Abstract
Sixty norovirus outbreaks that occurred in Pudong District, Shanghai in 2017 and affected 959 people were summarised. Of the outbreaks, 29 (48.3%), 27 (45.0%), and 4 (6.7%) occurred in kindergartens, primary schools, and middle schools, respectively. Although the total number of outbreaks peaked in March (13/60, 21.7%), outbreaks in kindergartens and primary schools peaked in April (6/29, 20.7%) and March (8/27, 29.6%), respectively. Primary schools had the highest median number of cases per outbreak (19) and the highest proportion of cases (54.6%). The male-to-female case ratio differed among school classifications, with the highest male case ratio (69.2%) occurring in middle schools. Primary symptoms also differed across the school classifications. Molecular virology analysis showed that a single viral strain caused each outbreak at each school. In turn, 50.6, 28.8, and 20.6% of cases were infected by GII.4, GII.2, and GII.17, respectively. Vomiting was seen in 98.2, 97.3, and 88.6% of the subjects infected with noroviruses GII.17, GII.4, and GII.2, respectively, and nausea in 73.6, 43.9, and 39.0%. In conclusion, noroviruses mainly affect primary school and kindergarten students. GII.4, GII.2, and GII.17 are the main epidemic strains in the local area, and the primary symptoms differed by age and genotype.
Similar content being viewed by others
Introduction
Noroviruses, previously known as Norwalk viruses, form a group of genetically diverse, positive-stranded non-enveloped RNA viruses belonging to the genus Norovirus, family Caliciviridae1,2. Their linear RNA genomes contain three open reading frames (ORFs)3,4. ORF1 encodes a large polyprotein, which is further cleaved by a viral protease into six non-structural proteins, including the viral polymerase1,2,3,4. ORF2 and ORF3 encode the major and minor capsid proteins VP1 and VP2, respectively1,2,3,4. Norovirus is a well-described cause of epidemic gastroenteritis across all age groups2,5. The U.S. Centres for Disease Control and Prevention (CDC) estimates that noroviruses are responsible for up to 60% of known-cause acute gastroenteritis cases2,6. The faecal–oral route is the main transmission mode, followed by other modalities, such as transmission via aerosolised viral particles in vomitus and through food, water, environmental contamination, and person-to-person1,2,3,4,5,6.
Students are frequently implicated in acute gastrointestinal outbreaks due to the concentrated population and close living environment in schools7. In China, outbreaks of infectious diarrhoeal disease also occur mainly in schools8. A systematic review and meta-analysis of data extracted from studies covering 85 outbreaks occurring from 1987 to 2014 showed that Shigella, pathogenic Escherichia coli, and norovirus were the most common pathogens to cause epidemic acute gastroenteritis in China9.
Thus far, complete data for whole-year norovirus outbreaks, regarding school type, season, primary symptoms, and genotype characteristics, remain rare in China. Shanghai is the largest city on the east coast of central China, with a total population of approximately 25 million. The Pudong District is located in eastern Shanghai with a population of 5.5 million, comprising 22.0% of the total population. In this report, we summarise 60 norovirus outbreaks that occurred in schools located in the Pudong District in 2017.
Materials and Methods
Ethics issues
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Internal Review Board of the Centre for Disease Control and Prevention of Pudong District, Shanghai. Written informed consent was obtained according to the guidelines of the National Ethics Regulation Committee.
Where, when, and who of outbreaks
Noroviruses cause inflammation of the stomach or intestines called acute gastroenteritis. The incubation period ranges from 12 to 72 h10. In this report, norovirus causing acute gastroenteritis was defined as sudden-onset vomiting or diarrhoea (i.e., abrupt-onset three or more loose or liquid stools above baseline in a 24-h period), with or without accompanying nausea, fever, or abdominal pain. An outbreak is defined as two or more cases with symptoms clustered in time and space.
This report summarises all norovirus outbreaks that occurred in kindergartens, primary schools, middle schools, and high schools in the Pudong District of Shanghai between January 1, 2017 and December 12, 2017. Norovirus outbreaks occurred in 29 kindergarten schools (students aged 4–6 years), 27 primary schools (students aged 7–11 years) and 4 middle schools (students aged 12–14 years) in this area in 2017. According to the relevant laws and regulations of the health authorities of China and Shanghai municipality, the sudden increase in the incidence of a disease or condition is reported to the local CDC. Infectious diarrhoeal disease is a key monitoring disease in schools. Each school reports students with diarrhoea, nausea, vomiting, abdominal pain, headache, and fever and/or chills, and the local CDC then carries out a field investigation. Investigation of the transmission source and route includes the collection of samples using rectal swabs of vomit, faeces, food, and water, and from the surfaces of public facilities. Environmental surface sampling was performed following the Guidelines for Environmental Infection Control in Health-Care Facilities (2003) recommended by the US CDC11. Briefly, the surfaces of classroom and bathroom doorknobs, faucets, desks, and chairs were lightly moistened with 1× phosphate-buffered saline (PBS; pH 7.4) containing 0.01% Tween™ 20 Surfact-Amps™ Detergent Solution (Thermo Fisher, Shanghai, China); cotton swabs were rolled across the moistened area quickly and rinsed in a 1.5-mL collection tube (Biovisualab, Shanghai, China) containing 0.5 mL PBS; all samples were kept on ice and submitted for pathogen detection within 6 h.
Investigation of the health management systems of schools includes consideration of vomitus disinfection, timely isolation, quality of daily disinfection, whether the sanitation facilities are adequate and reasonable, and management of public drinking water. All the parameters were presented as binary variables and recorded by a questionnaire.
Data collection
Age, sex, initial symptoms, symptoms of acute gastroenteritis, histories of ingesting potentially contaminated food (expired food and spoiled food) and water (expired bottled water and unboiled water), contact with diarrhoea patients, travel 1 week before the onset of diarrhoea, and the administration of antibiotics were recorded using a questionnaire survey. The school, location, case number, number of student cases, number of employee cases, reporting date, data of the first case, data of the last case, and sampling number, as well as the above data from the field investigation and subsequent molecular virological analysis, were recorded.
Norovirus detection
Faeces and vomit specimens were prepared as 10% (w/v) suspensions in distilled water and then centrifuged for 10 min at 10,000 × g in a 1.5-mL collection tube (Biovisualab) to remove any debris. The environmental surface samples were also centrifuged for 10 min at 10,000 × g in a 1.5-mL collection tube (Biovisualab) before viral RNA extraction. All centrifugation processes were carried out in a Thermo Scientific™ Sorvall™ Legend™ Micro 21 Microcentrifuge (Thermo Fisher Scientific, Shanghai, China) at 4 °C.
Viral RNA was extracted from the 140 μL suspensions using the QIAamp Viral RNA Mini Kit (QIAGEN, Venlo, Limburg, the Netherlands) according to the manufacturer’s instructions. Viral RNA was eluted with 60 μL RNase-free water containing 0.04% sodium azide (buffer AVE) and stored at −80 °C until polymerase chain reaction detection.
Real-time reverse-transcription polymerase chain reaction (RT-PCR) was used for the initial sample testing for noroviruses according to the method we described previously12. VP1 sequences were further amplified from real-time RT-PCR-positive samples. The primer pairs and probes used for sample screening were: forward, 5′-CARGARBCNATGTTYAGRTGGATGAG-3′; reverse, 5′-TCGACGCCATCTTCATTCACA-3′; and probe, 5′-TGGGAGGGCGATCGCAATCT-3′. The fragment encoding VP1 was amplified and sequenced using the forward primer 5′-CNTGGGAGGGCGATCGCAA-3′ and reverse primer 5′-CCRCCNGCATRHCCRTTRTACAT-3′. The expected PCR product size was 343 bp12.
The sequences of the VP1 regions were input into the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi), and the genogroup and genotype definition were decided according to the “Sequences producing significant alignments of 100 Blast Hits on the Query Sequence.” Alternatively, the genogroup and genotypes were determined by the Norovirus Typing Tool Version 2.0 (https://www.rivm.nl/mpf/typingtool/norovirus/how-to-use). The correlation among all viral sequences was inferred using the maximum likelihood method based on the JTT matrix-based model. All sequence analyses were performed using MEGA713,14.
Statistical analysis
Categorical data are presented as frequencies with percentages; continuous variables are presented as means ± standard deviations or medians and upper and lower quartiles. Differences among groups were examined using Fisher’s exact probability test, the chi-square test or one-way analysis of variance, according to the characteristics of data distribution. P < 0.05 was considered to indicate statistical significance.
Results
Summary of outbreaks
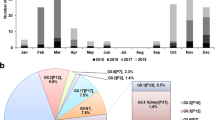
In 2017, 60 outbreaks occurred in 29 (48.3%) kindergarten schools, 27 (45.0%) primary schools, and 4 (6.7%) middle schools; no outbreak was reported in high schools or other settings. There were 949 students and 10 employees in the schools with norovirus infections; the overall infection rate and median attack rate were 2.1% and 2.7%, respectively. The percentages of affected males and females were 52.1% and 47.9%, respectively. The average duration between the first and last cases was 3.5 ± 1.9 days. The proportions of cases with vomiting, nausea, abdominal pain, fever, and diarrhoea ranged from 95.3% to 10.4% (Table 1). In addition, 1.1% (6/542) of samples from the surfaces of public facilities were positive for noroviruses. Investigation of the health management systems of schools revealed incomplete disinfection of vomitus, lack of timely isolation and incomplete daily disinfection in 73.3, 61.7, and 51.7% of schools, respectively; these deficiencies might promote the transmission of noroviruses.
Outbreaks distributed by month
When the outbreaks were plotted along a temporal axis, most occurred in the first half of the year, with a peak in March (Fig. 1A). When these data were further grouped by school type, the epidemic trend differed among types (Fig. 1B). The outbreaks in kindergartens and primary schools peaked in April (six outbreaks) and March (eight outbreaks), respectively; whereas only four outbreaks occurred in middle schools and two of which were in March.
Characteristics of norovirus outbreaks by school type
As shown in Table 2, the total number of cases and total student cases were concentrated mainly in the kindergartens and primary schools; the median numbers of cases per outbreak for kindergartens (12 cases) and middle schools (12 cases) were similar.
A sex difference existed in the proportion of cases: in middle schools, the proportion of male cases (69.2%) was significantly higher than that of female cases (30.8%).
The primary symptoms also differed among the school types. The manifestations in kindergartens and primary schools, ordered from high to low occurrence, were vomiting, nausea, abdominal pain, fever, and diarrhoea. In the middle schools, the rates of cases with vomiting (88.5%) and fever (5.8%) were significantly lower than those in kindergartens and primary schools. Conversely, the rates of cases with nausea, abdominal pain, and diarrhoea were significantly higher than those in kindergartens and primary schools.
The deficiencies in the health management systems in schools varied among agencies. Lack of timely isolation was a common deficiency across all school types, with rates of 50.0% to 66.7%. Incomplete disinfection of vomitus was the dominant deficiency in kindergarten and primary schools, and the lack of sanitary facilities was a dominant deficiency in middle schools.
The proportion of adult cases (employees such as teachers and nursery governesses) was significantly higher in kindergartens (6/10) than in primary (3/10) and middle (1/10) schools.
Characteristics of the norovirus outbreaks by genotype
Of the 60 outbreaks that occurred in 60 schools, viral VP1 sequencing of 9 to 20 specimens sampled from each school, corresponding to at least three independent individuals, was successfully completed in 50 schools (corresponding to 50 outbreaks). Of the 50 outbreaks with identified norovirus genotypes, 26 (52.0%), 14 (28.0%), 9 (18.0%), and 1 (2.0%) were caused by the GII.4, GII.2, GII.17, and GII.3 noroviruses, respectively. To determine the characteristics of the norovirus outbreaks by genotype, 49 outbreaks were regrouped as genotypes GII.2, GII.4, and GII.17 (the one GII.3 outbreak was not included in this analysis).
As shown in Table 3, GII.4 was the dominant epidemic strain across all school types; GII.2 and GII.17 had similar prevalence rates in middle and primary schools; GII.2 was the second common genotype to cause outbreaks in kindergartens. Although the prevalence rates of each viral genotype differed among school types, no statistical significance was observed for each school type.
In order, 50.6, 28.8, and 0.6% of the student cases were infected by genotype GII.4, GII.2, and GII.17, respectively. No significant difference in gender susceptibility was observed among genotypes. In addition, no difference in disease course or adult case rates existed among the three genotypes. The median number of cases of GII.2, GII.4, and GII.17 was seen in October, April, and March, respectively (Table 3).
Interestingly, the primary symptoms, such as vomiting and nausea, showed genotype differences. The proportion of nausea was highest in GII.17 infection, although vomiting was the most common symptom and its incidence ranged from 88.6% in GII.2 infection to 98.2% in GII.17 infection. Although abdominal pain, fever, and diarrhoea were common symptoms, no genotype-specific rate difference was observed among the three genotypes (Table 3).
Molecular phylogenetic characteristics of noroviruses
The viral sequences for each school were aligned to determine whether the outbreaks were caused by single or multiple strains. The alignment analysis showed 100% identity of the VP1 sequence obtained from each school; thus, one sequence from each school was selected to perform further viral correlation analysis among the schools. As shown in Fig. 2, for each genotype cluster, sub-clusters were formed for different agencies. Due to the high population density and complex community life relationships in Shanghai, we do not argue that these data can explain the virological linkage of outbreaks among schools. However, we are certain that GII.4, GII.2, and GII.17 can infect children in kindergarten, primary, and middle schools.
Molecular phylogenetic analysis by the maximum likelihood method. The evolutionary history was inferred using the maximum likelihood method based on the JTT matrix-based model. The tree with the highest log likelihood is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbour-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with a superior log likelihood value. The tree is drawn to scale, with branch lengths measured as the number of substitutions per site. The analysis involved 54 sequences. To avoid ethical issues, all schools are represented by codes consisting of abbreviations of the full school names.
Discussion
The illness caused by norovirus infection was initially described as “winter vomiting disease” in 1929 due to its seasonal predilection1,2,15. Our data suggested that age and viral genotype affect the seasonal predilection of norovirus outbreaks. In general, most outbreaks occurred in the first half of the year, with a peak in March. Outbreaks in kindergartens and primary schools peaked in April and March, respectively. The months in which the median number of GII.2, GII.4, and GII.17 outbreaks occurred were October, April, and March, respectively.
In 1968, an acute gastroenteritis outbreak occurred in an elementary school in Norwalk, OH, USA, and virologists identified the pathogen as norovirus2,16. Subsequently, nausea, vomiting, diarrhoea, and a low-grade fever were found to be the primary symptoms of norovirus infection2,16. Our data suggest that the primary symptoms were also associated with age and viral genotype: the manifestations in students in the kindergarten and primary schools, ordered from high to low proportion, were vomiting, nausea, abdominal pain, fever, and diarrhoea; among middle school students, the rates of vomiting (88.5%) and fever (5.8%) were significantly lower and the rates of cases with nausea, abdominal pain, and diarrhoea were significantly higher than among kindergarten and primary school students. In addition, the proportion of nausea was highest in GII.17-infected cases, although vomiting was the most common symptom, its concomitant probability ranged from 88.6% in GII.2-infected cases to 98.2% in GII.17-infected cases. Taken together, our data showed that diarrhoea is not a dominant symptom of norovirus infection and implied that age-specific host factors and genotype-specific viral factors play roles in the pathology of norovirus infection.
Human norovirus infections are caused, in decreasing order of frequency, by GII (mostly GII.4), GI, and, to a very limited extent, GIV genotypes2,17,18,19,20. However, this trend varies with time, place, and population in China. Since 2002, GII.4 viruses have been the most common genotype circulating in China9,21,22. However, GII.17, GII.P16/GII.2, and GII.6 variants have emerged in recent years and caused outbreaks in China21,23,24. The most prevalent genotypes in north and south China are GII.4, GII.1, and GII.3 and GII.4, GII.17, and GII.3, respectively9. The prevalent strains differ among populations due to differences in acquired immunity21,25. A systematic review has analysed the burden of acute gastroenteritis caused by norovirus infection in China since the year 20009. The integrated data from more than 200 original reports showed that GII.4 (70.4%), GII.3 (13.5%), GII.17 (11.9%), and GII.1 (4.0%) were the top four prevalent strains in China9. The outbreaks in 26 (52.0%), 14 (28.0%), 9 (18.0%), and 1 (2.0%) of the schools in this report were caused by GII.4, GII.2, GII.17, and GII.3 norovirus infections, respectively. Although GII.4 and GII.17 were the first and third most common genotypes in our report, consistent with the above integrated data; GII.3 is rare and GII.2 emerged as the second most common genotype identified in our report. These differences might be explained by the fact that the integrated data included people of all ages from different regions of China, while our data only examined the epidemic characteristics of a specific population in a certain period of time in a local area.
Limitations: in this report, we did not trace the first case to identify the transmission source, as the first symptomatic case does not equate to the one originally excreting or being infected with the virus. On the other hand, although the government has been concentrating on improving water and food supplies in schools, sporadic waterborne or foodborne acute gastroenteritis cannot be extinct. The high population density and complex community life relationships in China weaken the significance of transmission source identification in outbreak control and prevention. As almost all were self-reported cases, age, sex, and personal psychosocial characteristics might affect the accuracy of self-reporting data and we could not exclude possible bias in case reporting. BLAST analysis showed that our sequences were highly similar to many reference sequences submitted from China and abroad. We could not decipher traceability because we lacked long-term background data on norovirus epidemics in China. In the present study, the viral genogroup and genotype were determined by partial VP1 sequences; thus, we could not fully study the recombinant noroviruses. Although this method is commonly used26,27, increasing evidence has supported the proposal to adopt a dual nomenclature using both ORF1 and VP1 sequences28 because recombination is common and the recognition of recombinant viruses may be relevant to their epidemiological characteristics23,28,29,30.
In conclusion, noroviruses GII.4, GII.2, GII.17, and GII.3 were in order the common genotypes in a local area. Noroviruses mainly affect primary school and kindergarten students and the primary symptoms differed by age and viral genotype.
References
Kapikian, A. Z. et al. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10, 1075–1081 (1972).
Robilotti, E., Deresinski, S. & Pinsky, B. A. Norovirus. Clin. Microbiol. Rev. 28, 134–164 (2015).
Jiang, X., Wang, M., Wang, K. & Estes, M. K. Sequence and genomic organization of Norwalk virus. Virology 195, 51–61 (1993).
Lambden, P. R., Caul, E. O., Ashley, C. R. & Clarke, I. N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science 259, 516–519 (1993).
Goodgame, R. Norovirus gastroenteritis. Curr. Infect. Dis. Rep. 9, 102–109 (2007).
Patel, M. M., Hall, A. J., Vinjé, J. & Parashar, U. D. Noroviruses: a comprehensive review. J. Clin. Virol. 44, 1–8 (2009).
Götz, H. et al. Clinical spectrum and transmission characteristics of infection with Norwalk-like virus: findings from a large community outbreak in Sweden. Clin. Infect. Dis. 33, 622–628 (2001).
Ding, Z. et al. Infectious diarrheal disease caused by contaminated well water in Chinese schools: A systematic review and meta-analysis. J. Epidemiol. 27, 274–281 (2017).
Zhou, H. L. et al. Burden of acute gastroenteritis caused by norovirus in China: A systematic review. J. Infect. 75, 216–224 (2017).
Burkhart, D. M. Management of acute gastroenteritis in children. Am Fam Physician. 60, 2555–2563 (1999).
Centers for Disease Control and Prevention. Guidelines for Environmental Infection Control in Health-Care Facilities. https://www.cdc.gov/infectioncontrol/guidelines/environmental/background/sampling.html (2003).
Xue, C. et al. Molecular epidemiology of genogroup II norovirus infections in acute gastroenteritis patients during 2014–2016 in Pudong New Area, Shanghai, China. Gut Pathog. 10, 7 (2018).
Jones, D. T., Taylor, W. R. & Thornton, J. M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282 (1992).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Zahorsky, J. Hyperemesis hiemis or the winter vomiting disease. Arch. Pediatr. 46, 391–395 (1929).
Adler, J. L. & Zickl, R. Winter vomiting disease. J. Infect. Dis. 119, 668–673 (1969).
Noel, J. S., Fankhauser, R. L., Ando, T., Monroe, S. S. & Glass, R. I. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J. Infect. Dis. 179, 1334–1344 (1999).
Lindesmith, L. C. et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5, e31 (2008).
Desai, R. et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin. Infect. Dis. 55, 189–193 (2012).
Turcios, R. M., Widdowson, M. A., Sulka, A. C., Mead, P. S. & Glass, R. I. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998–2000. Clin. Infect. Dis. 42, 964–969 (2006).
Ao, Y. et al. Norovirus GII.P16/GII.2-Associated Gastroenteritis, China, 2016. Emerg. Infect. Dis. 23, 1172–1175 (2017).
Zhang, J. et al. Genotype distribution of norovirus around the emergence of Sydney_2012 and the antigenic drift of contemporary GII.4 epidemic strains. J. Clin. Virol. 72, 95–101 (2015).
Luo, L. F. et al. Acute gastroenteritis outbreak caused by a GII.6 norovirus. World J. Gastroenterol. 21, 5295–5302 (2015).
Lu, J. et al. Gastroenteritis Outbreaks Caused by Norovirus GII.17, Guangdong Province, China, 2014-2015. Emerg. Infect. Dis. 21, 1240–1242 (2015).
Parra, G. I. et al. Static and evolving norovirus genotypes: implications for epidemiology and immunity. PLoS Pathog. 13, e1006136 (2017).
Bruggink, L. D., Dunbar, N. L. & Marshall, J. A. Evaluation of the updated RIDA®QUICK (Version N1402) immunochromatographic assay for the detection of norovirus in clinical specimens. J. Virol. Methods 223, 82–87 (2015).
Rupprom, K., Chavalitshewinkoon-Petmitr, P., Diraphat, P. & Kittigul, L. Evaluation of real-time RT-PCR assays for detection and quantification of norovirus genogroups I and II. Virol. Sin. 32, 139–146 (2017).
Kroneman, A. et al. Proposal for a unified norovirus nomenclature and genotyping. Arch. Virol. 158, 2059–2068 (2013).
Yu, Y., Yan, S., Li, B., Pan, Y. & Wang, Y. Genetic diversity and distribution of human norovirus in China (1999–2011). Biomed. Res. Int. 2014, 196169 (2014).
Ji, L. et al. Rapid emergence of novel GII.4 sub-lineages noroviruses associated with outbreaks in Huzhou, China, 2008–2012. PLoS One 8, e82627 (2013).
Acknowledgements
This work was supported by the excellent young medical talents training program in Pudong New Area health system (PWRq2016–16).
Author information
Authors and Affiliations
Contributions
Y.W., L.H., X.Z. and W.Z. conceptualized the study, drafted the initial manuscript, and reviewed and revised the manuscript. L.P., C.X. and Q.L. collected data, carried out the initial analyses, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Hao, L., Pan, L. et al. Age, primary symptoms, and genotype characteristics of norovirus outbreaks in Shanghai schools in 2017. Sci Rep 8, 15238 (2018). https://doi.org/10.1038/s41598-018-33724-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33724-0
Keywords
This article is cited by
-
Epidemiology of GII.4 and GII.2 norovirus outbreaks in closed and semi-closed institutions in 2017 and 2018
Scientific Reports (2023)
-
Rotavirus and Norovirus Infections in Children Under 5 Years Old with Acute Gastroenteritis in Southwestern China, 2018–2020
Journal of Epidemiology and Global Health (2022)
-
An outbreak of gastroenteritis by emerging norovirus GII.2[P16] in a kindergarten in Kota Kinabalu, Malaysian Borneo
Scientific Reports (2020)
-
Epidemiological and clinical differences between sexes and pathogens in a three-year surveillance of acute infectious gastroenteritis in Shanghai
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.