeds were surface sterilized, sown on MS agar and stratified. After germination was induced by white light for 24 h, plates were incubated for 5 days under continuous red light (40 μmolm−2s−1). Hypocotyl length was measured every day up to five days, or at the 5th day. For ABA experiments, seeds were sown on MS agar with one layer of filter paper 595 (Schleicher & Schull, Dassel, Germany), and 48 h after stratification, seedlings were transferred to plates containing different ABA concentrations (0, 1, 10, 100 and 150 µM). Hypocotyl length was measured after 5 days. Seedlings were scanned and the hypocotyl length was measured using IMAGEJ software. For cell number measurement, four days-old wild type, dag1 and dag1DAG1-HA seedlings, grown on horizontal plates under continuous red light (40 μmolm−2s−1), were fixed in a ethanol/acetic acid mixture (6:1). The samples were cleared in 100% ethanol, incubated for 30 minutes at room temperature. Than the 100% ethanol has been removed and replaced with 70% ethanol, and incubated for 30 minutes. After removing ethanol, samples were placed in a chloral hydrate/glycerol/water mixture (8:1:2, g:ml:ml). Seedlings were mounted in chloral hydrate mixture and images of the samples were taken with a Nikon coolpix 990 camera mounted on Zeiss Axioskop 2 plus microscope equipped with DIC optics. For each sample, the number of cells in an epidermal cell file without stomata was counted. All experiments were performed with three biological replicates, each with three technical replicates.
RNA-seq
Sterilized seeds were sown on MS agar and stratified. After germination was induced by white light for 24 h, plates were incubated for four days under monochromatic continuous red light (40 μmolm−2s−1). Seedlings were collected and frozen in liquid nitrogen in the dark. For hypocotyls about 1000 seedlings grown in this condition have been dissected, then hypocotyls were frozen in liquid nitrogen in the dark. Three biological replicates for both whole seedlings and hypocotyls were processed. Total RNA was extracted and purified as reported below. Any contaminating genomic DNA was removed using on column DNAse digestion. RNA quality was verified on agarose gel and with Agilent Bioanalyzer 2100 (RNA Integrity Number (RIN) >8).
RNA-seq data processing and detection of differentially expressed genes
RNA-seq reads were mapped to the A.thaliana Tair10 genome assembly using STAR258 with default parameters. The gene and transcript annotation from the Ensembl Plant database (http://plants.ensembl.org) was provided during the alignment step. After filtering for uniquely mapped reads, gene-level read counts were obtained using the HTSeq-count algorithm59 and then processed using the edgeR package60. For each sample, raw gene counts were first converted into CPM (counts per million) and those having CPM < 1 in at least three samples (the minimum number of samples in a group) were filtered out in order to filter lowly expressed genes. Gene expression levels and fold-changes were estimated after TMM (Trimmed-Mean of M values) normalization61. Both common (all genes in all samples) and separate (tag-wise) dispersion parameters were estimated using the Cox-Reid model and integrated into a Negative Binomial generalized linear model (NB-GLM). Statistical significance of differential expression was assessed using a GLM-likelihood ratio test and the ‘Benjamini-Hochberg’ correction for multiple testing. A FDR adjusted p-value of 0.05 was set as threshold to define differentially expressed genes.
Gene ontology analysis
Functional annotation analysis of DE genes was performed using the Singular Enrichment Analysis tool (SEA) from the AgriGO ontology database (http://bioinfo.cau.edu.cn/agriGO/analysis.php 62). AgriGO Enrichment results were further processed using the ReviGO tool63 in order to reduce redundant GO terms and prioritize the statistically significant representative terms. ReviGo analysis was performed using default parameters: allowed similarity: Medium (0.7); database: whole UniProt; semantic similarity; SimRel. A Fisher’s exact test q-value of 0.05 was used as a threshold for significant enrichment.
Expression analysis
For RNA extraction, four days-old dag1 and wild type seedlings, grown under monochromatic red light, were harvested and immediately frozen in liquid nitrogen. Total RNA was isolated by grinding the tissues in liquid nitrogen. The samples were then vortexed for 3 min in the presence of an extraction buffer (0.1 MLiCl, 0.1 M Tris-HCl [pH 8], 0.01 M EDTA, 1% sodium dodecyl sulfate-phenol-chloroform mixture (1:1:1). Three phenol-chloroform extractions were then performed. RNA was precipitated overnight at 4 °C with 1 volume of 4 M LiCl, followed by a second precipitation with 0.1 volume of sodium acetate, pH 5.2. RT-qPCR assays were performed with SYRgreen I master using the LightCycler® 480 instrument (Roche, http://www.roche.com). A total of 1 μl of the diluted cDNA was used, along with the specific primers, listed in Table S3 (Supplementary Table S3). Relative expression levels were normalized with UBQ10 (At4g05320) reference gene.
Chromatin Immunoprecipitation (ChIP) assay
ChIP assay was performed with 5 days old-seedlings of the transgenic line overexpressing the DAG1-HA chimeric protein in a dag1 mutant background and with the dag1 mutant as a negative control. Seedlings (about 1gr) were washed with water, then resuspended with 3 ml extraction buffer 1 (0.4 M sucrose, 0.01 M Tris-HCl [pH 8], 5 mM β-mercaptoethanol, 1 mM PMSF, 1x protease inhibitors) and treated with 37% formaldehyde for 10 min under vacuum. The reaction was stopped with glycine 0.125 M. Samples were then harvested with a miracloth membrane and immediately frozen and ground in liquid nitrogen. Extraction buffer was added to the samples (30 ml) then filtered on a miracloth membrane. After a centrifugation (4000 g, 20 min), the pellet was resuspended in 1 ml extraction buffer 2 (0.25 M sucrose, 0.01 M Tris-HCl [pH 8], 10 mM MgCl2, 1%Triton x-100, 5 mM β-mercaptoethanol, 1 mM PMSF, 1x protease inhibitors). After 10 min on ice, samples were centrifuged (12000 g, 10 min, 4 °C). The pellet was resuspended in 0.3 ml extraction buffer 3 (1.7 M sucrose, 0.01 M Tris-HCl [pH 8], 2 mM MgCl2, 0.15%Triton x-100, 5 mM β-mercaptoethanol, 1 mM PMSF, 1x protease inhibitors), then samples were centrifuged again (1 h, 16000 g, 4 °C). The chromatin pellet was resuspended in 0.3 ml lysis buffer (0.05 M Tris-HCl [pH 8], 0.01 MEDTA, 1% SDS, 1 mM PMSF, 1x protease inhibitors). Chromatin was sheared by sonication. To an aliquot of each sample (0.1 ml) was added 0.9 ml ChIP buffer (1.1% Triton, 1.2 mM EDTA, 16.7 mM Tris-HCl [pH 8], 167 mM NaCl, 1 mM PMSF, 1x protease inhibitors).
The immunoprecipitation was performed using HA-probe antibody (Y-11, sc-805 Santa Cruz), or without antibodies as negative control, overnight at 4 °C. After reverse cross-linking, the enriched DNA levels were quantified by qPCR using specific primer sets (Supplementary Table S3). The Fold enrichment of a specific region was calculated respect to the negative control without antibody. The values are the average of two biological replicates presented with SD values. Significant fold enrichments were analyzed by t-test (*P ≤ 0,05).
Statistical analysis
Two-way ANOVA followed by Tukey’s HSD posthoc test were used for pairwise multiple comparison (Figs 1 and 7). For hypocotyl elongation assays, statistical significance is indicated by the use of different letters. The values of gene expression analysis and ChIP assays are the mean of three biological replicates presented with SD values. Significant differences were analyzed by t-test (*P ≤ 0,05; **P ≤ 0,01).
References
Casal, J. J., Luccioni, L. G., Oliverio, K. A. & Boccalandro, H. E. Light, phytochrome signaling and photomorphogenesis in Arabidopsis. Photochem Photobiol Sci 2, 625–636 (2003).
McClure, B. A. & Guilfoyle, T. Characterization of a class of small auxin-inducible soybean polyadenylated RNAs. Plant Mol Biol 9, 611–623, https://doi.org/10.1007/BF00020537 (1987).
Fendrych, M., Leung, J. & Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. Elife 5 https://doi.org/10.7554/eLife.19048 (2016).
Zhong, S. et al. A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22, 1530–1535, https://doi.org/10.1016/j.cub.2012.06.039 (2012).
Shi, H. et al. The Red Light Receptor Phytochrome B Directly Enhances Substrate-E3 Ligase Interactions to Attenuate Ethylene Responses. Dev Cell 39, 597–610, https://doi.org/10.1016/j.devcel.2016.10.020 (2016).
Zhong, S. et al. Ethylene-orchestrated circuitry coordinates a seedling’s response to soil cover and etiolated growth. Proc Natl Acad Sci USA 111, 3913–3920, https://doi.org/10.1073/pnas.1402491111 (2014).
Shi, H. et al. Seedlings Transduce the Depth and Mechanical Pressure of Covering Soil Using COP1 and Ethylene to Regulate EBF1/EBF2 for Soil Emergence. Curr Biol 26, 139–149, https://doi.org/10.1016/j.cub.2015.11.053 (2016).
McWha, J. A. & Jackson, D. L. Some growth promotive effects of abscisic acid. J Exp Bot 27, 1004–1008 (1976).
Takahashi, K. Abscisic Acid as a Stimulator for Rice Mesocotyl Growth. Nat New Biol 238, 92–93 (1972).
Barrero, J. M. et al. The ABA1 gene and carotenoid biosynthesis are required for late skotomorphogenic growth in Arabidopsis thaliana. Plant Cell Environ 31, 227–234, https://doi.org/10.1111/j.1365-3040.2007.01759.x (2008).
Humplík, J. F., Bergougnoux, V. & Van Volkenburg, E. To stimulate or inhibit? That is the question for the function of abscissic acid. Trends Plant Sci 22, 830–841, https://doi.org/10.1016/j.tplants.2017.07.009 (2017).
Hayashi, Y., Takahashi, K., Inoue, S. & Kinoshita, T. Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H(+)-ATPase in Arabidopsis thaliana. Plant Cell Physiol 55, 845–853, https://doi.org/10.1093/pcp/pcu028 (2014).
Oh, E. et al. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16, 3045–3058, https://doi.org/10.1105/tpc.104.025163 (2004).
Gabriele, S. et al. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene. AtGA3ox1. Plant J 61, 312–323, https://doi.org/10.1111/j.1365-313X.2009.04055.x (2010).
Papi, M. et al. Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev 14, 28–33 (2000).
Gualberti, G. et al. Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 14, 1253–1263 (2002).
Boccaccini, A. et al. The DAG1 transcription factor negatively regulates the seed-to-seedling transition in Arabidopsis acting on ABA and GA levels. BMC Plant Biol 16, 198, https://doi.org/10.1186/s12870-016-0890-5 (2016).
Gendreau, E. et al. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114, 295–305 (1997).
Smalle, J., Haegman, M., Kurepa, J., Van Montagu, M. & Straeten, D. V. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94, 2756–2761 (1997).
Zhao, Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61, 49–64, https://doi.org/10.1146/annurev-arplant-042809-112308 (2010).
Hernández-Sánchez, I. E., Maruri-López, I., Graether, S. P. & Jiménez-Bremont, J. F. In vivo evidence for homo- and heterodimeric interactions of Arabidopsis thaliana dehydrins AtCOR47, AtERD10, and AtRAB18. Sci Rep 7, 17036, https://doi.org/10.1038/s41598-017-15986-2 (2017).
Wu, X. et al. CML20, an Arabidopsis Calmodulin-like Protein, Negatively Regulates Guard Cell ABA Signaling and Drought Stress Tolerance. Front Plant Sci 8, 824, https://doi.org/10.3389/fpls.2017.00824 (2017).
Pandey, G. K. et al. ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol 139, 1185–1193, https://doi.org/10.1104/pp.105.066324 (2005).
Geilen, K. & Böhmer, M. Dynamic subnuclear relocalisation of WRKY40 in response to Abscisic acid in Arabidopsis thaliana. Sci Rep 5, 13369, https://doi.org/10.1038/srep13369 (2015).
Dubois, M. et al. Ethylene Response Factor6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol 162, 319–332, https://doi.org/10.1104/pp.113.216341 (2013).
Moore, M., Vogel, M. & Dietz, K. The acclimation response to high light is initiated within seconds as indicated by upregulation of AP2/ERF transcription factor network in Arabidopsis thaliana. Plant Signal Behav 9, 976479, https://doi.org/10.4161/15592324.2014.976479 (2014).
Rushton, D. L. et al. WRKY transcription factors: key components in abscisic acid signaling. Plant Biotechnol J 10, 2–11, https://doi.org/10.1111/j.1467-7652.2011.00634.x (2012).
De Paolis, A., Sabatini, S., De Pascalis, L., Costantino, P. & Capone, I. A rolB regulatory factor belongs to a new class of single zinc finger plant proteins. Plant J 10, 215–223 (1996).
Toufighi, K., Brady, S. M., Austin, R., Ly, E. & Provart, N. J. The Botany Array Resource: e-Northerns, Expression Angling, and promoter analyses. Plant J 43, 153–163, https://doi.org/10.1111/j.1365-313X.2005.02437.x (2005).
Boccaccini, A. et al. The DOF protein DAG1 and the DELLA protein GAI cooperate in negatively regulating the AtGA3ox1 gene. Mol Plant 7, 1486–1489, https://doi.org/10.1093/mp/ssu046 (2014).
Yanagisawa, S. & Schmidt, R. J. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J 17, 209–214 (1999).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37, W202–208, https://doi.org/10.1093/nar/gkp335 (2009).
Leivar, P. & Quail, P. H. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16, 19–28, https://doi.org/10.1016/j.tplants.2010.08.003 (2011).
Lee, N. & Choi, G. Phytochrome-interacting factor from Arabidopsis to liverwort. Curr Opin Plant Biol 35, 54–60, https://doi.org/10.1016/j.pbi.2016.11.004 (2017).
Leivar, P. et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18, 1815–1823, https://doi.org/10.1016/j.cub.2008.10.058 (2008).
Shin, J. et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci USA 106, 7660–7665, https://doi.org/10.1073/pnas.0812219106 (2009).
Boccaccini, A. et al. Independent and interactive effects of DOF affecting germination 1 (DAG1) and the Della proteins GA insensitive (GAI) and Repressor of ga1-3 (RGA) in embryo development and seed germination. BMC Plant Biol 14, 200, https://doi.org/10.1186/s12870-014-0200-z (2014).
Wakabayashi, K., Sakurai, N. & Kuraishi, S. Role of the outer tissue in abscisic acid-mediated growth suppression of etiolated squash hypocotyl segments. Physiologia Plantarum 75, 151–156, https://doi.org/10.1111/j.1399-3054.1989.tb06162.x (1989).
Kohnen, M. V. et al. Neighbor Detection Induces Organ-Specific Transcriptomes, Revealing Patterns Underlying Hypocotyl-Specific Growth. Plant Cell 28, 2889–2904, https://doi.org/10.1105/tpc.16.00463 (2016).
Xu, X., Chen, C., Fan, B. & Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18, 1310–1326, https://doi.org/10.1105/tpc.105.037523 (2006).
Wenke, K. et al. Volatiles of two growth-inhibiting rhizobacteria commonly engage AtWRKY18 function. Plant J 70, 445–459, https://doi.org/10.1111/j.1365-313X.2011.04891.x (2012).
Chen, H. et al. Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10, 281, https://doi.org/10.1186/1471-2229-10-281 (2010).
Liu, Z. Q. et al. Cooperation of three WRKY-domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA-responsive genes ABI4 and ABI5 in Arabidopsis. J Exp Bot 63, 6371–6392, https://doi.org/10.1093/jxb/ers293 (2012).
Chen, M., Schwab, R. & Chory, J. Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc Natl Acad Sci USA 100, 14493–14498, https://doi.org/10.1073/pnas.1935989100 (2003).
Huang, Y., Feng, C. Z., Ye, Q., Wu, W. H. & Chen, Y. F. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. PLoS Genet 12, e1005833, https://doi.org/10.1371/journal.pgen.1005833 (2016).
Ecker, J. R. The ethylene signal transduction pathway in plants. Science 268, 667–675 (1995).
Yu, Y. et al. Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet 9, e1004025, https://doi.org/10.1371/journal.pgen.1004025 (2013).
Yu, Y. & Huang, R. Integration of Ethylene and Light Signaling Affects Hypocotyl Growth In. Front Plant Sci 8, 57, https://doi.org/10.3389/fpls.2017.00057 (2017).
Li, Z. et al. The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68, 88–99, https://doi.org/10.1111/j.1365-313X.2011.04670.x (2011).
Das, D. et al. Ethylene- and Shade-Induced Hypocotyl Elongation Share Transcriptome Patterns and Functional Regulators. Plant Physiol 172, 718–733, https://doi.org/10.1104/pp.16.00725 (2016).
Gil, P. et al. Characterization of the auxin-inducible SAUR-AC1 gene for use as a molecular genetic tool in Arabidopsis. Plant Physiol 104, 777–784 (1994).
Sun, N. et al. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc Natl Acad Sci USA 113, 6071–6076, https://doi.org/10.1073/pnas.1604782113 (2016).
Oh, E. et al. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 3 https://doi.org/10.7554/eLife.03031 (2014).
Wu, M. F., Tian, Q. & Reed, J. W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133, 4211–4218, https://doi.org/10.1242/dev.02602 (2006).
Gutierrez, L. et al. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21, 3119–3132, https://doi.org/10.1105/tpc.108.064758 (2009).
Dure, L., Greenway, S. C. & Galau, G. A. Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry 20, 4162–4168 (1981).
Hundertmark, M. & Hincha, D. K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9, 118, https://doi.org/10.1186/1471-2164-9-118 (2008).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, https://doi.org/10.1093/bioinformatics/bts635 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, https://doi.org/10.1093/bioinformatics/btu638 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, https://doi.org/10.1093/bioinformatics/btp616 (2010).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25, https://doi.org/10.1186/gb-2010-11-3-r25 (2010).
Du, Z., Zhou, X., Ling, Y., Zhang, Z. & Su, Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38, W64–70, https://doi.org/10.1093/nar/gkq310 (2010).
Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6, e21800, https://doi.org/10.1371/journal.pone.0021800 (2011).
Acknowledgements
We would like to thank Dr. Luca Montagni for his helpful assistance. This research was supported by research grants from Ministero dell’Istruzione, Università e Ricerca, Progetti di Ricerca di Interesse Nazionale, and from Sapienza Università di Roma to P.C. and to P.V. and from Istituto Pasteur Italia-Fondazione Cenci Bolognetti to P.V.
Author information
Authors and Affiliations
Contributions
A.T., P.V., Rc. L. conceived the strategy. P.V. directed the project. P.V., Rc. L. and A.B. designed the experiments. Rc. L., A.B. and V.R. performed the RNA-seq, ChIP and qRT-PCR experiments. F.G. and Rb. L. analysed RNA-seq data, generated the DE gene list and performed all bioinformatics analysis. M.P. performed the microscopic analysis. P.V., F.G., Rb. L. and P.C. wrote the manuscript. A.B., Rb. L. and V.R. revised the manuscript. All Authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorrai, R., Gandolfi, F., Boccaccini, A. et al. Genome-wide RNA-seq analysis indicates that the DAG1 transcription factor promotes hypocotyl elongation acting on ABA, ethylene and auxin signaling. Sci Rep 8, 15895 (2018). https://doi.org/10.1038/s41598-018-34256-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34256-3
Keywords
This article is cited by
-
Fasciclin-like arabinogalactan gene family in Nicotiana benthamiana: genome-wide identification, classification and expression in response to pathogens
BMC Plant Biology (2020)
-
Inhibition of Polycomb Repressive Complex 2 activity reduces trimethylation of H3K27 and affects development in Arabidopsis seedlings
BMC Plant Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.