Abstract
Current electrotransfection protocols are well-established for decades and, as a rule, employ long micro-millisecond range electric field pulses to facilitate DNA transfer while application of nanosecond range pulses is limited. The purpose of this paper is to show that the transfection using ultrashort pulses is possible by regulating the pulse repetition frequency. We have used 200 ns pulses (10–18 kV/cm) in bursts of ten with varied repetition frequency (1 Hz–1 MHz). The Chinese Hamster Ovary (CHO) cells were used as a cell model. Experiments were performed using green fluorescent protein (GFP) and luciferase (LUC) coding plasmids. Transfection expression levels were evaluated using flow cytometry or luminometer. It was shown that with the increase of frequency from 100 kHz to 1 MHz, the transfection expression levels increased up to 17% with minimal decrease in cell viability. The LUC coding plasmid was transferred more efficiently using high frequency bursts compared to single pulses of equivalent energy. The first proof of concept for frequency-controlled nanosecond electrotransfection was shown, which can find application as a new non-viral gene delivery method.
Similar content being viewed by others
Introduction
Electroporation is a common method to facilitate intracellular delivery of membrane-impermeable molecules1,2,3. The array of applications includes cancer treatment4,5,6,7, drug delivery8,9, gene transfer10,11,12, food processing13 and biotechnology14,15. Such procedure requires specific pulse parameters (amplitude, duration, number of pulses, etc.) to trigger the desired electroporation effect, which varies between different cell types16,17,18,19,20. Recently, a new electroporation modality, which employs nanosecond, high intensity (tens to hundreds of kV/cm) electric field pulses became a subject of intensive investigations21,22,23.
Sub-microsecond or nanosecond range pulsed electric field (ns-PEF) allows countering multiple limitations which exist in conventional micro-millisecond range electroporation. Firstly, ns-PEFs offer better control of delivered energy, while maintaining primarily non-thermal treatment24,25,26. Also, nsPEFs extend the flexibility of electroporation, allowing to induce apoptosis27 and non-chemical triggering of Ca2+ channels28. Additionally, the manipulation of other cell functions is possible due to electric field induced displacement currents and the non-thermal interactions with subcellular structures29,30. Lastly, ns-PEF allows to minimize electrochemical reactions31 during the procedure and a more patient-friendly treatment with minimal muscle contractions32 is possible.
One of the electroporation applications is gene delivery both in vitro and in vivo10,12. Well established protocols for efficient electrotransfection have been already established for decades33,34,35,36,37. However, all of them require application of long micro-millisecond range pulses or combination of microsecond and nanosecond bursts to facilitate DNA transfer38. At the same time, electrophoresis has a crucial role during transfection39. However, not all of the aspects of how the electroporation mediates gene electrotransfer and expression in cells and tissues are known40. As a result, electrophoresis is a limiting parameter for application of ultrashort pulses. Nevertheless, new pulse delivery techniques emerge and the variation of pulse repetition frequency (PRF) appears to be a powerful tool for control of electroporation efficiency40,41,42,43. Low frequency protocols offer the possibility to induce the phenomenon of cell sensitization44, while higher frequency range allows to counter bioimpedance problems and reduce muscle contractions45,46. However, since the amplification of the external electric field in the cell membrane is frequency dependent and decreases in the sub-megahertz range47,48 the MHz region is still poorly focused, thus remaining challenging technologically, and therefore mostly a subject of theoretical analysis.
In our previous work, we have shown that it is possible to generate a high frequency pulse burst to achieve a threshold PRF (presumably unique for each cell type and other electroporation conditions), when the discharging (transmembrane potential relaxation) time of the membrane is longer than the delay time between the pulses49. As a result, the response of cells (determined by propidium iodide permeabilization assay) to high PRF was several-fold higher if compared to low frequency protocols. Recently, similar result was confirmed by Semenov et al., where authors of the study showed that the uptake of YO-PRO-1 is doubled when the nanosecond pulses are closely spaced50.
Taking into the account the membrane charge relaxation phenomenon (in high PRF region) and the capability to induce a more uniform exposure (Fig. 1), we have speculated that it is possible to achieve successful transfection using nsPEF, which has not been shown so far.
In this study, we expand the experimental coverage of high frequency, MHz range nanosecond electroporation, compare the results to conventional low pulse frequency (LPF) protocols and provide a proof of concept for successful frequency-controlled nanosecond electrotransfection.
Results
Electrotransfection using GFP coding plasmid
We have evaluated the transfection expression levels of GFP coding plasmid after application of 200 ns × 10 pulses bursts using different PRF protocols and compared the results to a single pulse (2 µs) of identical energy and to the standard 100 µs pulses (2 × 1.4 kV/cm × 100 µs), the latter serving as a positive control. The cells were treated with PEF and the fraction of GFP positive cells was evaluated the next day using flow cytometry. The dependence of the number of fluorescent (GFP positive) cells on the applied pulsing protocol is presented in Fig. 2.
As it can be seen in Fig. 2, the low frequency (<100 kHz) nanosecond pulse protocols were not effective (FC <4%) in the whole range of amplitudes (10–18 kV/cm). However, increase of the PRF to 0.1 and 1 MHz allowed to achieve a transfection efficiency in the 10–20% range, which was comparable with single pulse protocol (2 µs). The 2 × 1.4 kV/cm × 100 µs protocol resulted in up to 51 ± 7% GFP positive cells.
Taking into the account that the percentage of GFP positive cells may be manipulated by definition of gates in flow cytometry, we have also evaluated the total fluorescence intensity of the samples in order to quantify the transfection expression levels in terms of relative total GFP production. The results are summarized in Fig. 3.
As it can be seen in Fig. 3 a similar tendency of PRF influence on the electrotransfer persists, however the 2 µs protocol appeared to be significantly superior (2–3 fold) in the 16–18 kV/cm range if compared to the nanosecond pulse protocols in the 16–18 kV/cm range. At the same time, the positive control was more than 10-fold more fluorescent if compared to the highest intensity (18 kV/cm, 1 MHz) nanosecond range protocol.
Electrotransfection using LUC coding plasmid
Similar pulsing and comparison strategy was applied for electrotransfection using LUC coding plasmid. The cells were treated with PEF and the luciferase expression was evaluated after 24 h using luminometer (TECAN GeniosPro). The dependence of sample luminescence on the applied PEF parameters is presented in Fig. 4.
As it can be seen in Fig. 4, the transfection efficiency increased with the increase of PRF. Similar to the case of GFP, the highest expression levels were observed using the 1 MHz protocol, and a dose dependent response was apparent. The 10 kHz protocol was already resulting in low, but statistically significant increase of luminescence. Differently from GFP experiments, the 2 µs protocol was inferior to the high frequency (0.1–1 MHz) bursts in the whole range of investigated amplitudes. However, the positive control had the highest luminescence, which was more than 30-fold higher if compared to the highest intensity (18 kV/cm; 1 MHz) nanosecond pulse burst. Similar to the case of GFP, the low frequency nanosecond range protocols (<10 kHz) did not result in a statistically significant increase of LUC expression levels in the whole range of applied amplitudes.
Viability
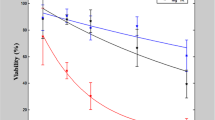
Lastly, the viability of the CHO cells after the PEF treatment using all of the applied protocols was evaluated. The results are summarized in Fig. 5.
As it can be seen in Fig. 5, the viability decrease after the 2 µs pulse was higher compared to the nanosecond pulse protocols, however, it was statistically significant only after the 18 kV/cm treatment. Similar reduction in viability (up to 26%) was also observed in the positive control. All of the applied treatment protocols resulted in a predominantly reversible electroporation of CHO cells.
Discussion
Electroporation is considered to be an effective non-viral gene delivery approach, which usually requires maintaining of a good balance between electrotransfection efficiency and cell viability51,52. The high potential of the gene therapy in clinical applications and the evolution of the whole electroporation area towards shorter pulse duration space53 requires development of new and effective gene electrotransfer protocols. Currently, it is believed that nanosecond range PEF are not suitable for transfection54, presumably due to lack of sufficient electrophoresis, while the micro-millisecond range pulse protocols are a well-established procedure33. Nevertheless, it was shown by Guo et al. that pretreating the cells with nsPEF before application of the microsecond range pulses can significantly increase transport of DNA38. It implies that the lack of electrophoretic force during nsPEF can be compensated by subsequent microsecond pulses to facilitate DNA electrophoretic transfer. Combined with other nsPEF effects such as cell swelling55, organelle membrane modulation30 and calcium mobilization28, the nsPEF can be effectively used for pretreatment of cells to increase DNA diffusion or stability38. However, transfection solely by nanosecond pulse protocols is currently considered unachievable54.
In this study, we present a proof of concept that the nanosecond range pulses can be successfully applied for transfection by manipulating pulse repetition frequency.
The high PRF (1 MHz) of nanosecond pulses allowed to achieve significant increase of the transfected cells (up to 17% of cells being GFP positive), which was not possible in 1 Hz–10 kHz range. The phenomenon could be partly justified by the accumulation of charge on the cell membrane when the relaxation time is longer compared to the delay between the pulses, which results in the increase of transmemebrane voltage potential and thus increased permeabilization rate43,49,50. However, it does not explain the increased DNA electrotransfer rate, which requires electrophoresis that is limited during short pulses. The hypothesis that the cells see the high frequency burst as a single pulse of equivalent energy43 was proposed by Steelman et al., however, our data suggests that it is not the only cause of this phenomenon. The LUC coding plasmid was transferred more efficiently using high frequency bursts compared to single pulses of equivalent energy. It could be the cause of the dielectric polarization, which is altered in high frequency bursts since the permittivity parameters are frequency-dependent56,57. The effect also could be caused by the increased average pore size and altered polarizability of the cells and/or biomolecules during high frequency bursts. The concept of induction of bigger pore radii during high pulse frequency (HPF) electroporation compared to single pulses of equivalent energy was proposed recently in silico58. When the PRF increases (or pulse interval decreases), the pore radius also tends to increase58, which can explain better DNA electrotransfer. The capability to reorient DNA fragments using high frequency pulsing was highlighted before59, while smaller DNA molecules reorient faster than larger DNA molecules, which partially can explain the differences in electrotransfer between LUC and GFP coding plasmids in our study. Lastly, dependent on the inner cell and outer medium conductivities the deformation of the cells during high frequency burst may take place60 and requires further study.
Also, in our work we have used the high frequency nanosecond range monopolar pulses with low influence of transient processes, which typically is a technological challenge. Since the electrophoretic transfer of molecules occurs only during the pulse, the prompt reversal of the field (due to transient process or deliberate bipolar pulse) may push them back out of the cell61,62. Therefore, in our work the electrophoretic transfer was also preserved during the burst and the cancelation effect was minimized. Application of symmetrical bipolar pulses or high frequency sine wave significantly reduces the efficiency of electroporation in the high PRF range62,63. Based on these assumptions, it could be presumed that the high frequency nanosecond range electrotransfection is possible using monopolar bursts only.
It should be noted that our pilot study is a proof of concept and optimization of pulsing parameters (i.e. number of pulses, amplitude, etc.) needs to be performed in the future. The applied MHz range protocols resulted in a predominantly reversible electroporation of CHO cells (>85% of cells survived using the highest intensity burst), therefore, the increase of the treatment energy and PEF amplitude seems possible. Additionally, we were able to observe different trends in transfection expression levels using GFP and LUC coding plasmids. The quantitative analysis revealed that cell transfection using LUC coding plasmid is higher during high frequency bursts compared to single pulses of equivalent energy (Refer to Fig. 4). Notably, it was not the case for GFP (Refer to Fig. 3) and the phenomenon is of particular interest since it may reveal possible differences in the electrotransfection mechanism. The plasmids also vary in size, which can attribute to the electrotransfer rate63,64,65,66, but the experimental data do not allow to form descriptive conclusions yet, thus, the study of this phenomenon is a matter of future works.
Nevertheless, the acquired data are of high relevance for development of new efficient nanosecond range transfection protocols, while optimization of the protocols and a detailed study of the observed frequency responses is required and will be covered in the future.
Material and Methods
Pulsed power setups
The experimental setup consisted of 3 kV, 100 ns−1 ms square wave high voltage pulse generator (VGTU, Vilnius, Lithuania)67 and a commercially available electroporation cuvette with 1 mm gap between electrodes (Biorad, Hercules, USA). The voltage (VC) that was applied to the cuvette was varied in the 0.14–1.8 kV range, corresponding to 1.4–18 kV/cm electric field.
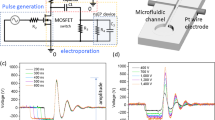
Three pulsing protocols were used: (1) 10 × 10–18 kV/cm 200 ns pulses of varied PRF (1 Hz–1 MHz); (2) 10–18 kV/cm single 2 µs square-wave pulse of identical energy to account for the effect of PRF and (3) 2 × 1.4 kV/cm × 100 µs (1 Hz) protocol that was used as a positive control for electrotransfection. An example of the high frequency burst waveform of the high frequency burst (10 × 200 ns, 1 MHz) is shown in Fig. 6.
As it can be seen in Fig. 6, the waveform features some overshoots and transient processes, which are due to the specific structure of the pulse forming circuit and the impedance of the load. However, due to very short duration of these oscillations and low voltage of the spikes (up to 300 V at 3 kV), the influence on the transmembrane potential and the results of this study is negligible.
Biological cells and electroporation
Chinese Hamster Ovary (CHO) cells were used as an electroporation object. Cells where grown in Dublecco’s modified Eagle’s medium (supplemented with FBS (10%), L-Glutamine and penicillin/streptomycin (1%) Sigma, St. Louis, MO, USA) at 37 °C in humidified 5% CO2 atmosphere in incubator. After trypsinization, CHO cells where suspended at concentration of 2 × 106 cells/ml in 270 mOsm and 7.1 pH electroporation medium. Electroporation medium was composed of sucrose (242 mM), Na2HPO4 (5.5 mM), NaH2PO4 (3 mM) and MgCl2 (1.7 mM). The measured specific conductivity of electroporation medium was 0.1 S/m at 25 °C. Cell suspension of 36 µl (2 × 106 cells/ml) in electroporation medium and 4 µl of pmaxGFP (Amaxa, Lonza, Switzerland) or pGL4.13-Luc2/SV40 (Promega, Madison, USA) plasmid at concentration (2 mg/ml) was mixed and put into electroporation cuvette with 1 mm gap between electrodes (Biorad, Hercules, USA). Then cell/plasmid suspension was treated with electric pulses. Afterwards 35 µl of the treaded suspension was taken and transferred into 1.5 ml Eppendorf tube for 10 min incubation at room temperature.
Flow cytometry and spectrophotometry
Growth medium at volume of 965 μl was added to 35 µl of cell suspension after incubation for 10 min post electroporation. Afterwards 900 μl of diluted cell suspension was transferred into a well of 24 well plate (TPP) and incubated for 24 hours. Then the cells where trypsinized, centrifuged at 200 × g and resuspended in 100 µl of PBS. Then the transfection (GFP positive cells) was evaluated using flow cytometer (BD Accuri C6, BD Biosciences, USA).
For the experiments with luciferase coding plasmid, 950 µl of growth medium was added 10 min post electroporation. Then 139 µl of treated cell suspension (~1 × 104 cells) were plated in the wells of microplate (Plastibrand, Wertheim, Germany) and allowed to grow in cell culture medium for 24 h. Luciferase protein activity was measured with the ONE-GloTM Luciferase Assay System (Promega, Madison, USA) using a luminometer (Tecan GENios Pro; MTX Lab Systems, Vienna, VA).
Viability assay
Clonogenic assay was performed for cell viability evaluation. 965 μl of growth medium was added to 35 µl of cell suspension after incubation for 10 min post electroporation. Afterwards, 100 μl of diluted cell suspension was taken and mixed with 900 μl of growth medium. Then 55 μl of the suspension was taken and placed in 40 mm diameter Petri dish containing 2 ml of growth medium. This way around 400 cells from each experimental point was grown for colony formation during the period of 6 days. Afterwards, cell colonies were fixed with 70% ethanol for 10 min and stained with crystal violet dye. For evaluation of results cell colonies where scanned with scanner (Canon CanoScan LiDE220). Colonies were counted using open source imaging software ImageJ (National Institutes of Health, USA).
Statistical analysis
One-way analysis of variance (ANOVA; P < 0.05) was used to compare different treatments. Tukey HSD multiple comparison test for evaluation of the difference was used when ANOVA indicated a statistically significant result (P < 0.05 was considered statistically significant). The data was post-processed in OriginPro software (OriginLab, Northhampton, MA, USA). All experiments have been performed at least in triplicate and the treatment efficiency was expressed as mean ± standard deviation.
Data Availability
Derived data supporting the findings of this study are available from the corresponding author V.N. on request.
References
Tsong, T. Y. Y. Electroporation of cell membranes. Biophys. J. 60, 297–306 (1991).
Ohmachi, M. et al. A modified single-cell electroporation method for molecule delivery into a motile protist, Euglena gracilis. J. Microbiol. Methods 130, 106–111 (2016).
Rems, L. & Miklavčič, D. Tutorial: Electroporation of cells in complex materials and tissue. J. Appl. Phys. 119 (2016).
Wagstaff, P. G. K. et al. Irreversible electroporation: state of the art. 2437–2446, https://doi.org/10.2147/OTT.S88086 (2016).
Miklavcic, D. & Davalos, R. V. Electrochemotherapy (ECT) and irreversible electroporation (IRE) -advanced techniques for treating deep-seated tumors based on electroporation. Biomed. Eng. Online 14, I1 (2015).
Daud, A. I. et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 26, 5896–5903 (2008).
Heller, L. C. & Heller, R. In Vivo Electroporation for Gene Therapy. Hum. Gene Ther. 17, 890–897 (2006).
Blagus, T. et al. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J. Control. Release 172, 862–871 (2013).
Becker, S., Zorec, B., Miklavčič, D. & Pavšelj, N. Transdermal transport pathway creation: Electroporation pulse order. Math. Biosci. 257, 60–68 (2014).
Lambricht, L. et al. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 5247, 295–310 (2015).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Spanggaard, I. et al. Gene therapy for patients with advanced solid tumors: a phase I study using gene electrotransfer to muscle with the integrin inhibitor plasmid AMEP. Acta Oncol. 56, 909–916 (2017).
Mahnič-Kalamiza, S., Vorobiev, E. & Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 247, 1279–1304 (2014).
Golberg, A. et al. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 9, 94 (2016).
Kotnik, T. et al. Electroporation-based applications in biotechnology. Trends Biotechnol. 33, 480–488 (2015).
Pucihar, G., Krmelj, J., Reberšek, M., Napotnik, T. B. & Miklavčič, D. Equivalent pulse parameters for electroporation. IEEE Trans. Biomed. Eng. 58, 3279–3288 (2011).
Phillips, M., Krishnan, H., Raju, N. & Rubinsky, B. Tissue Ablation by a Synergistic Combination of Electroporation and Electrolysis Delivered by a Single Pulse. Ann. Biomed. Eng. 44, 3144–3154 (2016).
Schoenbach, K. H. et al. Ion transport into cells exposed to monopolar and bipolar nanosecond pulses. Bioelectrochemistry 103, 44–51 (2015).
Brambach, B. C., Michels, A., Kettler, R. & Franzke, J. Charge dependent electroporation of Escherichia coli C600. J. Electroanal. Chem. 767, 108–113 (2016).
Haberl-Meglič, S., Levičnik, E., Luengo, E., Raso, J. & Miklavčič, D. The effect of temperature and bacterial growth phase on protein extraction by means of electroporation. Bioelectrochemistry 112, 77–82 (2016).
Chopinet, L. & Rols, M. P. Nanosecond electric pulses: A mini-review of the present state of the art. Bioelectrochemistry 103, 2–6 (2015).
Denzi, A. et al. Microdosimetric study for nanosecond pulsed electric fields on a cell circuit model with nucleus. J. Membr. Biol. 246 (2013).
Batista Napotnik, T., Reberšek, M., Vernier, P. T., Mali, B. & Miklavčič, D. Effects of high voltage nanosecond electric pulses on eucaryotic cells (in vitro): A systematic review. Bioelectrochemistry 110, 1–12 (2016).
Sano, M. B., Arena, C. B., DeWitt, M. R., Saur, D. & Davalos, R. V. In-vitro bipolar nano- and microsecond electro-pulse bursts for irreversible electroporation therapies. Bioelectrochemistry 100, 69–79 (2014).
Breton, M. & Mir, L. M. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics 33, 106–123 (2012).
Guionet, A. et al. Effect of nanosecond pulsed electric field on Escherichia coli in water: inactivation and impact on protein changes. J. Appl. Microbiol. 117, 721–728 (2014).
He, L., Xiao, D., Feng, J., Yao, C. & Tang, L. Induction of apoptosis of liver cancer cells by nanosecond pulsed electric fields (nsPEFs). Med. Oncol. 34 (2017).
Semenov, I., Xiao, S. & Pakhomov, A. G. Primary pathways of intracellular Ca2+ mobilization by nanosecond pulsed electric field. Biochim. Biophys. Acta - Biomembr. 1828, 981–989 (2013).
Schoenbach, K. H. et al. Bioelectric effects of intense nanosecond pulses. IEEE Trans. Dielectr. Electr. Insul. 14, 1088–1107 (2007).
Joshi, R. P. & Schoenbach, K. H. Bioelectric effects of intense ultrashort pulses. Crit. Rev. Biomed. Eng. 38, 255–304 (2010).
Chafai, D. E., Mehle, A., Tilmatine, A., Maouche, B. & Miklavčič, D. Assessment of the electrochemical effects of pulsed electric fields in a biological cell suspension. Bioelectrochemistry 106, 249–257 (2015).
Valdez, C., Jirjis, M. B., Roth, C. C., Barnes, R. A. & Ibey, B. L. Nanosecond electric pulses modulate skeletal muscle calcium dynamics and contraction. Proc. SPIE 10066, 100660X (2017).
Potter, H. & Heller, R. Transfection by electroporation. Curr. Protoc. Immunol. 2017, 10.15.1–10.15.9 (2017).
Chu, G., Hayakawa, H. & Berg, P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 15, 1311–1326 (1987).
Potter, H. Transfection by electroporation-1. Curr. Protoc. Mol. Biol. 9.3.1–9.3.6, https://doi.org/10.1002/0471142727.mb0903s62 (1996).
Kubiniec, R. T., Liang, H. & Hui, S. W. Effects of pulse length and pulse strength on transfection by electroporation. Biotechniques 8, 16–20 (1990).
Covello, G., Siva, K., Adami, V. & Denti, M. A. An electroporation protocol for efficient DNA transfection in PC12 cells. Cytotechnology 66, 543–553 (2014).
Guo, S. et al. Gene electrotransfer enhanced by nanosecond pulsed electric fields. Mol. Ther. - Methods Clin. Dev. 1, 14043 (2014).
Klenchin, V. A., Sukharev, S. I., Serov, S. M. & Chernomordik, L. V. & Chizmadzhev YuA. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys. J. 60, 804–811 (1991).
Escoffre, J. M. et al. What is (Still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Molecular Biotechnology 41, 286–295 (2009).
Pucihar, G., Mir, L. M. & Miklavčič, D. The effect of pulse repetition frequency on the uptake into electropermeabilized cells in vitro with possible applications in electrochemotherapy. Bioelectrochemistry 57, 167–172 (2002).
Lamberti, P., Romeo, S., Sannino, A., Zeni, L. & Zeni, O. The Role of Pulse Repetition Rate in nsPEF-Induced Electroporation: A Biological and Numerical Investigation. IEEE Trans. Biomed. Eng. 62, 2234–2243 (2015).
Steelman, Z. A., Tolstykh, G. P., Beier, H. T. & Ibey, B. L. Cellular response to high pulse repetition rate nanosecond pulses varies with fluorescent marker identity. Biochem. Biophys. Res. Commun. 478, 1261–1267 (2016).
Muratori, C., Pakhomov, A. G., Xiao, S. & Pakhomova, O. N. Electrosensitization assists cell ablation by nanosecond pulsed electric field in 3D cultures. Sci Rep 6, 23225 (2016).
Arena, C. B. et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 10, 102 (2011).
Siddiqui, I. A. et al. Induction of rapid, reproducible hepatic ablations using next-generation, high frequency irreversible electroporation (H-FIRE) in vivo. HPB 18, 726–734 (2016).
Kotnik, T., Kotnik, T., Miklavcic, D. & Miklavcic, D. Second-order model of membrane electric field induced by alternating external electric fields. IEEE Trans. Biomed. Eng. 47, 1074–81 (2000).
Silve, A., Guimerà Brunet, A., Al-Sakere, B., Ivorra, A. & Mir, L. M. Comparison of the effects of the repetition rate between microsecond and nanosecond pulses: Electropermeabilization-induced electro-desensitization? Biochim. Biophys. Acta - Gen. Subj. 1840, 2139–2151 (2014).
Novickij, V., Ruzgys, P., Grainys, A. & Šatkauskas, S. High frequency electroporation efficiency is under control of membrane capacitive charging and voltage potential relaxation. Bioelectrochemistry 119, 92–97 (2018).
Semenov, I., Casciola, M., Ibey, B. L., Xiao, S. & Pakhomov, A. G. Electropermeabilization of cells by closely spaced paired nanosecond-range pulses. Bioelectrochemistry 121, 135–141 (2018).
Zu, Y., Huang, S., Liao, W. C., Lu, Y. & Wang, S. Gold nanoparticles enhanced electroporation for mammalian cell transfection. J. Biomed. Nanotechnol. 10, 982–992 (2014).
Ruzgys, P. & Šatkauskas, S. Cell electrotransfection and viability dependence on electrotransfection and viability dependence on electoporation medium. Biologija 62, 98–104 (2016).
Weaver, J. C., Smith, K. C., Esser, A. T., Son, R. S. & Gowrishankar, T. R. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry 87, 236–243 (2012).
Chopinet, L. et al. Nanosecond electric pulse effects on gene expression. J. Membr. Biol. 246, 851–859 (2013).
Romeo, S., Wu, Y. H., Levine, Z. A., Gundersen, M. A. & Vernier, P. T. Water influx and cell swelling after nanosecond electropermeabilization. Biochim. Biophys. Acta - Biomembr. 1828, 1715–1722 (2013).
Bakewell, D., Vergara-Irigaray, N. & Holmes, D. Dielectrophoresis of Biomolecules. JSM Nanotechnol. Nanomedicine 1, 1–14 (2013).
Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 4 (2010).
Mi, Y., Xu, J., Tang, X., Yao, C. & Li, C. Electroporation simulation of a multicellular system exposed to high-frequency 500 ns pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 24, 3985–3994 (2017).
Ghourchian, H. & Elyasvandi, H. Capacitively-induced pulsed-field gel electrophoresis: a novel method for DNA separation. Med. Eng. Phys. 27, 723–727 (2005).
Dimova, R. et al. Giant vesicles in electric fields. Soft Matter 3, 817 (2007).
Pakhomov, A. G. et al. Cancellation of cellular responses to nanoelectroporation by reversing the stimulus polarity. Cell. Mol. Life Sci. 71, 4431–4441 (2014).
Valdez, C. M. et al. Asymmetrical bipolar nanosecond electric pulse widths modify bipolar cancellation. Sci. Rep. 7 (2017).
García-Sánchez, T., Merla, C., Fontaine, J., Muscat, A. & Mir, L. M. Sine wave electropermeabilization reveals the frequency-dependent response of the biological membranes. Biochim. Biophys. Acta – Biomembr, https://doi.org/10.1016/j.bbamem.2018.01.018 (2018).
Lesueur, L. L., Mir, L. M. & André, F. M. Overcoming the Specific Toxicity of Large Plasmids Electrotransfer in Primary Cells In Vitro. Mol. Ther. - Nucleic Acids 5, e291 (2016).
Liew, A. et al. Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Hum. Gene Ther. Methods 24, 289–97 (2013).
Čepurniene, K., Ruzgys, P., Treinys, R., Šatkauskiene, I. & Šatkauskas, S. Influence of plasmid concentration on DNA electrotransfer in vitro using high-voltage and low-voltage pulses. J. Membr. Biol. 236, 81–85 (2010).
Novickij, V. et al. High-frequency submicrosecond electroporator. Biotechnol. Biotechnol. Equip. 30 (2016).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
V.N., P.R., S.S. conceived the experiments and methodology. P.R., V.N. have conducted the experiments, processed and analysed the results. V.N. and J.N. have developed pulsed electric field system and adapted it for the high frequency experiments. V.N., P.R., S.S. interpreted the results. V.N., P.R., S.S., J.N. have wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruzgys, P., Novickij, V., Novickij, J. et al. Nanosecond range electric pulse application as a non-viral gene delivery method: proof of concept. Sci Rep 8, 15502 (2018). https://doi.org/10.1038/s41598-018-33912-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33912-y
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.