Abstract
Cigarette smoke (CS) exposure is one of the primary risk factors associated with the chronic mucous hypersecretion (CMH). The antiapoptotic protein, Bcl-2 sustains hyperplastic mucous cells, and the airway epithelium of ex-smokers with CMH as well as mice exposed to chronic CS showed increased Bcl-2 expression. Therefore, we investigated whether Bcl-2 plays a role in CS-induced mucous expression. Primary airway epithelial cells (AECs) of murine and human origin were treated with CS extract (CSE), and there was a concentration- and time-dependent increase in secretory mucin (MUC5AC), mucous regulator (SPDEF) and Bcl-2 expression. Using differentiated human AECs cultured on air-liquid interface, EGFR and ERK1/2 pathways were interrogated. Bcl-2 activity was blocked using a small molecule BH3 mimetic ABT-263 that disrupts the Bcl-2 interaction with pro-apoptotic proteins. The ABT-263 treatment resulted in the downregulation of CSE-induced mucus expression and disrupted the EGFR-signaling while inducing the apoptosis and the pro-apoptotic protein, Bik expression. This strategy significantly suppressed the mainstream CS-induced mucous phenotype in a 3-D human airway epithelium model. Therefore, the present study suggests that CS induces Bcl-2 expression to help promote mucous cell survival; and small molecule BH3 mimetics targeting Bcl-2 could be useful in suppressing the CS-induced mucous response.
Similar content being viewed by others
Introduction
Airway mucus secretion plays a key role in innate immune responses against inhaled toxicants and pathogens. However, in susceptible population there is abnormally high level of mucus production and accumulation in the airways, specifically in patients suffering from chronic mucus hypersecretion (CMH)1,2. The primary mechanisms associated with CMH are mucus overproduction and hypersecretion by the goblet or mucous cells and the decreased elimination of mucus. CMH prevalence varies from 3.5% to 12.7% in the general population but is much higher (~30%) in individuals with COPD1,3. In CMH patients, the airway epithelial responses are compromised due to dysregulated mucus production, increased mucous cell numbers and ineffective airway clearance1,4. This mucous phenotype is highly exacerbated in patients affected with severe COPD and the poorly controlled CMH leads to airway plugging and reduced lung functions5,6,7,8,9,10. Therefore, understanding the molecular mechanisms responsible for the increased differentiation and proliferation of hyperplastic mucous cells and resulting mucus overexpression and hypersecretion are crucial in developing CMH targeted therapeutics.
Cigarette smoke (CS) exposure is one of the primary risk factors associated with CMH and the debilitating mucus hyperproduction11,12. CS exposure alters the cell fate by affecting the cell proliferation and the cell death pathways13,14,15,16,17. One of the plausible mechanism could involve modulating the levels of Bcl-2, an anti-apoptotic protein that promotes cell survival13,18,19,20. In support of this, we have shown that airway inflammation induces Bcl-2 in airway epithelium and induced Bcl-2 sustains the survival of hyperplastic mucous cells14,15,20,21,22. Furthermore, our recent findings showed that Bcl-2 is one of the main drivers associated with the airway mucous responses14,15,20, therefore, the effect of CS exposure on Bcl-2 expression was investigated in this study. The secretory mucin that is primarily produced by mucous cells in the airway epithelium is MUC5AC, which is induced upon CS exposure and other airway injuries8,23,24. In chronic airway diseases such as COPD and asthma, the debilitating mucus or phlegm production is highly associated with increased numbers of mucous cells with increased mucin synthesis and secretion8 and this pathology is primarily driven by MUC5AC, as shown by a recent study25.
In an animal model of chronic CS exposure, we had observed increased expression of Bcl-2 mRNA in mice exposed to CS for 16 weeks with 4-fold higher number of airway epithelial cells (AECs) showing Bcl-2 immunopositivity in CS-exposed mice compared to air-exposed controls22. More importantly, bronchial biopsies from ex-smokers with CMH showed significantly increased Bcl-2 levels with 5-fold increased immunopositivity compared to control subjects20. Therefore, we investigated the role of Bcl-2 in CS-induced mucous expression using cultured murine and human airway epithelial cells and tested whether targeting Bcl-2 using a small molecule BH3 mimetic compound, ABT-263, could help in modulating CS-induced mucous expression.
Results
CS induces mucus and Bcl-2 levels in a concentration- and time-dependent manner in murine AECs
CS induces mucus production and mucous cell hyperplasia in airway epithelium13,16,26,27, nonetheless, the molecular mechanisms involved in CS-induced mucous expression remain elusive. We analyzed the effect of CS extract (CSE) on primary murine AECs by treating them with 0, 1, 10 and 100 µg/ml of CSE for 24 h. Cells were analyzed for the expression of a secretory mucin, Muc5ac8,28; a master transcriptional regulator of mucous response, Spdef or SAM pointed domain containing ETS transcription factor29; and Bcl-2, a key anti-apoptotic protein that sustains mucous cells14,15,20,21. There was a dose-dependent increase in Muc5ac mRNA levels with significant change following 10 and 100 µg/ml CSE exposure (Fig. 1A). A similar change was observed in Spdef mRNA levels (Fig. 1B), however CSE treatment induced Bcl-2 mRNA levels at all tested concentrations (Fig. 1C). Next, we assessed the expression kinetics of these mRNAs over 0, 3, 24, 48 and 72 h following 10 µg/ml CSE treatment. The Muc5ac mRNA levels were highest at 24 h post CSE treatment (Fig. 1D), and Spdef mRNA levels were increased within 3 h of CSE treatment (Fig. 1E). Bcl-2 mRNA levels peaked at 48 h post CSE exposure (Fig. 1F).
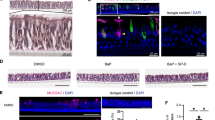
CS exposure induces mucous phenotype and Bcl-2 levels in murine airway epithelial cells (AECs). Primary murine AECs were treated with cigarette smoke extract (CSE) at 0, 1, 10, and 100 µg/ml for 24 h and the mRNA levels of Muc5ac (A) Spdef (B) and Bcl-2 (C) were analyzed by qRT-PCR. Murine AECs treated with 10 µg/ml CSE and cells were harvested at 0, 3, 24, 48 and 72 h and mRNA levels of Muc5ac (D) Spdef (E) and Bcl-2 (F) were quantified. (G) Representative micrographs showing Muc5ac (red) and Spdef (green) expression in murine AECs following CSE treatment in comparison with non-treated (NT) cells. Murine AECs were treated with CSE (10 µg/ml) for 48 h and immunostained for Muc5ac and Spdef, and the nuclei (blue) were stained with DAPI (Scale – 5 µ). (H) Representative micrographs showing Muc5ac (red) and Bcl-2 (green) expression in CSE-treated and treated (NT) murine AECs, nuclei were stained with DAPI (Scale – 5 µ). (I) Quantification of Muc5ac, Spdef and Bcl-2 immunopositive cells following CSE exposure. Approximately 300 cells from each treatment were analyzed to calculate the percentage of Muc5ac-positive (Muc5ac+), Spdef-positive (Spdef+) and Bcl-2-positive (Bcl-2+) cells. Data shown as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01; ***p < 0.001.
Because there was a modest (~2-fold) increase in the mRNA levels, we analyzed the protein expression in murine AECs at 48 h post 10 µg/ml CSE treatment. There was increased expression of Muc5ac, Spdef and Bcl-2 in a subset of murine AECs following 10 µg/ml CSE treatment (Fig. 1G and H). CSE treatment resulted in more than 10-fold higher number of Muc5ac- and Spdef-immunopositive cells compared to non-treated controls, along with 5-fold higher Bcl-2-immunopositive murine AECs (Fig. 1H). Therefore, these results suggest that CSE exposure results in a CSE concentration- and time-dependent increase in Muc5ac, Spdef and Bcl-2 levels in murine AECs.
Concomitant induction of MUC5AC and Bcl-2 by CSE in Human AECs
To characterize the CSE response in human cells, monolayers of human AECs grown in submerged cultured conditions were treated with 0, 1, 10 and 100 µg/ml of CSE for 48 h. There was a dose-dependent increase in MUC5AC (Fig. 2A) and SPDEF (Fig. 2B) mRNA levels, however, CSE treatment induced Bcl-2 mRNA levels at 1 and 10 µg/ml CSE concentration (Fig. 2C). Bcl-2 protein levels examined by western bot analysis showed >4-fold increase following 24 and 48 h of 10 µg/ml CSE treatment (Fig. 2D and E). Specifically, following in-situ immunostaining, all of the MUC5AC-positive cells showed higher Bcl-2 expression at 48 h post 10 µg/ml CSE treatment (Fig. 2F). There were 5-fold higher Bcl-2+ and 6-fold higher MUC5AC+ human AECs following CSE treatment (Fig. 2G). Thus, like murine AECs, CSE exposure resulted in a concomitant increase in both MUC5AC and Bcl-2 expression in human AECs.
CS exposure induces MUC5AC, SPDEF, and Bcl-2 levels in human AEC monolayers. Primary human AECs grown in submerged cultured conditions were treated with 0, 1, 10, and 100 µg/ml of CSE for 24 h and the mRNA levels of MUC5AC (A) SPDEF (B) and Bcl-2 (C) were analyzed by qRT-PCR. (D) Immunoblot analyses of Bcl-2 expression following CSE exposure. Differentiated human AECs were treated with 10 µg/ml CSE and cell lysates were analyzed at 0, 24 and 48 h post CSE exposure by western blot analysis for Bcl-2 protein levels with β-actin levels detected as the loading controls. (E) Relative quantities of Bcl-2 protein as determined by densitometric analysis and normalized to β-actin levels. (F) Representative micrographs showing MUC5AC (green) and Bcl-2 (red) immunopositivity in HAECs treated with CSE or left non-treated (NT). Human AECs were treated with CSE (10 µg/ml) for 48 h and immunostained for Bcl-2 and MUC5AC, and nuclei were stained with DAPI (Scale – 5 µ). (G) Quantification of human AECs immunopositive for MUC5AC and Bcl-2 following CSE exposure. The percentage of human AECs immunopositive for Bcl-2 (Bcl-2+) and MUC5AC (MUC5AC+) were calculated. Data shown as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01; ***p < 0.001.
CSE engages EGFR pathways to induce MUC5AC and Bcl-2 expression
To mimic the in-vivo physiology of AECs, we next analyzed the effect of CSE on air-liquid interface differentiated human AECs. At 48 h post 10 µg/ml CSE treatment, the MUC5AC (Fig. 3A), SPDEF (Fig. 3B) and Bcl-2 (Fig. 3C) mRNA levels were induced by CSE exposure. At protein levels too, there was increased MUC5AC expression (Fig. 3D) with a 4-fold higher number of MUC5AC+ cells at 48 h post 10 µg/ml CSE treatment compared to controls (Fig. 3E). We next investigated the pathways implicated in CS-induced mucus expression by analyzing EGFR (Epithelial Growth Factor Receptor) and ERK1/2 (Extracellular-signal Regulating Kinase 1/2) pathways23,30,31,32. The immunoblot analyses showed increased ERK1/2 phosphorylation at 24 and 48 h post CSE treatment whereas EGFR phosphorylation was observed only at 48 h post CSE treatment (Fig. 3F and G). EGFR mRNA levels were also an increased at 48 h post CSE treatment (Fig. 3H). These data thus suggest that CSE engages EGFR and ERK1/2 pathways to induce mucus and Bcl-2 expression.
CSE engages EGFR and ERK1/2 pathways to induce mucous phenotype and Bcl-2 levels in differentiated human AECs. Primary human AECs differentiated for 3 weeks on air-liquid interface were treated with 10 µg/ml of CSE for 48 h and the mRNA levels of MUC5AC (A), SPDEF (B) and Bcl-2 (C) were analyzed by qRT-PCR. (D) A 3-D representation of a micrograph showing cilia (green) and MUC5AC (red) immunopositivity in differentiated human AECs treated with CSE. Differentiated human AECs were treated with 10 µg/ml CSE for 48 h and were immunostained for acetylated-tubulin (ACT, in green) for cilia and MUC5AC (red), and nuclei were stained with DAPI (Scale – 5 µ). (E) Quantification of MUC5AC immunopositive cells following CSE exposure. Approximately 300 cells from each treatment were analyzed to calculate the percentage of MUC5AC-positive (MUC5AC+) cells. (F) Immunoblot analyses of EGFR and ERK1/2 signaling pathway following CSE exposure. Differentiated human AECs were treated with 10 µg/ml CSE and cell lysates were analyzed at 0, 24 and 48 h post CSE exposure by western blot analysis for phosphorylated ERK1/2, total ERK1/2, phosphorylated EGFR and total EGFR with β-actin levels as the loading controls. (G) Relative quantities of pERK1/2, ERK1/2, pEGFR and EGFR as determined by densitometric analysis where protein quantities were normalized to β-actin levels. (H) Fold-change in EGFR mRNA levels in human AECs treated with 10 µg/ml of CSE for 48 h. Data shown as mean ± SEM (n = 3); *p < 0.05; **p < 0.01; ***p < 0.001.
Small molecule BH3 mimetic, ABT-263, suppresses CSE-induced mucous expression by inducing apoptosis
Bcl-2 levels regulate epithelial cell survival in IL-13- and allergen-induced airway inflammation14 and in the present study, CSE treatment induced both MUC5AC and Bcl-2 expression. Therefore, we reasoned that blocking Bcl-2 might be helpful in restricting the CS-induced mucous response. We had tested various chemical and molecular approaches to inhibit Bcl-2 and found that ABT-263, a small molecule BH3-domain mimetic compound efficiently blocks Bcl-2 activity with fewer off-target effects14. Therefore, we tested the effect of ABT-263 on air-liquid interface differentiated human AECs. Pretreatment with ABT-263 (1uM) significantly suppressed the CSE-induced MUC5AC (Fig. 4A), SPDEF (Fig. 4B) and EGFR (Fig. 4C) mRNA as observed in CSE + ABT treatment group with no discernible effect in ABT-only treated cells. ABT-263 pretreatment suppressed the CSE-induced EGFR pathway by 2-fold in CSE + ABT condition compared to CSE only group and there were no changes in ERK1/2 pathway (Fig. 4D and E).
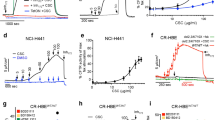
Blocking Bcl-2 with ABT-263 suppresses the CSE-induced mucous phenotype by suppressing EGFR signaling and inducing apoptosis and Bik expression. ABT-263 treatment suppresses CSE-induced MUC5AC (A), SPDEF (B) and EGFR (C) mRNA levels. Differentiated human AECs were treated with 1 µM ABT-263 for 2 h before treating with 10 µg/ml CSE and cells were harvested at 48 h post treatment. (D) Immunoblot analyses of pERK1/2, ERK1/2, pEGFR and EGFR following CSE exposure and ABT-263 treatment of differentiated human AECs. ALI-differentiated human AECs were treated with ABT-263 (1 µM) for 2 h before treating with 10 µg/ml CSE and cells were harvested at 48 h post treatment. (E) Relative quantities of pERK1/2, ERK1/2, pEGFR and EGFR as determined by densitometric analysis with protein quantities normalized to β-actin levels. (F) ABT-263 treatment augments apoptosis in CSE-exposed human AECs. Differentiated human AECs treated with ABT-263, CSE and CSE + ABT were harvested at 48 h post treatment, and analyzed for Annexin V-FITC and propidium iodide staining by Flow cytometry. (G) ABT-263 treatment induces the proapoptotic Bik mRNA levels that are suppressed by CSE exposure. (H) ABT-263 treatment of differentiated murine AECs suppresses the CSE-induced mucous secretory phenotype by modulating cell survival/death pathways. Primary murine AECs differentiated for 3 weeks on ALI were treated with ABT-263 (1 µM) and/or CSE (10 µg/ml) and the mRNA levels of Muc5ac, FoxA3, Egfr and Bik were analyzed by qRT-PCR. Data shown as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01; ***p < 0.001.
Because ABT-263 is a known apoptotic inducer, therefore, we examined the extent of apoptosis in our experimental setting by flow cytometric analysis of the Annexin V and propidium iodide stained cells. There were 4-fold higher apoptotic cells in human AECs pretreated with ABT-263 after CSE exposure (CSE + ABT) compared to CSE- or ABT-alone treated controls (Fig. 4F). Our previous studies have shown that Bik is a key proapoptotic protein in AECs that induces cell death14,16 and therefore, we analyzed the effect of ABT-263 on Bik levels. Compared to CSE treated cells, the Bik mRNA levels were upregulated in CSE + ABT treated cells (Fig. 4G). Similarly, in differentiated murine AECs (Fig. 4H), ABT-263 significantly suppressed the CSE-induced expression of Muc5ac, Egfr and FoxA3, another mucous-regulating transcription factor33. In addition, there were higher Bik mRNA levels in CSE + ABT treated murine AECs (Fig. 4H).
ABT-263 completely blocks CS-induced mucous in 3-D human airway tissue
In order to assess the efficacy of ABT-263 in blocking CS-induced mucous, we analyzed the 3-D EpiAirway tissue culture model (MatTek Corp, Ashland, MA). The 3-D human epithelial airway tissues were exposed to mainstream CS using a SCIREQ smoke machine (Montreal, QC, Canada) for three consecutive days and a group of tissues were treated with 1 µM ABT-263 treatment 2 h before exposures. Smoke exposures caused around 5-fold increase in both MUC5AC (Fig. 5A) and SPDEF (Fig. 5B) mRNA expression which was completely blocked by ABT-263 pretreatment. There was a significant increase in MUC5AC+ cell population (40% cells) following smoke exposure (Fig. 5C and D) that was suppressed in ABT-263 pretreated tissues (15% cells) with no discernible changes in ciliated cell population (Fig. 5C and E). Thus, blocking Bcl-2 by a small molecule BH3 mimetic, ABT-263 suppresses the smoke-induced mucous phenotype without affecting the ciliated epithelial cells.
BH3 mimetic ABT-263 blocks the CS-induced mucous phenotype in 3-D human airway tissue model via inducing apoptosis and Bik expression. 3-D tissue cultures of human airways were exposed to CS using a SCIREQ smoke machine (Montreal, QC, Canada) for 3 consecutive days and one group was treated with 1 µM ABT-263 2 h before exposures. ABT-263 treatment inhibited the CS-induced MUC5AC (A) and SPDEF (B) mRNA expression. (C) Representative micrographs showing cilia (green) and MUC5AC (red) immunopositivity in 3-D airway tissue culture treated with CS and/or ABT-263 compared to non-treated (NT) ones. 3-D airway tissues were immunostained for acetylated-tubulin (ACT, in green) for cilia and MUC5AC (red), and nuclei were stained with DAPI (Scale – 5 µ). Quantification of MUC5AC + mucous cells (D) and ACT + ciliated cells (E) where more than 300 cells from each treatment were analyzed. (F) ABT-263 treatment results in increased TUNEL-positivity in CSE-exposed human AECs. Human AECs treated with ABT-263 (1 µM) and/or CSE (10 µg/ml) were stained for TUNEL (green) and MUC5AC (red). (G) Quantification of human AECs immunopositive for MUC5AC (MUC5AC+) or TUNEL (TUNEL+) or both (MUC5AC+/TUNEL+) following ABT-263 and CSE treatment. (H) ABT-263 increases Bik expression and caspase 3 activation in CSE-exposed AECs. Human AECs were immunostained for Bik (red) and cleaved caspase 3 (CC3, shown in green). (I) Quantification of human AECs immunopositive for Bik (Bik+) or CC3 (CC3+) or both (Bik+/CC3+) following ABT-263 and CSE treatment. Data shown as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01; ***p < 0.001.
ABT-263 induces Bik expression and apoptosis in CSE-induced mucous cells
To determine whether CSE-induced mucous cells were undergoing apoptosis, human AECs pretreated with ABT-263 and exposed to CSE for 48 h were analyzed by TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay and were co-stained for MUC5AC (Fig. 5F). There were >10-fold higher number of TUNEL-positive cells in CSE + ABT treated cells with majority of MUC5AC+ cells showing TUNEL positivity as represented by MUC5AC+TUNEL+ cell counts (Fig. 5G). Next, we analyzed whether Bik expression is associated with the apoptotic AECs by co-staining the CSE + ABT and CSE-treated cells for Bik and activated or cleaved caspase 3 (CC3), a late-stage apoptosis indicator (Fig. 5H). There were higher number of cells expressing Bik (Bik+) in CSE + ABT treatment condition with most of Bik+ cells were undergoing apoptosis as detected by the CC3 immunostaining and are represented as Bik+CC3+ cell counts (Fig. 5I). These data suggest that ABT-263 treatment of CSE exposed cells suppresses mucous expression by upregulating Bik levels and augmenting apoptosis.
Discussion
In this study, we report that CS exposure induces MUC5AC mucin and SPDEF, the mucous master regulator with a concomitant induction in Bcl-2 levels in both human and murine airway epithelial cells. The treatment with a small molecule BH3 mimetic compound, ABT-263, attenuated the CS-induced mucus expression in both human and murine cells. Furthermore, we observed that ABT-263 treatment attenuated the CS-engaged EGFR signaling to help upregulate apoptosis and the proapoptotic Bik levels in order to suppress the CS-induced mucus expression.
CS exposure has been shown to drive chronic mucus production6,11,28,34,35,36 and alters the cell fate by affecting the cell proliferation and the cell death pathways13,16,26,27. During the lung development, a network of transcription factors including thyroid transcription factor-1 (TTF1), forkhead box protein A2 (FOXA2) and A3 (FOXA3), and SPDEF, drive the fate of respiratory airway epithelial cells29,37,38. This cybernetic transcriptional network regulates the proliferation, differentiation and function of airway epithelial cells including mucous, ciliary and basal cells. Among these transcription factors, SPDEF is the important regulator of the growth and differentiation of mucous cells and mucus production29 and its levels are upregulated in airway mucous cells of patients with chronic airway diseases29 and in animal models of allergen exposure17. In our studies using CSE exposure model, both murine and human airway epithelial cells showed a dose-dependent response in SPDEF levels, one of the first reports showing the direct effect of CS on this mucous master regulator. It is notable that human airway epithelial cells were more sensitive to CSE exposure than murine counterparts because 1 µg/ml CSE, the lowest dose tested, significantly induced both MUC5AC and SPDEF levels in humans with no discernable changes in murine airway epithelial cells. Moreover, even at the higher CSE dose there was only modest increase in murine Muc5ac and Spdef expression compared to human AECs. This could be due to the inherent differences between human and murine airway epithelial physiology and the mucous regulatory network as discussed in detail elsewhere8,39. In our previous studies, we observed that murine AECs inherently show moderate mucous response due to a mucous-limiting p53 genotype that negatively affects Bcl-2 mRNA half-life and SPDEF transcriptional activity15. Nonetheless, Bcl-2 seems to be important for mucous expression in both human and murine airway epithelial cells because ABT-263 treatment blocked the mucous expression in cells of both origin. With respect to our studies, the absence of SPDEF in a mice model was shown to attenuate the allergen-induced mucous cell development and mucus production29,40. Similarly, knockdown of SPDEF with small interfering RNA (siRNA) in the human bronchial epithelial cell line was found to significantly reduce the expression of IL-13-induced MUC5AC expression41. Recently, in order to achieve a long-lasting mucus inhibition Song et al. have successfully used epigenetic silencing of SPDEF to downregulating mucous expression in human lung epithelial cells42. These observations together with our data suggest that SPDEF could be a potential therapeutic target for regulating CS-induced mucus hypersecretion and future studies will help determine the feasibility of this proposition.
Cigarette smoke components engage several signaling events that lead to increased differentiation and proliferation of mucous cells leading to chronic mucus production43,44 and here, we observed that EGFR might be involved in CSE-induced mucin expression. In addition, CS induced inflammatory mediators secreted from airway epithelial cells might be providing a feed-forward to the EGFR-mediated mucin expression in an autocrine or paracrine manner because various EGFR ligands are present in AECs in an inactive form45. Furthermore, CS stimulates reactive oxygen species or ROS production that induce TGFα and activation of TNFα-converting enzyme (TACE)-mediated mucin expression30,46,47. Similarly, dual oxidase-1 (DUOX-1) is also expressed by airway epithelium that could activate TACE to engage EGFR-mediated mucin expression48. More importantly, EGFR pathways has been identified as key proinflammatory mediators that aide in epithelial and mucous cell hyperplasia23,49,50,51,52,53 and are associated with the survival of airway epithelial cells in response to a viral challenge49,54. We observed an increased phosphorylation of EGFR following CSE exposure that could be implicated in the subsequent induction of both Bcl-2 and MUC5AC. These findings are consistent with other studies, which report TGFα-induced activation of EGFR mediates Bcl-2 induction52,53. Moreover, the external stimuli including exposure to CS, LPS, ozone, or allergen that upregulate Bcl-2 expression, also activate EGFR in airway epithelial cells47,54. Recently, we reported that when airway epithelial cells were pre-incubated with the specific EGFR and ERK1/2 inhibitors there was a significant suppression of the allergic inflammation induced expression of MUC5AC and Bcl-214. However, in the present study we failed to see any significant changes in ERK1/2 signaling following ABT treatment although CSE exposure caused increased ERK1/2 phosphorylation. There are other ERK-independent pathways engaged by CS and CS-induced ROS to help induce MUC5AC including JNK and AP-1 signal transducers31. Further studies are required to delineate the CSE-engaged EGFR signaling mediator(s) that result in mucus expression specifically the one blocked by BH-3 mimetic compound treatment.
Collectively, the present study suggests that Bcl-2 and MUC5AC may be regulated by identical or overlapping pathways implicating EGFR as one of the upstream mediators. Besides epithelial cell proliferation and survival signaling, EGFR mediates MUC5AC synthesis following exposure to CS-, LPS-, or microbial infection30,55. Therefore, EGFR activation might be one the primary mechanism responsible for the induction of mucous cell differentiation52,53 following CS exposure to help sustain hyperplastic mucous cells. However, therapeutic approaches targeting EGFR receptors face considerable safety and efficacy challenges in clinical trials because COPD subjects poorly tolerated EGFR inhibitor, BIBW 2948, and more so, there was no discernible reduction in mucin production56. Therefore, downstream mediators of EGFR signaling may offer a more effective target for developing novel mucolytic therapeutics.
One such target could be Bcl-2, which is critical for airway inflammation induced mucous cell hyperplasia14,20,21. Bcl-2 is a founding member of a family of proteins that maintain cellular homeostasis by regulating programmed cell death pathways like apoptosis and autophagy18,57. Bcl-2 protects cells against a wide range of cell death stimuli by stabilizing the mitochondrial and endoplasmic reticulum (ER) membrane, and preventing permeabilization and release of death mediators58,59. Bcl-2 is present in the outer mitochondrial and ER membranes, and inactivates BH3-domain consisting pro-apoptotic members of the Bcl-2 family58,59. Here, using a BH3 mimetic compound, ABT-263, which blocks Bcl-2 activity by competing for the BH-3 binding domain with proapoptotic proteins resulting in increased apoptosis; we were able to suppress the CSE-induced mucus expression. Moreover, among the CSE-exposed differentiated airway epithelial cells, there was increased apoptosis in ABT-263-treated ones compared to non-treated controls accompanied by increased Bik levels and reduced EGFR phosphorylation. Bik is the known proapoptotic protein in airway epithelium because Bik-deficient mice fail to resolve allergen-induced mucous expression60. In airway epithelium of patients with chronic airway diseases, there is significant reduction in Bik mRNA compared to controls16. Therefore, ratio of Bik and Bcl-2 levels could be crucial in determining the epithelial and mucous cell fate because blocking Bcl-2 with ABT-263 results in Bik-mediated cell death.
The present study posits several limitations that merit further discussion. Firstly, the CS extract is primarily consisted of the solution phase extract and do not replicate the actual gaseous and particulate exposure conditions. In addition, there are wide variations in the CS extraction methods. In our studies, we employed both organic and aqueous extraction method to prepare the CS extract and observed that 10 µg/ml concentration was optimal to observe mucous response without altering cell growth. This concentration when extrapolated was equivalent to a 100 µg/mm3 of actual CS exposure; the levels comparable to those active smokers get exposed to. Secondly, in this study, we have only observed the effect of ABT-263 in cell culture model and to establish any clinical relevance of our findings, the preclinical animal model testing is required. However, like humans where CS-mediated effects take decades, the mouse models also require longer duration of CS exposure to observe any phenotypic changes in airway responses. From our previous time-course in-vivo studies in a mouse model of CS exposure20, we had observed that only after 16 weeks of exposure there was an observable mucous response. These longer exposure conditions might need a continuous sustained release of ABT-263 to avoid daily treatment and added irritation to animals. We are currently developing a sustained-release regimen of ABT-263 to help conduct these studies. Nonetheless, our in-vitro data using 3-D human airway tissues and differentiated AECs do provide a strong support for the utility of ABT-263 in blocking CS-induced mucous conditions. With the current success of ABT-263 in the treatment of human cancer and other chronic diseases61,62, it will be safer to presume that these BH3 mimetics might find the utility in regulating mucous pathologies associated with chronic airway diseases.
Methods
Cell Culture
Murine AECs were isolated from mouse trachea and cultured on plastic plate or Transwell membranes (Corning, New York, NY) as described previously63. All experimental procedures were carried out in accordance with FIU institutional guidelines and regulations. Briefly, tracheas from C57Bl/6 (Jackson Laboratory) mice were excised, connective tissue was cleared, and tracheas were cut open lengthwise. Cleaned tracheas were incubated in Pronase solution (DMEM, 1.4 mg/mL Pronase, and 0.1 mg/mL DNase) overnight at 4 °C to dissociate AECs from BL. Enzymatic activity was stopped with 10% FBS (Invitrogen, Carlsbad, CA), and cells were collected by gently rocking trachea in DMEM/Ham H12 media (Invitrogen), followed by centrifugation at 400 g for 10 minutes at 4 °C. Cell clumps were dissociated by using 5 mL of declumping solution (DMEM and 2 mmol/L EDTA) and plated at 100,000 cells/well on collagen-coated plates. Primary human AECs were kindly provided by Dr. Scott Randell at the Marsico Lung Institute/Cystic Fibrosis Research Center at the University of North Carolina, Chapel Hill, USA. Lung tissues were procured under protocol #03-1396 approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board; informed consents were obtained from all subjects and AECs were procured as previously described64. The AECs were maintained in bronchial epithelial growth medium (BEGM, Lonza, Walkersville, MD). For air-liquid interface culture, AECs were seeded on Transwell membranes and differentiated for 14 days. Following treatments, the membrane quarters were used for RNA and protein isolation and were fixed in 4% paraformaldehyde for immunostaining. All the methods were performed in accordance with the institutional guidelines and regulations approved by FIU.
Preparation of Cigarette Smoke Extract and Treatment
The cigarette smoke particulate matter from research-reference filtered cigarettes (3R4F; University of Kentucky, KY, USA) collected on Cambridge glass fiber filters were kindly provided by Dr. P. Kuehl (Lovelace Biomedical, Albuquerque, NM). The CS extract amount obtained was determined by weight increase of the filter. CSE was prepared by dissolving the collected smoke particulates in BEGM media and dimethyl sulfoxide (DMSO) to yield a 200 µg/ml (w/v) solution and aliquots were stored at −80 °C.
Cigarette Smoke Exposure
The 3-D EpiAirway tissue cultures (MatTeck Corp, Ashland, MA) were exposed to mainstream CS using a SCIREQ smoke machine (Montreal, QC, Canada). Four 3R4F research-reference filtered cigarettes (University of Kentucky) were smoked with a puff volume of 35 ml per 2 sec for every minute and blown over tissue culture rate of 5 ml/min in basic conformity with ISO 3308 (International Organization for Standardization, 2012a). Total 32 puffs were recorded for a duration of approximately 35 minutes of exposure per day. Smoke exposures were performed for 3 consecutive days with1 µM ABT-263 treatment 2 h before exposures and tissues were analyzed at 48 h post last exposure.
Immunostaining and Fluorescent Imaging Analysis
The murine and human AECs grown on Labtek-II slides (ThermoFisher Inc.) were fixed in 4% paraformaldehyde and washed in 0.05% v Brij-35 in PBS (pH 7.4) and immunostaining was performed as described previously14. Briefly, the cells were blocked using a solution containing 3% BSA, 1% Gelatin and 1% normal donkey serum with 0.1% Triton X-100 and 0.1% Saponin and were stained with antibodies to MUC5AC (Millipore Inc., Burlington, MA), Spdef (Santa Cruz Biotech, Dallas, TX), Bcl-2 (Santa Cruz Biotech, Dallas, TX), Bik (Abcam, Cambridge, MA) and cleaved caspase 3 (Cell Signaling Tech., Danvers, MA) or isotype controls. The immunolabelled cells were detected using respective secondary antibodies conjugated fluorescent dyes (Jackson ImmunoResearch Lab Inc., West Grove, PA) and mounted with 4′,6-diamidino-2-phenylindole (DAPI) containing Fluormount-GTM (SouthernBiotech, Birmingham, AL) for nuclear staining. Immunofluorescent images were captured using BZX700 Microscopy system (Keyence Corp., Japan) and analyzed using NIH Image J software.
Flowcytometric Quantification of Apoptosis
Apoptotic cells were quantified by fixing cells in 2% paraformaldehyde and staining with Annexin V-FITC conjugate (BD Biosciences Inc., San Jose, CA) and propidium iodide (Sigma-Aldrich Inc., St. Louis, MO) for 30 min at 4 °C. Stained cells were washed and resuspended in 0.2% BSA/PBS and nearly 10,000 cells per sample were analyzed using BD FACS Canto® Flow Cytometer (BD Biosciences Inc., San Jose, CA) and the data was analyzed using FlowJo analysis software (Tree Star Inc., Ashland, OR).
Quantitative Real-Time RT-PCR
Total RNA was isolated from the snap-frozen cells using RNAeasy kit (Qiagen, Germantown, MD) as per manufacturer’s instruction. RNA concentration was determined using the Synergy HTX Multi-Mode reader (BioTek, Winooski, VT) and cDNA were synthesized using iScript advanced cDNA kit (BioRad, Hercules, CA). The primer/probe sets for MUC5AC, SPDEF, FOXA3, and Bcl-2 were obtained either from BioRad (Hercules, CA) or Qiagen (Germantown, MD) and cDNA amplified by q-PCR using the iTaq SYBR-green Master Mix (BioRad, Hercules, CA) in the Agilent Stratagene Mx3000P Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). Relative quantities were calculated by normalizing averaged CT values to GAPDH or β-Actin to obtain ΔCT, and the fold-change (ΔΔCT) over the controls were determined as described previously15.
Western Blot Analysis
Cell extracts were prepared using RIPA buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 1% NP-40, 0.25% Deoxycholate, 0.1% SDS, 1 mM EDTA and 1% protease inhibitor cocktail). Protein concentration was determined by BCA kit (Pierce; Rockford, IL) and 50 µg protein was analyzed by western blotting as described previously15. Antibodies to Bcl-2, EGFR, p-EGFR, ERK1/2 and p-ERK1/2 were all from Cell signaling technologies (Danvers, MA) and β-actin antibody was from Sigma co. (St. Louis, MO). Proteins were detected using ECL and visualized by chemiluminescence (Perkin Elmer, Waltham, MA) using the BioRad Chemidoc Imaging system (Hercules, CA). The full-length immunoblots are available online as supplemental information.
In-Situ Apoptosis Detection by TUNEL
Paraformaldehyde-fixed cells were washed in 0.05% v Brij-35 in PBS (pH 7.4) and processed for TUNEL labelling as per manufacturer’s instruction using TACS•XL® In-Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD). The TdT-labelled cells were detected using fluorescently-conjugated secondary antibodies (Jackson ImmunoResearch Lab Inc., West Grove, PA). The cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) containing Fluormount-G (SouthernBiotech, Birmingham, AL) to visualize nuclei. Images were captured with BZX700 Microscopy system (Keyence Corp., Japan) and analyzed by NIH Image J software.
Statistical Analysis
Grouped results were expressed as means ± SEM. Data were analyzed using GraphPad Prism Software (GraphPad Software, Inc., San Diego, CA). Grouped results were analyzed using two-way analysis of variance. When significant main effects were detected (P < 0.05), Fishers least significant difference test was used to determine differences between groups.
References
Kim, V. & Criner, G. J. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187, 228–237 (2013).
Button, B., Anderson, W. H. & Boucher, R. C. Mucus Hyperconcentration as a Unifying Aspect of the Chronic Bronchitic Phenotype. Ann Am Thorac Soc 13 S 2, S156–162 (2016).
de Oca, M. M. et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 40, 28–36 (2012).
Burgel, P. R. & Martin, C. Mucus hypersecretion in COPD: should we only rely on symptoms? Eur Respir Rev 19, 94–96 (2010).
Lumsden, A. B., McLean, A. & Lamb, D. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax 39, 844–849 (1984).
Prescott, E., Lange, P. & Vestbo, J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 8, 1333–1338 (1995).
Saetta, M. et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 161, 1016–1021 (2000).
Fahy, J. V. & Dickey, B. F. Airway mucus function and dysfunction. N Engl J Med 363, 2233–2247 (2010).
Aikawa, T., Shimura, S., Sasaki, H., Ebina, M. & Takishima, T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101, 916–921 (1992).
Evans, C. M., Kim, K., Tuvim, M. J. & Dickey, B. F. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15, 4–11 (2009).
David, G. L., Koh, W. P., Lee, H. P., Yu, M. C. & London, S. J. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax 60, 1052–1058 (2005).
Domagala-Kulawik, J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 59(Suppl 6), 19–34 (2008).
Nyunoya, T. et al. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am J Respir Cell Mol Biol 50, 471–482 (2014).
Chand, H.S., Mebratu, Y.A., Kuehl, P.J. & Tesfaigzi, Y. Blocking Bcl-2 resolves IL-13-mediated mucous cell hyperplasia in a Bik-dependent manner. J Allergy Clin Immunol (2017).
Chand, H. S. et al. A genetic variant of p53 restricts the mucous secretory phenotype by regulating SPDEF and Bcl-2 expression. Nat Commun 5, 5567 (2014).
Mebratu, Y. A., Schwalm, K., Smith, K. R., Schuyler, M. & Tesfaigzi, Y. Cigarette smoke suppresses Bik to cause epithelial cell hyperplasia and mucous cell metaplasia. Am J Respir Crit Care Med 183, 1531–1538 (2011).
Park, J. W., Ryter, S. W. & Choi, A. M. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD 4, 347–353 (2007).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15, 49–63 (2014).
Strasser, A., Cory, S. & Adams, J. M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 30, 3667–3683 (2011).
Chand, H. S. et al. Intracellular insulin-like growth factor-1 induces Bcl-2 expression in airway epithelial cells. J Immunol 188, 4581–4589 (2012).
Harris, J. F. et al. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am J Respir Crit Care Med 171, 764–772 (2005).
Chand, H.S., Woldegiorgis, Z., Schwalm, K., McDonald, J. & Tesfaigzi, Y. Acute Inflammation Induces IGF-1 to Mediate Bcl-2 and Muc5ac Expression in Airway Epithelial Cells. Am J Respir Cell Mol Biol (2012).
Takeyama, K. et al. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 280, L165–172 (2001).
Rose, M. C. & Voynow, J. A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86, 245–278 (2006).
Kesimer, M. et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl J Med 377, 911–922 (2017).
Barrett, E. G., Wilder, J. A., March, T. H., Espindola, T. & Bice, D. E. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med 165, 1410–1418 (2002).
Simet, S. M. et al. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am J Respir Cell Mol Biol 43, 635–640 (2010).
Nadel, J. A. Mucous hypersecretion and relationship to cough. Pulm Pharmacol Ther 26, 510–513 (2013).
Chen, G. et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119, 2914–2924 (2009).
Shao, M. X., Nakanaga, T. & Nadel, J. A. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol 287, L420–427 (2004).
Gensch, E. et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem 279, 39085–39093 (2004).
Xiao, J. et al. Role of extracellular signal-regulated kinase 1/2 in cigarette smoke-induced mucus hypersecretion in a rat model. Chin Med J (Engl) 124, 3327–3333 (2011).
Chen, G. et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189, 301–313 (2014).
Evans, C. M. & Koo, J. S. Airway mucus: the good, the bad, the sticky. Pharmacol Ther 121, 332–348 (2009).
Waked, M., Salame, J., Khayat, G. & Salameh, P. Correlates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional study. Int J Chron Obstruct Pulmon Dis 7, 577–585 (2012).
Ghosh, R. et al. Air pollutants, genes and early childhood acute bronchitis. Mutat Res 749, 80–86 (2013).
McCauley, H. A. & Guasch, G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 21, 492–503 (2015).
Wang, G. et al. Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers. BMC Med Genomics 5, 21 (2012).
Thai, P., Loukoianov, A., Wachi, S. & Wu, R. Regulation of airway mucin gene expression. Annu Rev Physiol 70, 405–429 (2008).
Rajavelu, P. et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 125, 2021–2031 (2015).
Yu, H., Li, Q., Kolosov, V. P., Perelman, J. M. & Zhou, X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes 17, 83–92 (2010).
Song, J. et al. Targeted epigenetic editing of SPDEF reduces mucus production in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 312, L334–L347 (2017).
Evans, C. M. et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun 6, 6281 (2015).
Kim, V. et al. Small airway mucous metaplasia and inflammation in chronic obstructive pulmonary disease. COPD 5, 329–338 (2008).
Citri, A. & Yarden, Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7, 505–516 (2006).
Khan, E. M., Lanir, R., Danielson, A. R. & Goldkorn, T. Epidermal growth factor receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. Faseb J 22, 910–917 (2008).
Baginski, T. K., Dabbagh, K., Satjawatcharaphong, C. & Swinney, D. C. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol 35, 165–174 (2006).
Shao, M. X. & Nadel, J. A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J Immunol 175, 4009–4016 (2005).
Tyner, J. W. et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116, 309–321 (2006).
Hua, Z. et al. Requirement for MyD88 signaling in B cells and dendritic cells for germinal center anti-nuclear antibody production in Lyn-deficient mice. J Immunol 192, 875–885 (2014).
Takeyama, K. et al. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 164, 1546–1552 (2000).
Takeyama, K., Tamaoki, J., Kondo, M., Isono, K. & Nagai, A. Role of epidermal growth factor receptor in maintaining airway goblet cell hyperplasia in rats sensitized to allergen. Clin Exp Allergy 38, 857–865 (2008).
Casalino-Matsuda, S. M., Monzon, M. E. & Forteza, R. M. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 34, 581–591 (2006).
Alevy, Y. G. et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest 122, 4555–4568 (2012).
Kanai, K. et al. Cigarette smoke augments MUC5AC production via the TLR3-EGFR pathway in airway epithelial cells. Respiratory investigation 53, 137–148 (2015).
Woodruff, P. G. et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181, 438–445 (2010).
Delbridge, A. R., Grabow, S., Strasser, A. & Vaux, D. L. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nature reviews 16, 99–109 (2016).
Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL-2 family reunion. Mol Cell 37, 299–310 (2010).
Levine, B., Sinha, S. & Kroemer, G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4, 600–606 (2008).
Mebratu, Y. A., Dickey, B. F., Evans, C. & Tesfaigzi, Y. The BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of activated ERK1/2 to mediate IFNgamma-induced cell death. J Cell Biol 183, 429–439 (2008).
Kipps, T. J. et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma 56, 2826–2833 (2015).
Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci Transl Med 9 (2017).
You, Y., Richer, E. J., Huang, T. & Brody, S. L. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 283, L1315–1321 (2002).
Fulcher, M. L. & Randell, S. H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 945, 109–121 (2013).
Acknowledgements
The authors thank Drs H. Unwalla and S. Chinnapaiyan for their help with CS exposures using SCIREQ machine; and Ruben Castro, Suvarna Prasad and Emellyn Arellano for assistance on the selected experiments. UNC CF Tissue Procurement and Cell Culture Core is supported by NIH P30DK065988 and CF Foundation BOUCHE15R0 grants. The work has been supported by NIH R21 AI117560 (H.S.C) and American Lung Association RG306208 (H.S.C.).
Author information
Authors and Affiliations
Contributions
S.S.H. conducted the experiments, analyzed the data and wrote the manuscript; S.G. and S.S. conducted the experiments and analyzed the data; R.J., C.-A.H. and M.S. analyzed the data; H.S.C. designed the studies, analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, S.S., George, S., Singh, S. et al. A Small Molecule BH3-mimetic Suppresses Cigarette Smoke-Induced Mucous Expression in Airway Epithelial Cells. Sci Rep 8, 13796 (2018). https://doi.org/10.1038/s41598-018-32114-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32114-w
Keywords
This article is cited by
-
Comparative transcriptomics in human COPD reveals dysregulated genes uniquely expressed in ferrets
Respiratory Research (2022)
-
Differential gene expression of 3D primary human airway cultures exposed to cigarette smoke and electronic nicotine delivery system (ENDS) preparations
BMC Medical Genomics (2022)
-
Acetylcholinesterase Inhibitor Pyridostigmine Bromide Attenuates Gut Pathology and Bacterial Dysbiosis in a Murine Model of Ulcerative Colitis
Digestive Diseases and Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.