Abstract
A recent study has pointed out to CD32a as a potential biomarker of HIV-persistent CD4 cells. We have characterized the level and phenotype of CD32+ cells contained in different subsets of CD4 T-cells and its potential correlation with level of total HIV-DNA in thirty HIV patients (10 typical progressors naïve for cART, 10 cART-suppressed patients, and 10 elite controllers). Total HIV-DNA was quantified in different subsets of CD4 T-cells: Trm and pTfh cells. Level and immunephenotype of CD32+ cells were analyzed in these same subsets by flow cytometry. CD32 expression in Trm and pTfh subsets was similar in the different groups, and there was no significant correlation between the level of total HIV-DNA and the level of CD32 expression in these subsets. However, total HIV-DNA level was correlated with expression of CD127 (rho = −0.46, p = 0.043) and of CCR6 (rho = −0.418, p = 0.027) on CD32+ cells. Our results do not support CD32 as a biomarker of total HIV-DNA content. However, analyzing the expression of certain markers by CD32+ cells could improve the utility of this marker in the clinical setting, prompting the necessity of further studies to both validate our results and to explore the potential utility of certain markers expressed by CD32+ cells.
Similar content being viewed by others
Introduction
The existence of HIV reservoirs is the main barrier to HIV eradication1. At cellular level HIV latency is mainly found in CD4 T cells with a resting memory phenotype2,3,4, especially in certain subsets such as peripheral follicular T helper cells5. Due to the long half life of CD4 subsets harbouring proviral HIV-DNA6 as well to additional mechanisms of reservoir maintenance as homeostatic proliferation7 or clonal expansion8 of latently infected cells, the kinetics of reservoir decline is so slow that purging it with antiretroviral therapy alone is not feasible6. Moreover, the different approaches proposed so far have failed in significantly diminishing the size of the HIV reservoir, likely due to different reasons9,10.
One of such reasons lies in the difficulty of precisely measuring the HIV reservoir size11,12, what has hindered the ability to compare HIV reservoir size in different groups of patients and to estimate the effectiveness of therapeutic approaches aimed to diminish it. Current assays are based either on detection of proviral HIV-DNA content in different types of cells such as peripheral blood mononuclear cells (PBMCs), CD4 cells or subsets of CD4 cells, or on the quantification of virus growth in culture (quantitative viral outgrowth assay, qVOA) that is considered the gold-standard13. An inherent drawback of these assays is that since they do not detect HIV at single-cell level, they are not able to identify the phenotype of every single cell carrying latent HIV, what is crucial for our understanding of cell types involved in the maintenance of the reservoir. Although the majority of cellular reservoir resides in CD4 cells with resting memory phenotype, the great majority of cells with this phenotype do not carry HIV. Thus, identifying a cell marker specific for CD4 cells carrying latent HIV would be of great interest not only for the understanding of cellular reservoirs but also as an easily scalable high-throughput assay to precisely measure the in vivo frequency of latently infected cells, that could be implemented in clinical trials aimed to purge the HIV reservoir14.
In this regard, a very recent paper has pointed to CD32a as a potential biomarker of latently infected CD4 cells15. FcγRII (CD32) is a low-affinity cell surface receptor of the immunoglobulin G (IgG) Fc fragment involved in immune response regulation. In human cells three different isoforms have been defined, two activating receptors (CD32a and CD32c), and one inhibitory receptor (CD32b)16. CD32 marker is mainly expressed on B-cells, monocytes, granulocytes, platelets and endothelial cells, whereas the expression of this receptor on CD4 T cells is controversial and it seems to be associated to CD4 T cell activation17,18,19. Hovewer, using an in vitro infection system, the authors found that CD32a was induced selectively in resting CD4 cells latently infected with HIV but not on those cells actively replicating HIV. Moreover, the content of proviral HIV-DNA was several hundred-fold higher in purified CD4+CD32a+ compared to CD4+CD32a− cells from patients under antiretroviral therapy. From these results the authors conclude that CD32a is a good potential biomarker of persistently infected cells15.
To test this hypothesis, in the present study we have characterized the levels and phenotype of CD32+ cells contained in different subsets of CD4 T-cells, and its potential correlation with total HIV-DNA content in two groups of HIV patients with HIV replication control (spontaneously or through cART) and in a group of progressor HIV patients with uncontrolled HIV replication.
Results
Characteristics of study population
Table 1 shows the main characteristics of HIV patients enrolled in this study. Briefly, elite controllers (EC), patients with undetectable HIV plasma viremia (pVL) in the absence of antiretroviral therapy (cART) (n = 10), and treated patients (TX) (n = 10), cART-suppressed patients maintaining undetectable pVL, were not significantly different in terms of age, CD4 counts and time since HIV diagnosis (years between the date of HIV diagnosis and the sample collection), although proportion of males was higher in TX compared to EC group. Median length of EC status in EC group was 6 [3–12] years and median length of cART in TX group was 12 [9–16] years. Plasma HIV load and CD4 counts in TP group (typical progressor patients naïve for cART and with high levels of pVL, n = 10) were 4.79[4.46–4.91] log HIV-RNA copies/mL and 599 [518–832] cells/uL respectively. Levels of HIV-DNA in resting memory CD4 T (Trm) and peripheral T follicular helper (pTfh) cells were lowest in EC patients (381 [74–1002] and 88 [73–291] copies/million cells in Trm and pTfh respectively) and highest in TP patients (6768 [3674–12673] and 7737 [4921–9044] copies/million cells in Trm and pTfh respectively), with an intermediate level in TX group (1197[619–1623] and 723 [393–1199] copies/million cells in Trm and pTfh respectively). Moreover, when comparing only EC and TX patients, levels of HIV-DNA in pTfh cells were significantly lower in EC (p = 0.025) and a similar trend was found for Trm cells although the difference was not statistically significant (p = 0.063).
Levels of CD32 expression on different CD4 subsets
Levels of CD32 expression in total CD4, Trm and pTfh cells were analyzed by multiparameter flow cytometry. Supplementary Fig. S1 shows a representative example of CD32 staining in the different groups of subjects analyzed. In all subjects analyzed the population of CD32+ cells consisted mainly of CD32+dim cells and to a much lesser extent of CD32+bright cells. Interestingly, the majority of CD32+bright expressed the activation marker HLADR, except in Trm cells that by definition where HLADR-negative (Supplementary Fig. S1).
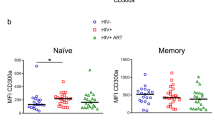
In the global comparison (Kruskal-Wallis test), levels of total CD32+ cells (dim + bright) in the different CD4 T cells subsets analyzed were not significantly different between the different groups of individuals (Fig. 1, left graph). Overall, levels of total CD32+ cells tended to be higher in TP compared to TX, EC and HC groups in total CD4 T cells (median values: 10.1%, 6.6%, 9.2% and 9.2% respectively) and pTfh cells (median values: 4.6%, 3.3%, 2.7% and 3.5% respectively), although differences were not statistically significant. Similar results were obtained when comparing EC versus TX groups. In Trm cells, EC showed the highest CD32 expression compared to TX, TP and HC groups (median values: 4.4%, 1.6%, 3.7% and 1.9% respectively). Levels of CD32+bright cells were much lower compared to total CD32+ cells, especially in the Trm subset of CD4 cells (Fig. 1, right graph) in agreement with the finding that most CD32+bright expressed the activation marker HLADR (Supplementary Fig. S1). Levels of CD32+bright in total CD4 T cells and in Trm cells were similar in the different groups of subjects analyzed (median values in total CD4 T cells: 0.079%, 0.075%, 0.073% and 0.048%; median values in Trm cells: 0.011%, 0.001%, 0.003% and 0.004% for EC, TX, TP and HC groups respectively), but in pTfh subset, levels of CD32+bright cells were significantly higher in uninfected subjects (0.47%) compared to EC, TX and TP groups (0.21%, 0.12% and 0.17% respectively, p = 0.009) (Fig. 1, right graph).
Box-plots showing the levels of CD32+ (dim + bright) (left graph) and of CD32+bright (right graph) cells in different subsets of CD4+ T-cells from uninfected subjects (green boxes), elite controller (blue boxes), cART-suppressed (red boxes) and cART naive (yellow boxes) HIV patients. P-values for the global comparison (Kruskall-Wallis test) are shown. Dotted line in the right graph represents the threshold for detection of CD32+bright cells (0.01% of cells), established using the fluorescence minus one (FMO) control. ns: non significant (p > 0.05).
Intragroup comparisons were performed to ascertain differences in CD32 expression by different subsets of CD4 T-cells (Supplementary Fig. S2). Regarding levels of CD32+ cells, a similar profile was observed in all study groups, with higher levels of CD32 expression in total CD4 cells compared to the rest of CD4 subsets, being the differences statistically significant in all groups of subjects except in TP patients. Interestingly, levels of CD32+bright cells showed a different profile with lower levels in total CD4 cells compared to pTfh subset, and with the lowest levels observed in Trm subset.
Phenotype of CD32+ cells
Several aspects of CD32+ cells were assessed by multiparameter flow cytometry. Supplementary Fig. S3 shows heat map diagrams showing the differences observed in the level of expression of the different markers analyzed, comparing the different groups of HIV patients with the group of uninfected subjects. CD32+ cells from total CD4 cells of TP patients exhibited several alterations, mainly increased levels of senescence (CD28−CD57+ subset) and activation (HLADR+CD127−subset), increased expression of PD1 marker and decreased expression of CD127 and CXCR3 markers. A similar profile of alterations was observed in CD32+ cells from Trm cells of TP patients. CD32+ from pTfh of TP patients also presented some immune alterations though to a lesser extent. Many of these phenotypic alterations were statistically significant compared to uninfected subjects (Supplementary Fig. S4).
CD32+ cells from HIV patients with suppressed viremia (TX and EC groups) presented lesser immune alterations when compared to uninfected subjects. Overall, CD32+ cells from EC patients presented lower number of alterations than CD32+ cells from TX patients and the profile of alterations was not the same for TX and EC groups with some differences between them. In TX patients, CD32+ cells from total CD4 and from Trm cells presented increased levels of senescence and decreased expression of CD127 and of CCR6, whereas in EC patients the only alterations were an increased expression of PD1 and a decreased expression of CD127 and of CD28. Very few alterations were observed in CD32+ cells from pTfh subset in both TX and EC patients. Some of these alterations were statistically significant compared to uninfected subjects (Supplementary Fig. S4). Lastly, the phenotypic profile of CD32+ cells was compared between EC and TX groups of patients. There were some differences, although most of them were not statistically significant, likely due to the small sample size (Fig. 2). Overall, CD32+ cells from total CD4 and from Trm cells of EC patients expressed higher levels of CCR6 marker, whereas an increased expression of HLADR and a diminished expression of CD28 was observed in CD32+ cells from pTfh cells of EC patients compared to those of TX patients (Fig. 2). Further studies with larger cohorts of patients are needed to corroborate these findings.
The differential expression of several markers in the CD32+ cells from different groups of patients could be associated to the different phenotype of HIV patients included in the study, some with high plasma viral load and others with undetectable viremia, and thus could equally affect both CD32+ and CD32-negative subsets. To address this we analyzed the phenotype of CD32-negative cells and compared with the phenotype of CD32+ cells. Heat map diagrams showing the ratio of the expression level of the different markers between CD32+ and CD32-negative cells (Supplementary Fig. S5) demonstrate clear differences in the phenotypic profile between these two subsets of cells. Independently of the group of subjects (EC, TX, TP or HC), CD32+ cells were more senescent (CD28−CD57+), more exhausted (PD1+) and more activated (HLADR+ and/or CD38+) than CD32-negative cells. In contrast CD32+ cells showed a lower expression of CD28 and CD127 compared to CD32-negative cells. These differences were observed in total CD4 T cells, in Trm and in pTfh cells.
Correlation between CD32 expression and total HIV-DNA content
In the whole population of HIV patients (EC, TX and TP groups together), we explored the potential correlations between CD32 expression in different subsets of CD4 cells (total CD4 cells, Trm cells and pTfh cells) and the total HIV-DNA levels in Trm and pTfh subsets of CD4 cells. There were no significant correlations (Supplementary Table S6).
However there were several significant correlations between the phenotype of CD32+ cells and total HIV-DNA level in the different subsets of CD4 cells (Supplementary Table S6). Expression of CD127 by CD32+ cells from total CD4 T cells was correlated with HIV-DNA levels in Trm cells; on the other hand, significant correlation between CD127 and CD57 expression on CD32+ cells from total CD4 T cells and total HIV-DNA in pTfh cells was observed. A similar profile was found for the phenotype of CD32+ cells from Trm cells, with CD127 and CD57 markers being the ones that showed the highest correlations with HIV-DNA levels in Trm cells (Supplementary Table S6).
Since the expression levels of these phenotypic markers varied across the different groups of HIV patients (TP with high level of plasma viremia versus EC and TX with undetectable plasma viremia), a partial correlation coefficient was calculated after adjusting by this factor. After doing this, the majority of significant correlations disappeared (data not shown). The only correlations that remained significant after adjusting by type of patient were the CCR6 expression on CD32 cells from total CD4 cells with HIV-DNA level in Trm cells and the CD127 expression on CD32 cells from total CD4 cells with HIV-DNA level in pTfh cells (Fig. 3). Interestingly, CCR6 expression on CD32-negative CD4 T cells was also significantly correlated with total HIV-DNA level in Trm cells (data not shown), suggesting that CCR6 marker is associated to HIV-DNA content independently of CD32 expression and supporting a more relevant role for CCR6 than for CD32 in the HIV-DNA content of the Trm cells. In contrast to CCR6, no correlation between CD127 expression on CD32-negative CD4 T-cells and total HIV-DNA was observed (data not shown). Thus, the expression of CD127 seems to be associated to total HIV-DNA only in CD32+ cells.
Scatter-plots showing the correlations between phenotype of CD32+ cells and levels of total HIV-DNA in different subsets of CD4 cells, in the whole population of HIV patients (upper row) and in patients with undetectable plasma viremia (lower row). Partial correlation coefficients and p-values after adjusting by type of patient are shown inside the plots.
Interestingly, the same analysis only in the groups of patients with undetectable plasma HIV viremia (EC and TX groups) revealed that only the expression of CCR6 marker on CD32+ cells was correlated with levels of HIV-DNA in Trm cells and this correlation remained significant after adjusting by type of patient (Fig. 3). The same was observed for CCR6 in CD32-negative CD4 T-cells (data not shown).
Discussion
The present study was designed to test the suitability of CD32 molecule as a marker of cells carrying latent HIV and thus as a surrogate measure of HIV-DNA content. For this purpose, we measured levels of CD32 in total CD4 cells and in subsets of CD4 cells carrying persistent HIV in different groups of HIV-infected patients, and checked the potential correlations between CD32 expression and levels of total HIV-DNA in different subsets of CD4 cells. The main findings of our study are: a) There were no significant differences in the level of CD32 expression by CD4 cells between different groups of HIV patients stratified according to the level of HIV viral suppression; b) Levels of CD32 expression did not correlate with levels of total HIV-DNA; c) Expression of certain markers by CD32 cells did correlate with the levels of total HIV-DNA; d) CD32+ cells present a differential phenotype compared to CD32- cells, highlighting the increased expression of HLA-DR what could support that this CD32+ subpopulation is involved in productive HIV infection more than in latent infection.
CD32 has been recently proposed as a potential marker of cells carrying latent HIV15 and this has raised great expectations since an easily measurable marker of HIV latency would be an invaluable tool in the fight against HIV reservoirs. Briefly, the findings in the study by Descours et al. pointing to CD32 as a potential marker of HIV reservoir were: selective induction of CD32 after infection of resting CD4 cells; several hundred-fold enrichment of HIV-DNA in CD4 cells expressing CD32, especially in the very rare CD32bright population; several thousand-fold enrichment of inducible replication-competent virus in CD32+ cells. However, as the authors point out, not all HIV-DNA was present in CD4 cells expressing CD32 and not all CD32+ cells carried HIV-DNA.
To test the potential utility of CD32 as marker of cells carrying latent HIV, we first compared the levels of CD32+ cells in different subsets of CD4 cells harbouring proviral HIV-DNA and in different groups of HIV patients with different levels of HIV control and of total HIV-DNA levels, under the assumption that CD32 expression will parallel the levels of total HIV-DNA. However, our results demonstrate similar levels of CD32 expression in the different groups of patients analyzed, in spite of the different levels of total HIV-DNA observed in these groups of patients, in agreement with very recently published studies20,21. This finding was independent of the CD4 cell subset analyzed (total CD4, Trm or pTfh subsets) and is in agreement with a very recent report showing only very slight differences in the levels of CD32 expression when comparing HIV patients (either viremic or aviremic) and uninfected subjects22. Moreover, in our study we were able to clearly differentiate two populations of CD32+ cells in the majority of individuals analyzed: a dim population comprising the great majority of CD32+ cells and a very scarce bright population, in agreement with the study of Descours et al.15. Interestingly the CD32+bright population presented the highest enrichment in HIV-DNA in the study of Descours et al.15. Thus we tested if the levels of CD32+bright cells differed among HIV patients according to levels of total HIV-DNA. As for total CD32+ cells, we did not find significant differences between the different groups of patients. Lastly, we compared levels of CD32 in different subsets of CD4 cells and found that CD32 was not preferentially expressed on those subsets where the majority of latently infected cells reside (Trm and pTfh cells), being the expression on total CD4 cells higher than in Trm or pTfh subsets of CD4 cells, suggesting that other subsets of CD4 cells such as naïve cells and/or activated cells express even higher levels of CD32 than CD4 cells with memory phenotype, what is in agreement with a very recent study showing a positive correlation between CD32 expression and activation levels of T cells22. Taken together, our results regarding the levels of CD32+ cells do not support CD32 as a surrogate marker of latently HIV infected CD4 cells.
Next, we analyzed the potential correlations between the level of CD32 expression and the levels of total HIV-DNA in different subsets of CD4 cells and found no significant correlations, in agreement with recent reports20,21,22. Only a slight trend of correlation between CD32 expression on pTfh cells and total HIV-DNA levels in this subset was found, that disappeared after adjusting by the type of patient (with or without detectable HIV viremia). This lack of correlation is in accordance with the results regarding the levels of CD32 and reinforces the lack of support to CD32 as a surrogate marker of latently HIV-infected cells in HIV patients with different degrees of viral replication.
We also evaluated the expression of several phenotypic markers by CD32+ cells in order to check for the existence of phenotypic differences between the different groups of HIV patients that could be associated with the levels of total HIV-DNA. CD32+ cells from TP patients presented several phenotypic alterations as compared to cells from uninfected subjects. Overall, CD32+ cells from HIV patients with uncontrolled viral replication (TP) presented a more differentiated, activated and senescent phenotype as well as diminished expression of chemokine receptors CXCR3 and CXCR5. Some but not all of these alterations were normalized in patients with undetectable plasma viremia (TX and EC) and interestingly there were some differences in the phenotypic profile of CD32+ cells between TX and EC patients in spite of similar levels of HIV suppression. These phenotypic differences between groups of patients were specific for CD32+ cells, since the majority of these differences did not exist when analyzing the phenotype of CD32-negative cells, suggesting that the disease phenotype (uncontrolled versus controlled viral replication) does not equally affect the phenotype of CD32+ and CD32-negative subsets of T cells. Moreover, we found some interesting phenotypic differences between CD32+ and CD32-negative cells suggesting that, compared to CD32-negative, CD32+ cells present a characteristic phenotype that could favor productive HIV infection in this cell subpopulation as has been recently suggested23.
Of the many phenotypic markers analyzed on CD32+ cells, CD127 and CCR6 were among the markers showing the highest differences between groups of patients. Moreover, CD127 and CCR6 were the only markers that showed a significant correlation with levels of total HIV-DNA after adjusting by patient´s group. Both correlations were inverse and thus lower levels of CD127 or CCR6 expression on CD32+ cells were associated with higher levels of total HIV-DNA, suggesting a role for these markers in HIV reservoir. CD127 is the receptor for IL7, a cytokine with a pivotal role in T-cell homeostasis24 and in CD4 memory cells survival and expansion25. The role of IL7 in HIV persistence is controversial and previous studies have reported both a latency-reversing effect26,27, but also priming for HIV-reservoir maintenance due to the effect of IL7 on survival and expansion of infected CD4 cells28,29,30. The inverse association we found between CD127 expression by CD32+ cells and total HIV-DNA content suggest that cells not expressing CD127, and thus not able to respond to IL7, could have a higher contribution to HIV reservoir. We did not measure total HIV-DNA in cells according to CD127 expression and thus we couldn´t confirm this hypothesis. In contrast to our hypothesis, a previous study reported that the majority of HIV-DNA in CD4 T cells is present in CD127+ cells31, although they did not compare HIV-DNA levels between CD127+ with CD127- cells and thus the question is still open.
CCR6 expression was inversely correlated to levels of total HIV-DNA in the whole population of HIV patients. Interestingly this correlation was also observed in HIV patients with undetectable HIV plasma viremia (EC and TX groups together), suggesting a role for this marker in HIV reservoir in patients with complete suppression of viral replication. CCR6 plays an important role in redirecting T cells to gut-associated lymphoid tissue (GALT)32 and is considered a marker of Th17 cells33. Different findings support a multifaceted role for CCR6 in HIV pathogenesis, contributing to both HIV dissemination and to immunity against HIV34. Regarding HIV reservoirs, two recent studies have shown that CCR6+ CXCR3+35 or CCR6+36 cells are enriched in HIV-DNA, supporting a role for CCR6 in the maintenance of HIV persistence37. The inverse association we found between CCR6 and total HIV-DNA seems counterintuitive with these studies. However, other mechanisms not related to differential content of HIV-DNA in CCR6+ versus CCR6- could explain the inverse association. For example, longer survival and proliferation of CCR6+ cells38 could dilute the contribution of CCR6-expressing cells to HIV-DNA and this would explain the inverse correlation. Also, given that CCR6 is a marker of Th17 cells, the inverse correlation could simply reflect the fact that those patients with lower total HIV-DNA (EC) are also able to maintain a more preserved mucosal immunity as indicated by a more preserved Th17 population39. Lastly, the relatively low correlation coefficient may suggest a spurious correlation biased by other factors not controlled in our study.
There are some controversial issues in our study. First, Descours et al. described the selective induction of CD32a after HIV infection of resting CD4 T cells; however, there is no an available antibody specific for CD32a. The only antibody designed to detect the CD32a isoform has shown a very low resolution40. The anti-CD32 antibody clone FUN-2 used in our study and in the study by Descours et al.15 does not distinguish between different CD32 isoforms (CD32a, CD32b and CD32c)20,23,41 and thus, we cannot distinguishing them by flow cytometry. Therefore, a particular isoform of CD32 cannot be associated with enrichment in HIV-DNA. Second, the measurement of total HIV-DNA and the absence of virus growth assays to evaluate replication-competent virus in our study could raise concerns; however, several authors recently noted the relevance of measuring total HIV-DNA as a marker of viral reservoir dynamics with clinical implications42,43. The authors suggested that both integrated and total HIV-DNA provides an inducible and functional reservoir and predicts ex vivo viral outgrowth43,44. Additionally, it has been highlighted that the establishment of HIV latency and virus production from unintegrated genomes follows direct infection of resting CD4+ T-cells45. Moreover, the use of ddPCR to quantify the total HIV-DNA levels has been associated with greater precision and reproducibility to quantify low HIV-DNA levels compared to other techniques measuring integrated HIV-DNA46.
In summary, ours is the first study performing an in-deep characterization of CD32+ cells in HIV patients with different levels of HIV suppression and cell-associated HIV-DNA with the aim to ascertain the suitability of CD32 as a surrogate marker of total HIV-DNA levels. Overall the results of our study do not support the use of CD32 as marker of cells carrying latent HIV and sheds light on the phenotype of CD32+ cells, highlighting important phenotypic differences potentially associated with HIV persistence and prompting the necessity of further studies with larger cohort of HIV patients to both validate our results and to explore the potential utility of certain markers expressed by CD32+ cells that could potentially improve the utility of this marker in the clinical setting.
Materials and Methods
Study population
This is a cross-sectional study including patients with chronic HIV-1 infection distributed in three different groups: 10 elite controller (EC group) patients with undetectable HIV plasma viremia (pVL) in the absence of antiretroviral therapy (cART); 10 cART-suppressed patients maintaining undetectable pVL (TX group); and 10 typical progressor patients naïve for cART and with high levels of pVL (TP group). Patients were recruited from four different hospitals in Madrid, Spain, during a period of 12 months. Inclusion criteria for HIV patients have been described elsewhere47. A group of 10 uninfected subjects were also included as control group (HC group). The study protocol was approved by the Ethical review board of the Instituto de Investigación Sanitaria-Fundación Jiménez Díaz, Madrid, Spain in accordance with the declaration of Helsinki. All individuals participating in the study signed an informed consent form.
Purification of resting memory CD4 T (Trm) and peripheral follicular helper T (pTfh) cells
Starting form fresh peripheral blood mononuclear cells (PBMCs), two different subsets of CD4 T-cells were purified: resting memory CD4 T (Trm) cells (defined as CD4+CD45RO+CD25−CD69−HLADR−) and peripheral T follicular helper (pTfh) cells (defined as CD4+ CD45RO+ CXCR5+). For this purpose, immunomagnetic separation was employed as has been described elsewhere47. Purity of each CD4 subset was tested by flow cytometry using a Sony Spectral flow cytometer. Median [Interquartile range] purity for the Trm cells in the whole population of subjects analyzed was 95%[93–97%] and for the pTfh cells was 92%[90–93%].
Measurement of cell-associated HIV DNA
Proviral HIV DNA was quantified in Trm and pTfh cells from each HIV patient. Total cellular DNA was extracted using a QiAmp DNA Mini-Kit following manufacturer instructions. Content of HIV DNA (expressed as copies of HIV-DNA per million cells) was quantified using an ultrasensitive digital droplet PCR (ddPCR) as has been already described47.
Immunophenotypic analysis
Levels of CD32 expression in Trm, pTfh, and total CD4 cells, as well as phenotype of CD32+ cells, were analyzed by multiparametric flow cytometry. For this purpose, three different panels of monoclonal antibodies were designed to stain PBMCs, Trm and pTfh cells respectively. Each panel included 11 different monoclonal antibodies plus a cell viability dye. Using these staining panels, different characteristics of CD32+ cells were analyzed, including senescence, apoptosis, activation, exhaustion, homing potential, response to homeostatic cytokines (CD127 marker, receptor of IL7 cytokine involved in B and T cell development), as well as expression of chemokine receptors CXCR3, CCR6 and CXCR5. A list of monoclonal antibodies included in each staining panel is shown in Supplementary Table S7 and a detailed description of staining and analysis protocol in Supplementary Methods.
Statistical analysis
The main characteristics of the study groups are expressed as median [interquartile range]. Differences between the different groups of subjects for the different parameters analyzed were tested using non-parametric tests (Kruskall-Wallis, Mann-Whitney U-test and Wilcoxon signed-rank as appropriate). Spearman´s Rho correlation coefficient was used to test the existence of correlations between continuous variables and Chi-square test for the association between categorical variables. All statistical analyses were performed using SPSS software version 15 (SPSS Inc., Chicago, IL, USA). Heat map diagrams were realized using GraphPad Prism version 7 (GraphPad Software, CA, USA). All p-values were two-tailed and were considered significant when lower than 0.05.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
References
Barton, K., Winckelmann, A. & Palmer, S. HIV-1 Reservoirs during Suppressive Therapy. Trends Microbiol 24, 345–355 (2016).
Chun, T. W. et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 94, 13193–13197 (1997).
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Soriano-Sarabia, N. et al. Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol 88, 14070–14077 (2014).
Pallikkuth, S. et al. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T-cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J Virol 90, 2718–2728 (2015).
Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9, 727–728 (2003).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15, 893–900 (2009).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014).
Rasmussen, T. A. & Lewin, S. R. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr. Opin. HIV AIDS 11, 394–401 (2016).
Archin, N. M. & Margolis, D. M. Emerging strategies to deplete the HIV reservoir. Curr Opin Infec Dis 27, 29–35 (2014).
Massanella, M. & Richman, D. Measuring the latent reservoir in vivo. J Clin Invest 126, 464–472 (2016).
Bruner, K. M., Hosmane, N. N. & Siliciano, R. F. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 23, 192–203 (2015).
Eriksson, S. et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLOS Pathogens 9, e1003174 (2013).
Iglesias-Ussel, M. et al. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ cells in virally suppressed subjects. J Virol 87, 9148–9158 (2013).
Descours, B. et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 543, 564–567 (2017).
Nimmerjahn, F. & Ravetch, J. V. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8(1), 34–47 (2008).
Engelhardt, W., Matzke, J. & Schmidt, R. E. Activation-dependent expression of low affinity IgG receptors Fc gamma RII(CD32) and Fc gamma RIII(CD16) in subpopulations of human T lymphocytes. Immunobiology 192(5), 297–320 (1995).
McLain, L. & Dimmock, N. J. A human CD4+ T-cell line expresses functional CD64 (Fc gamma RI), CD32 (Fc gamma RII), and CD16 (Fc gamma RIII) receptors but these do not enhance the infectivity of HIV-1-IgG complexes. Immunology 90(1), 109–114 (1997).
Sandilands, G. P. et al. Differential expression of CD32 isoforms following alloactivation of human T cells. Immunology 91(2), 204–211 (1997).
Martin, G. E., et al. Enrichment of the HIV reservoir in CD32+ CD4 T cells occurs early in blood and tissue. BioRxiv, 169342 (2017).
Martin, G. E. et al. CD32-Expressing CD4 T Cells Are Phenotypically Diverse and Can Contain Proviral HIV DNA. Front Immunol 9, 928 (2018).
Wittner, M. et al. CD32 expression of different memory T cell subpopulations in the blood and lymph nodal tissue of HIV patients and healthy controls correlates with immune activation. J Acquir Immune Defic Syndr 77(4), 345–349 (2018).
Abdel-Mohsen, M. et al. CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci Transl Med 10 (437); https://doi.org/10.1126/scitranslmed.aar6759 (2018).
Fry, T. J. & Mackall, C. L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol 174, 6571–6576 (2005).
Kondrack, R. M. et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med 198, 1797–1806 (2003).
Wang, F. X. et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest 115, 128–137 (2005).
Scripture-Adams, D. D., Brooks, D. G., Korin, Y. D. & Zack, J. A. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol 76, 13077–13082 (2002).
Vandergeeten, C. et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121, 4321–4329 (2013).
Yin, Y. et al. Interleukin 7 up-regulates CD95 protein on CD4+ T cells by affecting mRNA alternative splicing. Priming for a synergistic effect on VIH-1 reservoir maintenance. J Biol Chem 290, 31–45 (2015).
Katlama, C. et al. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS 30, 221–230 (2016).
McBride, K. et al. The majority of HIV type 1 DNA in circulating CD4+ T lymphocytes is present in non-gut-homing resting memory CD4+ T cells. AIDS Res Hum Retroviruses 29, 1330–1339 (2013).
Wang, C., Kang, S. G., Lee, J., Sun, Z. & Kim, C. H. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol 2, 173–183 (2009).
Singh, S. P., Zhang, H. H., Foley, J. F., Hedrick, M. N. & Farber, J. M. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 180, 214–221 (2008).
Lee, A. Y. & Korner, H. CR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J Gen Virol 98, 338–344 (2017).
Khoury, G. et al. Persistence of HIV DNA in CXCR3+CCR6+ memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS 30, 1511–1520 (2016).
Gosselin, A. et al. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 31, 35–48 (2017).
Wacleche, V. S. et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 13, 59–84 (2016).
Muranski, P. et al. Th17 cells are long lived and retain stem cell-like molecular signature. Immunity 35, 972–985 (2011).
Hartigan-O'Connor, D. J., Hirao, L. A., McCune, J. M. & Dandekar, S. Th17 cells and regulatory T cells in elite control over HIV and SIV. Curr Opin HIV AIDS 6, 221–227 (2011).
Veri, M. C. et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcγ-receptor IIB (CD32B) from the activating Fcγ-receptor IIA (CD32A): Biochemical, biological and functional characterization. Immunology 121, 392–404 (2007).
Noto, A. et al. CD32(+) and PD-1(+) Lymph Node CD4 T Cells Support Persistent HIV-1 Transcription in Treated Aviremic Individuals. J Virol, https://doi.org/10.1128/JVI.00901-18 (2018).
Avettand-Fènoël, V. et al. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin Microbiol Rev 29(4), 859–80 (2016).
Kiselinova, M. et al. Integrated and Total HIV-1 DNA Predict Ex Vivo Viral Outgrowth. PLoS Pathog 12(3), e1005472 (2016).
Petitjean, G. et al. Unintegrated HIV-1 provides an inducible and functional reservoir in untreated and highly active antiretroviral therapy-treated patients. Retrovirology 4, 60 (2007).
Chan, C. N. et al. HIV-1 latency and virus production from unintegrated genomes following direct infection of resting CD4 T cells. Retrovirology 13, 1 (2016).
Trypsteen, W., Kiselinova, M., Vandekerckhove, L. & De Spiegelaere, W. Diagnostic utility of droplet digital PCR for HIV reservoir quantification. J Virus Erad 2(3), 162–169 (2016).
García, M. et al. Peripheral T follicular helper cells make a difference in HIV reservoir size between elite controllers and patients on successful cART. Sci Rep 7, 16799 (2017).
Acknowledgements
We would like to thank all patients and healthy donors who participated in the study. This study has been funded by projects CP14/00198, PI16/01769, and RD16/0025/0013 integrated in the State Plan for Scientific and Technical Research and Innovation and co-funded by ISCIII-Sub-Directorate General for Research Assessment and Promotion and European Regional Development Fund (ERDF). N Rallón is a Miguel Servet investigator from the Spanish Carlos III Institute of Health (ISCIII), grant CP14/00198, Madrid, Spain. María Angeles Navarrete-Muñoz was funded by RD16/0025/0013 and the Intramural Research Scholarship from IIS-FJD. Clara Restrepo was funded by project RD16/0025/0013. M García is a predoctoral student co-funded by CP14/00198 project and the Intramural Research Scholarship from IIS-FJD.
Author information
Authors and Affiliations
Contributions
N.R. and J.M.B. conceived and designed the study. M.Go., M.F., A.C., J.C.L.-B., F.dl. H., V.E. and C.B. contributed to recruitment and follow-up of patients. M.Ga., M.A.N.-M. and C.R. carried out the experimental work. M.M. and J.M. L. contributed to flow cytometry experiments. N.R., J.M.B. and M.Ga. performed the statistical analysis. N.R., J.M.B. and M.Ga. drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García, M., Navarrete-Muñoz, M.A., Ligos, J.M. et al. CD32 Expression is not Associated to HIV-DNA content in CD4 cell subsets of individuals with Different Levels of HIV Control. Sci Rep 8, 15541 (2018). https://doi.org/10.1038/s41598-018-33749-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33749-5
Keywords
This article is cited by
-
Transcriptional signature of resting-memory CD4 T cells differentiates spontaneous from treatment-induced HIV control
Journal of Molecular Medicine (2020)
-
Strategies to eradicate HIV from infected patients: elimination of latent provirus reservoirs
Cellular and Molecular Life Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.