Abstract
Bacterial microcompartments (BMCs) are promising natural protein structures for applications that require the segregation of certain metabolic functions or molecular species in a defined microenvironment. To understand how endogenous cargos are packaged inside the protein shell is key for using BMCs as nano-scale reactors or delivery vesicles. In this report, we studied the encapsulation of RuBisCO into the α-type carboxysome from Halothiobacillus neapolitan. Our experimental data revealed that the CsoS2 scaffold proteins engage RuBisCO enzyme through an interaction with the small subunit (CbbS). In addition, the N domain of the large subunit (CbbL) of RuBisCO interacts with all shell proteins that can form the hexamers. The binding affinity between the N domain of CbbL and one of the major shell proteins, CsoS1C, is within the submicromolar range. The absence of the N domain also prevented the encapsulation of the rest of the RuBisCO subunits. Our findings complete the picture of how RuBisCOs are encapsulated into the α-type carboxysome and provide insights for future studies and engineering of carboxysome as a protein shell.
Similar content being viewed by others
Introduction
Bacterial microcompartments (BMCs) are natural proteinaceous cell organelles that encapsulate functionally relevant enzymes to execute designated steps of biological pathways1,2,3,4. BMCs serve as a vesicular scaffold for the co-localization and physical separation of pathway enzymes from the rest of the cytosolic contents. The benefits and the biological significances of BMCs are often inferred from the functions of the encapsulated cargos. The most well studied BMCs include carboxysomes that participate in CO2 fixations in phototrophic and some chemoautotrophic bacteria5,6,7 and metabolosomes, such as Pdu and Eut BMCs that harbor the 1,2-propanediol and ethanolamine degradation pathways in Salmonella enterica8,9. Carboxysome plays a major role in the CO2-concentrating mechanism (CCM) through its selectively permeable protein shell to enhance the lumen concentration of CO2, which is produced by the encapsulated carbonic anhydrase (CA) and further serves as the substrate of the co-localized ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)10,11. The local environment with elevated CO2 level accelerates the overall rate of CO2 fixation by benefitting bacterial RuBisCOs, which generally have higher kcat and Km values than plant enzymes12. In addition to providing kinetic advantages, the protein shells of metabolosome-type of BMCs are considered as the major barrier to limit the free diffusion of cytotoxic pathway intermediates, such as propionaldehyde and acetaldehyde, to the cytosol and therefore ensure the fitness of cells.
Encapsulation by naturally occurring BMCs presents an elegant solution to a general orthogonality problem that is encountered in various formats in cellular engineering, such as how to break the diffusion-controlled kinetic barriers and how to minimize noise in a genetic circuit. BMCs are emerging as a promising type of protein scaffold structure in synthetic biology13,14,15,16. The practical application of BMCs relies on solving a basic research question: how functional proteins are selectively encapsulated into the protein shell. The answer is key to the introduction of foreign protein cargos into artificial BMC as nano-scale bioreactors or as delivery vesicles17,18,19,20,21. Mechanistic studies on protein encapsulation into the Pdu and Eut BMCs of S. enterica revealed that the N-termini of functional proteins, such as PduA and EutG, serve as signal peptides to interact with shell proteins and are responsible for the formation of BMCs around the cargos18,22,23. A similar mechanism was also uncovered in metabolosomes from other bacterial species, albeit the signal peptide may reside on the C-termini19,24. This knowledge guided efforts toward the engineering of artificial interaction motifs for foreign cargo protein encapsulation in BMC25,26. Studies on the biogenesis of the β-type carboxysome revealed a slightly altered approach for the encapsulation of RuBisCO and other proteins. A separate encapsulation peptide (EP), which is encoded by the ccmN gene, forms a tether between the cargo and the shell proteins27. Structural comparison revealed a common amphipathic α-helix motif that is shared between the signal peptides of metabolosomes and the EP of β-type carboxysome28. However, regardless of recent progress in understanding the role of csoS2-encoded scaffold proteins, CsoS2A and CsoS2B, in the nucleation of RuBisCO assembly, molecular details on how RuBisCO is encapsulated inside the shell of the α-type carboxysome are still murky29. In particular, sequence analysis of gene clusters of the α-carboxysomes failed to identify an EP-like sequence28, which indicates a different mechanism of cargo packaging.
The objective of the present work is to understand the encapsulation mechanism of α-type carboxysome through the study of interactions between BMC shell/scaffold proteins and cargo proteins. The carboxysome from Halothiobacillus neapolitan (ATCC 23641) was used as a model system in this study. The Halothiobacillus neapolitan encodes the form 1A/α-RuBisCO, which consists of eight large (CbbL, 52.6 kDa) and eight small (CbbS, 15.8 kDa) subunits (L8S8, PDB: 1SVD). The core of the enzyme is made up of four functional dimers of CbbL arranged around a 4-fold axis, and capped at each end by four noncatalytic CbbS proteins. The CbbL is built up of an N domain and a C domain, which harbors the major structure of the active site. A few residues of the N domain from the adjacent subunit of the dimer complete the substrate binding cavity30. Intrigued by the encapsulation function of N-terminal sequences of metabolosome enzymes, we seek to investigate whether the N-terminal domain of the large subunit participates in the encapsulation of RuBisCO into the α-type carboxysome. In this study, we present experimental evidences to demonstrate that the N domain of CbbL is essential for the encapsulation of the enzyme. Its interaction with the major shell protein has an apparent Kd in the submicromolar range. In silico docking experiments reveal that a short α-helix structural motif embedded in the middle of the N-domain may play a key role in the interaction. Our data also show that the previously identified interaction between scaffold protein CsoS2 and RuBisCO is engaged through the RuBisCO small subunit, CbbS. Overall, this study reveals new details of protein-protein interaction in the process of RuBisCO encapsulation into the α-type carboxysome and provides insights for future studies and applications of BMCs.
Results and Discussion
Encapsulation of wild-type CbbL into the heterologously expressed carboxysome
We first established a technical platform for the studies, including the expression, isolation, and detection of carboxysome, shell proteins, and/or cargo proteins. The cso gene cluster together with the csoS1D gene from H. neapolitan (ATCC 23641) was cloned behind an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible promoter on a pSC101-based vector to afford plasmid pZS-23641 (Fig. 1A)31. E. coli cells transformed with this plasmid were cultured at room temperature in media containing IPTG. Lysate from the harvested cells was subjected to a series of centrifugation steps to obtain fractions of different sedimentation coefficients. The formation of carboxysomes was confirmed by transmission electron microscopy (TEM) analysis of purified fraction (Fig. 1B). The average size of purified carboxysome was around 100 nm, which is consistent with a previous report32. Majority of the purified carboxysome had clear and continuous boundaries, which indicates intact physical structures. A small percentage of the sample appeared to be broken presumably due to the lengthy experimental procedure. SDS-PAGE analysis showed protein bands that correlate to the sizes of major shell proteins (CsoS1A/1C, CsoS1B) and encapsulated proteins (CbbL, CbbS, CsoS2A, and CsoS2B) in purified carboxysome (Fig. 2A, lane 2). Bands of minor shell proteins (CsoS1D, CsoS4A, and CsoS4B) and encapsulated carbonic anhydrase (CsoS3) could not be unambiguously identified, which is consistent with a previous report32.
Encapsulation of full-length CbbL and domains of CbbL. (A) SDS-PAGE analysis of purified carboxysomes (original image, Fig. S5). M, protein size markers; lane 1, from cells transformed with plasmid pZS-23641-MBP-CbbL(C)6xHis; lane 2, from cells transformed with pZS-23641; (B) Immunoprecipitation of purified carboxysome (original image, Fig. S6). I, samples from intact carboxysome; B, samples from broken carboxysome; (C) Confocal fluorescent microscope analysis of E. coli cells. Top panel are cells transformed with pZS-23641-CbbL-mCherry. Middle panel are cells transformed with pZS-23641-CbbL(C)-mCherry. Bottom panel are cells transformed with pZS-23641-CbbL(N)-mCherry. For each panel: left, fluorescent image; center, white-field image; right, overlap image.
To facilitate the encapsulation study, plasmid pZS-23641-CbbL6xHis was constructed to install a six-histidine tag to the C terminus of the CbbL protein encoded by the cso cluster. Modified carboxysomes were purified from E. coli cells and subjected to immunoprecipitation experiments22. Samples of intact and broken carboxysome were incubated with anti-6xHis antibody, which was subsequently precipitated by protein A agarose at low centrifugation speed. Obtained supernatant fractions of the two samples contained either the intact carboxysome or proteins of broken carboxysome. Released proteins from the agarose beads were analyzed as the precipitate fractions. Western blots using the anti-6xHis antibody detected the CbbL-6xHis protein in supernatant fractions from both intact and broken carboxysome samples, which confirmed that the protein was expressed from the modified cso gene cluster (Fig. 2B). Furthermore, the CbbL-6xHis protein was only detected in the precipitate fraction of the broken carboxysome, but not in the sample of the intact carboxysome (Fig. 2B). These observations support the conclusion that the CbbL-6xHis protein was inaccessible to anti-6xHis antibody when it was encapsulated into the intact protein shell of carboxysome.
N domain of CbbL (CbbL(N)) is essential for encapsulation
We hypothesized that the N domain of CbbL is necessary for its encapsulation into functional carboxysome. To this end, we initially sought to apply the same immunoprecipitation method to examining the encapsulation of CbbL protein that lacks the N domain (a.a. 1–136). However, protein expression analysis showed that the C domain of CbbL (CbbL(C), a.a. 137–473) was mainly expressed as inclusion bodies (Fig. S1A). We were unable to separate the inclusion bodies from the carboxysome fraction using sucrose gradient ultracentrifugation method. As a result, the C domain of CbbL was detected in both the broken and the intact fraction of the purified carboxysome in the immunoprecipitation experiment, which complicated the interpretation of the results (Fig. S1B). To solve the problem, we improved the solubility of the C domain by fusing it to the C-terminus of the maltose binding protein (MBP) (Fig. S1C). Plasmid was then constructed by replacing CbbL6xHis with MBP-CbbL(C)6xHis gene. Carboxysome was purified from E. coli strain transformed with plasmid pZS-23641-MBP-CbbL(C)6xHis. In comparison to the SDS-PAGE of wild-type carboxysome derived from pZS-23641, protein bands of major shell proteins and encapsulated proteins (CsoS2A and CsoS2B) derived from pZS-23641-MBP-CbbL(C)6xHis were still visible (Fig. 2A, lane 1). However, a band with the size of MBP-CbbL(C)6xHis could not be unambiguously identified. Immunoprecipitation experiment to detect the MBP-CbbL(C)6xHis protein in the purified carboxysome also showed negative result (Fig. 2B). These data indicated that the C domain of CbbL was not encapsulated in the carboxysome. Close inspection of SDS-PAGE of the wild-type (Fig. 2A, lane 2) and 23641-MBP-CbbL(C)6xHis carboxysomes (Fig. 2A, lane 1) also revealed a significantly reduced presence of the small subunit of RuBisCO, CbbS, when the N domain of CbbL was not expressed.
As it is possible that the MBP itself and not the lack of the N domain prevented the encapsulation of the MBP-CbbL(C)6xHis fusion protein, we designed and conducted another set of experiments. In these experiments, a red fluorescent protein (mCherry) was fused either to the intact or to the C domain of CbbL. The fluorescence of E. coli strains transformed with pZS-23641-CbbL-mCherry or pZS-23641-CbbL(C)-mCherry was studied under confocal microscope (Fig. 2C). Fluorescence foci formation was clearly observed in cells expressing CbbL-mCherry (Fig. 2C, top panel), which is a sign of localization in the cytosol due to the encapsulation by the carboxysome32. On the other hand, evenly dispersed fluorescent signals were observed in cells expressing the CbbL(C)-mCherry fusion protein (Fig. 2C, middle panel). Above data suggest that the N domain of CbbL is essential for its packaging into the carboxysome.
To further investigate whether the N domain of CbbL is capable of localizing foreign proteins to carboxysome, we fused either MBP6xHis or mCherry6xHis to the C-terminus of the domain. Carboxysome was purified from E. coli cells transformed with pZS-23641-CbbL(N)-MBP6xHis (Fig. 3A, lane 2). In comparison with purified wild-type carboxysome (Fig. 3A, lane 1), a new protein band corresponding to the size of CbbL(N)-MBP6xHis fusion protein was observed. Western blot experiment confirmed the protein band contained His tag (Fig. S2B). The intensity of this band is significantly lower than the band of CbbL in the wild-type carboxysome, although the two samples of purified carboxysome contained similar amounts of shell proteins (CsoS1A/1B/1C) and scaffold proteins (CsoS2A/2B) (Fig. 3). The purified carboxysome was then subjected to immunoprecipitation experiment (Fig. 3B), which showed that the CbbL(N)-MBP6xHis fusion protein was located to the purified carboxysome (Fig. 3B). We further studied the distribution of mCherry protein in E. coli cells expressing pZS-23641-CbbL(N)-mCherry (Fig. 2C, bottom panel). Significant heterogeneity in fluorescence distribution was observed within the cell population. Analysis showed that around 35% of the fluorescence signal formed foci in 23641-CbbL(N)-mCherry sample, while 100% of the signals formed foci in 23641-CbbL-mCherry sample and less than 5% in 23641-CbbL(C)-mCherry sample. Above data support the conclusion that the N domain of CbbL is capable of directing foreign protein to carboxysome, although the efficiency is low due to apparent stochastic effect. The expression of the N domain of CbbL also largely recovered the encapsulation of small subunit of RuBisCO, CbbS (Fig. 3A).
N domain of CbbL directs foreign proteins to carboxysome. (A) SDS-PAGE analysis of purified carboxysomes (original image, Fig. S7). M, protein size markers; lane 1, from cells transformed with plasmid pZS-23641-CbbL6xHis; lane 2, from cells transformed with pZS-23641-CbbL(N)-MBP6xHis; (B) Immunoprecipitation of purified carboxysome (original image, Fig. S8). I, samples from intact carboxysome; B, samples from broken carboxysome.
Scaffold protein CsoS2 interacts with CbbS
To understand how the N domain of CbbL participates in the encapsulation process, we first examined its interaction with CsoS2 protein. It is known that CsoS2 is absolutely conserved in organisms that express α-carboxysomes. Recent studies demonstrated that this structurally flexible protein interacts with both major shell proteins and RuBisCO29. It was further hypothesized that CsoS2 serves as a scaffold to recruit shell proteins and RuBisCO prior to the assembly of carboxysome29. However details on the interaction between CsoS2 and RuBisCO have not been studied. To probe whether the N domain of CbbL plays any role in this process, we conducted pull-down assays using cells that co-expressed different combinations of CsoS2 (with N-6xHis tag) and CbbL/CbbS proteins (Fig. 4). When CsoS2 and CbbS were co-expressed in E. coli cells, the fraction purified using immobilized Ni resin contained both CsoS2 and CbbS (Fig. 4, lane 3). On the other hand, the co-purification was not observed for CbbL when it was expressed together with CsoS2 (Fig. 4, lane 2). Furthermore, when the RuBisCO complex, including both CbbL and CbbS, was co-expressed with CsoS2, CbbL was also observed in the pull-down fraction (Fig. 4, lane 1). The results support the notion that scaffold CsoS2 recruits RuBisCO through its interaction with the small subunit (CbbS) of the RuBisCO, which eliminates the possibility that the role of CbbL(N) in RuBisCO encapsulation is through its interaction with CsoS2 proteins.
Pull-down assay between 6xHis-CsoS2s and CbbL/CbbS. In each lane, the following carboxysome-related proteins were co-expressed with 6xHis-CsoS2s: lane 1, CbbL and CbbS; lane 2, CbbL; lane 3, CbbS; lane 4, none. M, protein size markers. Original image is provided as Fig. S9 in Supplemental Information.
The N domain of CbbL (CbbL(N)) interacts with major shell proteins
The lack of direct interaction between CsoS2 proteins and CbbL(N) promoted us to investigate whether the N domain of CbbL interacts with any shell proteins of carboxysome. To co-express CbbL(N) and shell proteins in E. coli for pull-down assays, two plasmids were constructed. The first plasmid encodes a fusion protein of CbbL(N) and mCherry with a C-terminal 6xHis tag. The fluorescence protein was fused to the C-terminus of CbbL(N) and used as a visual signal to facilitate the protein purification. All genes that encode the H. neapolitan shell proteins, including CsoS1A/1B/1C/1D and CsoS4A/4B, were cloned into a second plasmid, pZS-23641-CsoS41. Above two plasmids were co-transformed into E. coli for protein expression and the cell extract was subjected to the pull-down assay. By comparing to a control sample of the CbbL(N)-mCherry-6xHis protein that was purified separately in parallel, an additional band was unambiguously identified in the SDS-PAGE of the pull-down sample (Fig. S3). Proteomics analysis confirmed that the identified band consisted of CsoS1A and CsoS1C with 91% sequence coverage for each protein. Since the two shell proteins are of the same length (98 a.a.) and differ only in two amino acid residues, they could not be resolved in our SDS-PAGE experiments.
To verify our above observations and to exclude the possibility that interactions between CsoS1A/1C and mCherry led to the pull-down effect, we generated a control construct in which the 6xHis tag was fused directly to the C terminus of CbbL(N). Plasmids encoding individual shell protein were also constructed. Another round of pull-down assays were conducted between Cbb(N)-6xHis and each shell protein separately. The results confirmed that the N domain of CbbL interacts with shell proteins CsoS1A and CsoS1C (Fig. 5). In addition, interaction between CbbL(N) and CsoS1B was also detected (Fig. 5). On the other hand, no pull-down effect was observed for CsoS1D, CsoS4A, and CsoS4B (Fig. 5). It was estimated in previous reports that CsoS1A/1B/1C (Pfam00936, BMC-H)33,34 exist in 3510 copies per carboxysome, while the copy numbers of CsoS1D (BMC-T) and CsoS4A/4B (Pfam 03319, BMC-P) were too low to be determined in wild-type carboxysomes purified from H. Neapolitan35. Interactions between the N domain of CbbL and the three major shell proteins indicate possible role that is played by CbbL(N) during the encapsulation of RuBisCO.
Pull-down assay between CbbL(N)-6xHis and shell proteins of H. neapolitan carboxysome. In addition to CbbL(N)-6xHis, cells also expressed the following carboxysome-related proteins: lane 1, CsoS4B; lane 2. CsoS4A; lane 3. CsoS1D; lane 4. CsoS1C; lane 5. CsoS1B; lane 6. CsoS1A; lane 7. none. M, protein size markers. Original image is provided as Fig. S10 in Supplemental Information.
Structural motifs of CbbL(N) that is responsible for the interaction
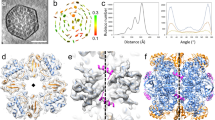
To investigate whether the entire N domain (a.a. 1–136) of CbbL contribute to the interaction with major shell proteins, we expressed five truncated segments of CbbL(N) based on its secondary structural units (Fig. 6A). Segment 1–20 represented the flexible N terminus (red, Fig. 6A) that was mainly ‘invisible’ in the X-ray structure. Segment 1–73 contained one of the central β strand followed by one large and one short α helices (yellow, Fig. 6A). Segment 1–100 covered two additional central β strands (green, Fig. 6A), while segment 1–122 covered the last α helix (blue, Fig. 6A) and 1–136 included an additional β strand (cyan, Fig. 6A). Each segment was fused to the N terminus of MBP that contained a C-terminal 6xHis tag. The interaction between truncated CbbL(N) with major shell proteins CsoS1A and CsoS1C was studied using pull-down assays (Fig. S4). The ligand binding ratio between the CbbL(N) segment and the shell protein was calculated using signals from densitometry measurement in the assays. The relative binding strength of a truncated segment was expressed as the percentage of its ligand-binding ratio to that of the entire N domain (Fig. 6B). Data indicated that the unstructured N-terminal peptide that contains the first 20 amino acids played insignificant role in the binding of CbbL(N) with the shell proteins, which is different from the observations of signal peptides in functional proteins of the Pdu and Eut metabolosomes. For CsoS1A and CsoS1C, the relative binding strength with truncated N domain continuously increased and reached maxima when CbbL(1–122) was used. Only a small difference in binding strength was observed between CbbL(1–122) and CbbL(N).
Interaction of truncated CbbL(N) with major shell proteins of H. neapolitan carboxysome. (A) Structure of CbbL(N) colored according to the truncated segments; (B) The relative binding strength between segments of CbbL(N) and shell proteins; (C) The binding affinity assay between CsoS1C and CbbL(1–136); (D) In silico simulation of interaction between hexamer of CsoS1C and CbbL(1–136). The hexamer of CsoS1C is colored in gold and purple. CbbL(N) is colored in blue, while the short α helix (a.a. 63–67) is colored in pink.
Due to the high sequence homology between CsoS1A and CsoS1C and their similar behaviors in the pull-down assay against truncated CbbL(N) protein, further studies on the interaction between the N domain of CbbL and major shell proteins focused on CsoS1C. The binding affinity between CbbL(N) and CsoS1C was examined by semi-quantitative ELISA experiments36. In brief, wells of microtiter plates were coated with MBP tagged CbbL(1–136), into which different concentrations of the CsoS1C-6xHis protein were applied. Following removal of the unbound proteins by washing, the amount of CsoS1C that bound to CbbL(1–136) was quantified by immunoassays. The interaction between CbbL(1–136) and CsoS1C showed a strong binding affinity with an apparent Kd of 120.5 ± 4.6 nM.
In silico docking experiment was subsequently conducted to simulate the possible mode of interaction between CbbL(N) and CsoS1C. The hexameric structure of CsoS1C (PDB: 3H8Y)37 and the CbbL (PDB: 1SVD) were used in rigid body docking in the ZDOCK module of Discovery Studio suite. The top three conformations with the lowest energy all showed possible interaction between a short α helix formed by residues 63 to 67 of CbbL(N) and the convex side of the CsoS1C hexamer (Fig. 6D). The simulation result supported observations in CbbL(N) truncation experiments where significant interactions were first observed when fragment 1–73 was examined. The identified short α helix is held between α helix (a.a. 42–54) and β sheet (a.a. 76–82), which indicates that additional structural units may further constraint the short α helix for optimal interaction with the shell proteins.
Conclusion
BMC is a promising type of protein scaffold for applications in synthetic biology, drug delivery and material science. Understanding the assembly mechanism of BMCs is critical for further engineering efforts. This research focused on studying the interactions between subunits of RuBisCO and shell/scaffold proteins of the α-type carboxysome from H. neapolitan. Our data showed that the small subunit (CbbS) of RuBisCO interacts with the scaffold proteins (CsoS2), while the N domain of the large subunit (CbbL(N)) of RuBisCO interacts with all major shell proteins CsoS1A, 1B and 1C with submicromolar binding affinity. Computer simulation revealed a possible mode of interaction between the CbbL(N) domain and shell proteins. The findings reveal molecular details of protein-protein interactions in the process of α-type carboxysome assembly and further support previous hypothesis of simultaneous assembly of the shell and encapsulation of RuBisCO enzymes38. In addition to completing the picture of α-type carboxysome assembly, findings from this work also provide insights for future engineering of carboxysome for biotechnological applications.
Methods
Material and general methods
Primers (Table S1) were ordered from Sigma. Plasmids (Table S2) were constructed using standard molecular biology techniques39. DNA sequencing services were provided by Eurofins MWG Operon. Restriction enzymes, Antarctic phosphatase (AP) and T4 DNA ligase were purchased from New England Biolabs. KOD hot start DNA polymerase was purchased from EMD Millipore. All solutions were prepared in deionized water that was further treated by Barnstead Nanopure® ultrapure water purification system (Thermo Fisher Scientific). LB medium (1 L) contained Bacto tryptone (10 g), Bacto yeast extract (5 g), and NaCl (10 g). Antibiotics were added where appropriate to following final concentrations: ampicillin, 100 mg L−1, chloramphenicol, 34 mg L−1, kanamycin, 50 mg L−1.
Expression and purification of carboxysome
Plasmid encoding the wild-type or a modified carboxysome was transformed into E. coli NEB®5-alpha. Cells were cultured in LB media with ampicillin at 37 °C until the OD600nm reached 0.6. Following the addition of IPTG to a final concentration of 0.2 mM, expression of carboxysome was carried out at room temperature for 14 h. Cells were collected by centrifugation, then resuspended in TEMB buffer (5 mM Tris-HCl, pH 8.0, 1 mM EDTA, 10 mM MgCl2, 20 mM NaHCO3). Purification of carboxysome followed a reported protocol40. Obtained pellet of carboxysome was first washed with ultrapure water, then resuspended in TEMB.
Pull-down assays
Two plasmids, each encoded one protein of interest, were co-transformed into E. coli BL21(DE3). Transformed cells were cultured in LB medium with appropriate antibiotics at 37 °C until OD600 reached 0.6. Protein expression was induced with IPTG at 0.4 mM and carried out at 30 °C for 12 h. The cells were harvested by centrifugation at 5,000 g for 10 min. Collected cells were suspended in TEMB with 200 mM NaCl, then lysed by sonication. Following removal of cell debris by centrifugation at 16,000 g for 20 min, the supernatant was applied to a Ni Sepharose 6 Fast Flow (GE Healthcare) column. The column was eluted stepwise with five column volumes of buffers containing 5 mM, 50 mM, then 250 mM of imidazole. All elution fractions were analyzed by SDS-PAGE to test for co-elution of proteins with and without the 6xHis tag.
Immunoprecipitation
Protein A agarose beads (PierceTM, ThermoFisher Scientific) were first washed with PBS buffer, then diluted tenfold in PBS prior to mixing with 6xHis tag monoclonal antibody (InvitrogenTM, ThermoFisher Scientific). The bead suspension was incubated at 4 °C for 1 h with gently shaking. Purified carboxysome was first treated with Ni Sepharose 6 Fast Flow resin to remove His-tagged protein that was leaked out of the carboxysome during the sample preparation process. To obtain broken carboxysome, purified sample was subjected to sonication using Model 120 sonic dismembrator (FisherbrandTM) at 50% amplitude for 2 min. Samples of broken or intact carboxysome were mixed with antibody-treated protein A beads. Following incubation at 4 °C for 1 h with gentle shaking, the beads were collected by centrifugation at 2,500 g for 2 min. The supernatant was saved as the supernatant fractions for further analysis. The beads were washed three times with 0.5 mL of IP buffer (25 mM Tris-HCl, pH 7.2, 150 mM NaCl). To elute the protein, the beads were suspended in 50 μL of glycine-HCl buffer (150 mM, pH 2.94) and incubated for 5 minutes. Following centrifugation at 2,500 g for 2 min, the supernatant was collected as the sediment fraction from the immunoprecipitation step. The supernatant and sediment fractions were analyzed by Western blots for the presence of 6xHis tag using the 6xHis tag monoclonal antibody together with the goat anti-mouse IgG-HRP conjugate (BioRad) and Opti-4CN detection kit (BioRad).
Enzyme-linked immunosorbent assay (ELISA)
The semi-quantitative ELISA experiments followed reported protocols with minor modifications36. In brief, MaxiSorp 96 well ELISA plates were coated with 100 μL of MBP-tagged CbbL(1–136) proteins (30 nM in 0.1 M Na2CO3 (pH 9.6)) at 4 °C for 12 h. After removal of the coating solution, blocking buffer (1 mM CaCl2 and 1% BSA in TBS buffer) was added and the plates were incubated at room temperature for 1 h. Following three times of washing with washing buffer (1 mM CaCl2 and 0.05% Tween 20 in TBS buffer), solutions of CsoS1C-6xHis protein at varied concentrations (5 nM to 5 μM in blocking buffer) were applied to each well. The plates were incubated for 1 h at room temperature, then washed three times to remove unbound protein. Detection of bound protein used the 6xHis tag monoclonal antibody together with the goat anti-mouse IgG-HRP conjugate and the 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate. Following the reaction, the absorbance at 450 nm was measured to quantify the activities of HRP. Dissociation constants were calculated by curve fitting with Hill Equation using Prism 7 (Graphpad).
Proteomics analysis
Gel band sample was washed, then digested with trypsin overnight at 37 °C. Tryptic peptides were extracted from the gel pieces, dried down, and re-dissolved in 25 μL of 2.5% acetonitrile, 0.1% formic acid. The digests were run on a Q-Exactive-HF mass spectrometer (ThermoFisher Scientific) equipped with a U3000 RSLCnano LC system. All MS/MS data were analyzed using Mascot (Matrix Science, v 2.5.1). Mascot was set up to search the databases with a fragment ion mass tolerance of 0.060 Da and a parent ion tolerance of 10 ppm. Modifications of residues, including deamination of asparagine and glutamine, oxidation of methionine and carbamidomethyl of cysteine, were specified as variable modifications. Scaffold (Proteome Software Inc., v 4.7.5) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 80.0% probability and protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides, with a false discovery rate less than 1%.
Microscopy methods
For transmission electron microscopy analysis, purified carboxysome were first fixed with glutaraldehyde (4% in PBS buffer) for 30 min at room temperature, washed three time with PBS, then adsorbed for 1 min to a carbon-coated grid. The samples were stained with 1.0% uranyl acetate in deionized water. The grids were imaged using 80 kv accelerating voltage using a Hitachi H7500 transmission electron microscope. For imaging using fluorescent microscope, E. coli cells transformed with either pZS-23641-CbbL-mCherry or pZS-23641-CbbL(C)-mCherry were cultured, induced for carboxysome expression, then collected by centrifugation at 5,000 g for 10 min. Cells were washed three times with PBS buffer, then fixed with paraformaldehyde (4% in PBS buffer) for 30 min at room temperature. Cells were then washed, resuspended in PBS, loaded on a glass slide, then imaged using Nikon A1R-Ti-2 confocal microscope. Texas red channel was excited at 595 nm and imaged at 620 nm. The percentage of localization of fluorescence signal is analyzed using ImageJ41.
References
Cannon, G. C. et al. Microcompartments in Prokaryotes: Carboxysomes and Related Polyhedra. Applied and Environmental Microbiology 67, 5351–5361 (2001).
Kerfeld, C. A., Heinhorst, S. & Cannon, G. C. Bacterial Microcompartments. Annual Review of Microbiology 64, 391–408 (2010).
Chowdhury, C., Sinha, S., Chun, S., Yeates, T. O. & Bobik, T. A. Diverse Bacterial Microcompartment Organelles. Microbiology and Molecular Biology Reviews: MMBR 78, 438–468 (2014).
Kerfeld, C. A., Aussignargues, C., Zarzycki, J., Cai, F. & Sutter, M. Bacterial microcompartments. Nature Reviews Microbiology (2018).
Gantt, E. & Conti, S. F. Ultrastructure of Blue-Green Algae. Journal of Bacteriology 97, 1486–1493 (1969).
Shively, J. M., Ball, F. L. & Kline, B. W. Electron Microscopy of the Carboxysomes (Polyhedral Bodies) of Thiobacillus neapolitanus. Journal of Bacteriology 116, 1405–1411 (1973).
Shively, J. M., Ball, F., Brown, D. H. & Saunders, R. E. Functional Organelles in Prokaryotes: Polyhedral Inclusions (Carboxysomes) of Thiobacillus neapolitanus. Science 182, 584 (1973).
Bobik, T. A., Havemann, G. D., Busch, R. J., Williams, D. S. & Aldrich, H. C. The Propanediol Utilization (pdu) Operon of Salmonella enterica Serovar Typhimurium LT2 Includes Genes Necessary for Formation of Polyhedral Organelles Involved in Coenzyme B(12)-Dependent 1,2-Propanediol Degradation. Journal of Bacteriology 181, 5967–5975 (1999).
Kofoid, E., Rappleye, C., Stojiljkovic, I. & Roth, J. The 17-Gene Ethanolamine (eut) Operon of Salmonella typhimurium Encodes Five Homologues of Carboxysome Shell Proteins. Journal of Bacteriology 181, 5317–5329 (1999).
Long, B. M., Rae, B. D., Rolland, V., Förster, B. & Price, G. D. Cyanobacterial CO2-concentrating mechanism components: function and prospects for plant metabolic engineering. Current Opinion in Plant Biology 31, 1–8 (2016).
Turmo, A., Gonzalez-Esquer, C. R. & Kerfeld, C. A. Carboxysomes: metabolic modules for CO2 fixation. FEMS Microbiology Letters 364, fnx176–fnx176 (2017).
Shih, P. M. et al. Biochemical characterization of predicted Precambrian RuBisCO. Nature Communications 7, 10382 (2016).
Way, J. C., Collins, J. J., Keasling, J. D. & Silver, P. A. Integrating Biological Redesign: Where Synthetic Biology Came From and Where It Needs to Go. Cell 157, 151–161 (2014).
Chessher, A., Breitling, R. & Takano, E. Bacterial Microcompartments: Biomaterials for Synthetic Biology-Based Compartmentalization Strategies. ACS Biomaterials Science & Engineering 1, 345–351 (2015).
Young, E. J. et al. Engineering the Bacterial Microcompartment Domain for Molecular Scaffolding Applications. Frontiers in Microbiology 8, 1441 (2017).
Frank, S., Lawrence, A. D., Prentice, M. B. & Warren, M. J. Bacterial microcompartments moving into a synthetic biological world. Journal of Biotechnology 163, 273–279 (2013).
Parsons, J. B. et al. Synthesis of Empty Bacterial Microcompartments, Directed Organelle Protein Incorporation, and Evidence of Filament-Associated Organelle Movement. Molecular Cell 38, 305–315 (2010).
Choudhary, S., Quin, M. B., Sanders, M. A., Johnson, E. T. & Schmidt-Dannert, C. Engineered Protein Nano-Compartments for Targeted Enzyme Localization. PLoS ONE 7, e33342 (2012).
Lawrence, A. D. et al. Solution Structure of a Bacterial Microcompartment Targeting Peptide and Its Application in the Construction of an Ethanol Bioreactor. ACS Synthetic Biology 3, 454–465 (2014).
Slininger Lee, M. F., Jakobson, C. M. & Tullman-Ercek, D. Evidence for Improved Encapsulated Pathway Behavior in a Bacterial Microcompartment through Shell Protein Engineering. ACS Synthetic Biology 6, 1880–1891 (2017).
Plegaria, J. S. & Kerfeld, C. A. Engineering nanoreactors using bacterial microcompartment architectures. Current Opinion in Biotechnology 51, 1–7 (2018).
Fan, C. et al. Short N-terminal sequences package proteins into bacterial microcompartments. Proceedings of the National Academy of Sciences of the United States of America 107, 7509–7514 (2010).
Fan, C. & Bobik, T. A. The N-Terminal Region of the Medium Subunit (PduD) Packages Adenosylcobalamin-Dependent Diol Dehydratase (PduCDE) into the Pdu Microcompartment. Journal of Bacteriology 193, 5623–5628 (2011).
Kinney, J. N., Salmeen, A., Cai, F. & Kerfeld, C. A. Elucidating Essential Role of Conserved Carboxysomal Protein CcmN Reveals Common Feature of Bacterial Microcompartment Assembly. The Journal of Biological Chemistry 287, 17729–17736 (2012).
Jakobson, C. M., Kim, E. Y., Slininger, M. F., Chien, A. & Tullman-Ercek, D. Localization of Proteins to the 1,2-Propanediol Utilization Microcompartment by Non-native Signal Sequences Is Mediated by a Common Hydrophobic Motif. The Journal of Biological Chemistry 290, 24519–24533 (2015).
Jakobson Christopher, M., Slininger Lee Marilyn, F. & Tullman‐Ercek, D. De novo design of signal sequences to localize cargo to the 1,2‐propanediol utilization microcompartment. Protein Science 26, 1086–1092 (2017).
Cameron, J. C., Wilson, S. C., Bernstein, S. L. & Kerfeld, C. A. Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway. Cell 155, 1131–1140 (2013).
Aussignargues, C., Paasch, B. C., Gonzalez-Esquer, R., Erbilgin, O. & Kerfeld, C. A. Bacterial microcompartment assembly: The key role of encapsulation peptides. Communicative & Integrative Biology 8, e1039755 (2015).
Cai, F. et al. Advances in Understanding Carboxysome Assembly in Prochlorococcus and Synechococcus Implicate CsoS2 as a Critical Component. Life 5, 1141–1171 (2015).
Branden, C. I., Lindqvist, Y. & Schneider, G. Protein engineering of rubisco. Acta Crystallographica Section B 47, 824–835 (1991).
Lutz, R. & Bujard, H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Research 25, 1203–1210 (1997).
Bonacci, W. et al. Modularity of a carbon-fixing protein organelle. Proceedings of the National Academy of Sciences of the United States of America 109, 478–483 (2012).
Yeates, T. O., Crowley, C. S. & Tanaka, S. Bacterial Microcompartment Organelles: Protein Shell Structure and Evolution. Annual Review of Biophysics 39, 185–205 (2010).
Lassila, J. K., Bernstein, S. L., Kinney, J. N., Axen, S. D. & Kerfeld, C. A. Assembly of Robust Bacterial Microcompartment Shells Using Building Blocks from an Organelle of Unknown Function. Journal of Molecular Biology 426, 2217–2228 (2014).
Heinhorst, S., Cannon, G. C. & Shively, J. M. In Complex Intracellular Structures in Prokaryotes. (ed. J. M. Shively) 141–165 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2006).
Lawrie, J., Song, X., Niu, W. & Guo, J. A high throughput approach for the generation of orthogonally interacting protein pairs. Scientific Reports 8, 867 (2018).
Tsai, Y., Sawaya, M. R. & Yeates, T. O. Analysis of lattice-translocation disorder in the layered hexagonal structure of carboxysome shell protein CsoS1C. Acta Crystallographica Section D 65, 980–988 (2009).
Iancu, C. V. et al. Organization, Structure, and Assembly of α-Carboxysomes Determined by Electron Cryotomography of Intact Cells. Journal of Molecular Biology 396, 105–117 (2010).
Sambrook, J. F., Russell, D. W. & Editors Molecular cloning: A laboratory manual, third edition. (Cold Spring HarborLaboratory Press, 2000).
So, A. K. C. et al. A Novel Evolutionary Lineage of Carbonic Anhydrase (ɛ Class) Is a Component of the Carboxysome Shell. Journal of Bacteriology 186, 623–630 (2004).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
This work was supported by the New Faculty Startup Fund to W.N. from the Department of Chemical and Biomolecular Engineering in University of Nebraska - Lincoln, the Layman Awards by the Office of Research & Economic Development of University of Nebraska - Lincoln (to W.N.), and by Grant CBET 1438332 (to W.N. and J.G.) from NSF. The authors would like to acknowledge assistance from the following individuals at the University of Nebraska-Lincoln: Dr. You Zhou, (Microscopy Core Research Facility of the Center for Biotechnology) for TEM and confocal microscopy analysis; Drs Sophie Alvarez and Mike Naldrett (Proteomics and Metabolomics Facility of the Center for Biotechnology) for proteomics analysis.
Author information
Authors and Affiliations
Contributions
W.N. and Y.L. designed the study. Y.L., X.H., W.L., J.M., L.K. and W.N. constructed the plasmids, performed protein purifications and conducted pull-down assays. Y.L., J.L. and W.N. purified carboxysomes and conducted immunoprecipitation assays. Y.L. and X.H. conducted ELISA assays. W.N., J.G. and Y.L. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., He, X., Lim, W. et al. Deciphering molecular details in the assembly of alpha-type carboxysome. Sci Rep 8, 15062 (2018). https://doi.org/10.1038/s41598-018-33074-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33074-x
Keywords
This article is cited by
-
Intrinsically disordered CsoS2 acts as a general molecular thread for α-carboxysome shell assembly
Nature Communications (2023)
-
Multivalent interactions between CsoS2 and Rubisco mediate α-carboxysome formation
Nature Structural & Molecular Biology (2020)
-
Encapsulation mechanisms and structural studies of GRM2 bacterial microcompartment particles
Nature Communications (2020)
-
Bacterial microcompartments: catalysis-enhancing metabolic modules for next generation metabolic and biomedical engineering
BMC Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.