Abstract
Parkinson’s disease (PD) alters cortico-basal ganglia-thalamic circuitry and susceptibility to an illusion of bodily awareness, the Rubber Hand Illusion (RHI). Bodily awareness is thought to result from multisensory integration in a predominantly cortical network; the role of subcortical connections is unknown. We studied the effect of modulating cortico-subcortical circuitry on multisensory integration for bodily awareness in 24 PD patients treated with subthalamic nucleus (STN) deep brain stimulation (DBS), in comparison to 21 healthy volunteers, using the RHI experiment. Typically, synchronous visuo-tactile cues induce a false perception of touch on the rubber hand as if it were the subject’s hand, whereas asynchronous visuo-tactile cues do not. However, we found that in the asynchronous condition, patients in the off-stimulation state did not reject the RHI as strongly as healthy controls; patients’ rejection of the RHI strengthened when STN-DBS was switched on, although it remained weaker than that of controls. Patients in the off-stimulation state also misjudged the position of their hand, indicating it to be closer to the rubber hand than controls. However, STN-DBS did not affect proprioceptive judgements or subsequent arm movements altered by the perceptual effects of the illusion. Our findings support the idea that the STN and subcortical connections have a key role in multisensory integration for bodily awareness. Decision-making in multisensory bodily illusions is discussed.
Similar content being viewed by others
Introduction
Bodily awareness is thought to result from the integration of multiple sensory cues with an internal sense of time and body image1. Much of the evidence for this theory comes from Rubber Hand Illusion (RHI). In the classic RHI experiment, synchronous visual and tactile cues (from stroking the subject’s hidden hand and a visible rubber hand) typically induce a false perception of touch on the rubber hand as if it were the subject’s hand, whereas asynchronous visual and tactile cues do not elicit this illusion (measured by questionnaire)2. Also, subjects mislocalize their unseen hand closer to the position of the rubber hand in the synchronous condition compared with the asynchronous condition (measured as “proprioceptive drift”). fMRI studies of the RHI have implicated prefrontal, premotor and intraparietal cortices and the cerebellum in a neural network for multisensory integration in body awareness3,4,5. However, little is known about the subcortical structures that may underpin this network.
People with Parkinson’s disease (PD) have a similar RHI experience to healthy controls in the synchronous condition but fail to reject the RHI experience as strongly in the asynchronous condition6. PD patients also have increased proprioceptive drift towards the rubber hand independent of visual-tactile synchrony6. The pathophysiological basis of PD is striatal dopaminergic degeneration resulting in altered cortico-basal ganglia-thalamic circuitry7. PD is treated with dopaminergic drugs and high-frequency deep brain stimulation (DBS) of the subthalamic nucleus (STN) or globus pallidus interna (GPi), which is thought to work by modulating cortico-basal ganglia-thalamic circuitry8. Previously, we found that dopaminergic drugs did not affect RHI experience or proprioceptive drift6. This study aims to explore possible subcortical involvement in bodily awareness by examining the effect of STN-DBS on the RHI in people with PD.
Based on our previous findings in PD patients without STN-DBS6, we hypothesized that STN-DBS treated patients would also have: first, higher illusion scores on the RHI questionnaire than controls in the asynchronous condition, with no differences in the synchronous condition, and second, greater proprioceptive drift than controls in both stroking conditions.
Our third hypothesis was that when STN-DBS is switched on, patients will have lower illusion scores in the asynchronous condition compared with when STN-DBS is switched off. Rather than being all-or-nothing, the subjective RHI experience exists on a spectrum—it is strongest when temporal mismatch between visual and tactile cues is less than 300 ms, weakens with increasing mismatch, and is eliminated when mismatch is greater than 600 ms9. Therefore, noisy or inaccurate temporal processing of visual and tactile cues in PD could weaken the asynchronous signal, resulting in the higher illusion scores reported in our previous study. PD patients have well-characterised temporal deficits, including increased somatosensory temporal discrimination thresholds10 and inaccurate estimation of temporal inter-stimulus intervals11,12. STN-DBS improves temporal estimation of auditory intervals in PD13,14. Extrapolating from these studies, STN-DBS could potentially improve perception of the asynchronous signal and hence “normalize” illusion scores in the asynchronous condition.
Our fourth hypothesis was that switching STN-DBS on would decrease proprioceptive drift in both synchronous and asynchronous conditions. Although proprioceptive drift was initially considered a behavioural correlate of subjective experience2, the two phenomena can be dissociated; just viewing a rubber hand in peri-personal space produces proprioceptive drift similar to that of the synchronous stroking condition, without evoking the subjective RHI15. Rohde et al.15 proposed that ‘visual capture of proprioception’ (visuo-proprioceptive integration and spatial recalibration) produces proprioceptive drift and is attenuated by asynchronous visuo-tactile cues. PD patients have well-characterised proprioceptive deficits16,17 and increased dependence on vision18,19, which may make them more susceptible to visual capture, resulting in the increased proprioceptive drift reported in our previous study6. STN-DBS improves proprioceptive accuracy in kinaesthesia20 and proprioceptive-tactile integration in haptic perception21 in PD patients, and may help to “normalize” their proprioceptive drift. To differentiate the effect of STN-DBS implantation surgery on the RHI from that of STN stimulation itself, we tested eligible subjects before and after they underwent implantation surgery.
Lastly, we included a reaching task at the end of each RHI trial to assess the influence of somatic illusions on movement. For healthy subjects, the literature is mixed on whether the RHI affects the trajectory of reaching tasks22,23,24 or pointing accuracy25,26. We had expected the RHI to have a greater influence on the subsequent actions of PD patients than controls because patients have proprioceptive deficits that compromise reaching accuracy27. We found no such effect in either patients or controls in our previous study6. However, most of those patients were mildly affected. The STN-DBS-treated PD patients in this study have more advanced disease. Our fifth hypothesis, therefore, was that the reach-to-grasp performance of STN-DBS-treated patients would differ between the synchronous and asynchronous condition, in addition to the expected improvement in motor function from STN-DBS27,28.
Methods
Participants
We recruited 24 patients with idiopathic PD treated with STN-DBS from our Movement Disorders Clinic, excluding those with clinically significant sensory or cognitive deficits. 12 of these patients had only postoperative testing. The other 12 patients had STN-DBS implantation surgery within the time frame of this study and were opportunistically tested pre- and postoperatively. Preoperative data from eight of these 12 patients and data from 21 healthy controls (aged 50–80) in this study has been published as part of a previous study6. Participant characteristics are shown in Table 1. Compared with the PD patients in this study, controls were older and showed less symptomatology on the Hospital Anxiety and Depression Scale (HADS) and the Apathy Scale, but Montreal Cognitive Assessment (MoCA) scores were similar. This study was approved by the Monash Health Research Ethics Committee (HREC 12350B) and carried out in accordance with national ethical guidelines. All subjects gave written informed consent.
For the entire patient cohort, the median disease duration from diagnosis was 13.5 years (IQR 10–20 years). The median duration of STN-DBS treatment was 16.5 months (IQR 7–38 months) with a minimum of 5 months. Intraoperative semi-microelectrode recordings were performed to guide placement within the anatomical boundaries of the STN. Postoperatively, all patients had a CT brain scan within 24 hours, which was then fused with their preoperative MRI brain scan to confirm lead placement within the STN (Fig. 1A). A neurologist (CD) assessed motor severity (on- and off-stimulation) using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale Part III (UPDRS).
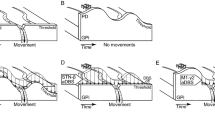
(A) Example postoperative CT fused with the preoperative MRI (sagittal 3D FLAIR sequences with 1 mm slices obtained on a Siemens Verio 3 Tesla unit). Orthogonal planes intersecting through a right Medtronic 3387 lead are shown (S: superior; I: inferior; L: left; R: right; A: anterior; P: posterior). White dots map the subthalamic nucleus (STN) and substantia nigra (SN) according to intraoperative semi-microelectrode recordings. (B) Graphical timeline of a trial: 1. Subject verbally indicates their pre-stroking estimate of middle finger position, 2. Investigator either synchronously or asynchronously (randomised) strokes the subject’s hand (shaded = hidden) and a visible rubber hand, 3. Subject verbally indicates their post-stroking estimate of middle finger position, 4. Subject reaches for a cylinder now illuminated in the far corner of the lateral compartment. At the end of each trial, the subject fills in the Rubber Hand Illusion questionnaire (not pictured). (C) Graphical timeline of the study. Pre-DBS implantation surgery, patients were tested in on and off-medication states. Post-DBS implantation surgery, patients were tested in on and off-stimulation states. Controls were also tested twice. (B) is from Ding, C. et al.6 Parkinson’s disease alters multisensory perception: Insights from the Rubber Hand Illusion, Neuropsychologia by Elsevier Science Ltd. Reproduced with permission of Elsevier Science Ltd in the format Journal/magazine via Copyright Clearance Center.
In the 12 subjects with pre-operative testing, the median interval between pre- and postoperative testing was 12.5 months (IQR 9.75–17.25 months). Although subjects took less dopaminergic medication postoperatively (preoperative levodopa equivalent daily dose (LEDD): Mdn (IQR) = 1350 (1090–1973); postoperative LEDD: Mdn (IQR) = 417 (0–508), V = 75, p = 0.005), there was no difference in motor severity in respective on-treatment states (preoperative on-medication UPDRS: Mdn (IQR) = 15 (11.5–16); postoperative on-stimulation UPDRS: Mdn (IQR) = 14 (9.25–16.25), V = 22, p = 1) or off-treatment states (preoperative off-medication UPDRS: Mdn (IQR) = 31.5 (18–28); postoperative off-stimulation UPDRS: Mdn (IQR) = 33 (22.5–41), V = 25.5, p = 0.878). Implantation surgery did not significantly affect scores on the MoCA, HADS or Apathy Scale (Supplementary Table A).
Experiment design
The RHI set-up used in this study is detailed in our previous paper6 and the graphical timeline of a trial is reproduced in Fig. 1B. Briefly, subjects sat in front of a black box with three compartments and a dark glass top that allowed them to view the contents of each compartment only when individually illuminated. In each trial, the hand being tested was placed in the corresponding lateral compartment, while an identically aligned rubber hand was placed in the central compartment. Each trial began with the subject making a ‘baseline estimate’ of the position of their unseen middle finger. They did this by verbally directing the investigator to move a marker along the length of the box until they were satisfied that the marker was directly in front of their middle finger. Then, the central compartment was illuminated to reveal the rubber hand. The subject was instructed to watch the rubber hand as the investigator stroked it alongside their hidden hand by using two identical paint brushes (brush area 0.5 cm × 2 cm). In the synchronous condition, the experimenter stroked corresponding fingers on the real and rubber hands in unison. In the asynchronous condition, the experimenter alternately stroked non-corresponding fingers on the real and rubber hands (i.e., such that there was both temporal and spatial asynchrony between the seen and felt touch). Using a timer, we varied the temporal interval in the asynchronous condition between 1 and 3 s. After two minutes of stroking, the light was switched off to hide the rubber hand once more. The subject then made a verbal ‘post-stroking estimate’ of the position of their hidden middle finger. Next, a cylinder in the far corner of the lateral compartment was illuminated, and the subject was instructed to touch the cylinder. Their hand was hidden from view until it neared the cylinder. At the end of each trial, the subject took their hand out of the box and completed a RHI questionnaire that assessed their perceptual experiences during that trial.
A testing session comprised two trials of the synchronous condition and two trials of the asynchronous condition in randomised order. Patients were tested on the more severely affected hand; in controls the hand tested was matched to patients, ensuring each group had the same number of left and right-sided trials. All subjects completed at least two testing sessions Fig. 1C. For STN-DBS patients, one session commenced after stimulation had been switched off for 30 min (‘off-stimulation’) while the other session commenced after stimulation had been switched on at usual settings for at least 30 min. In a counterbalanced, crossover design, half the STN-DBS patients were tested on-stimulation first, while the other half were tested in the reverse order. Patients took their usual anti-Parkinson medication in the on- and off-stimulation states.
To make a comprehensive comparison of pre- and postoperative on and off-states achieved by medication and STN-DBS respectively, in randomised order, we aimed to test the 12 preoperative patients whilst they were taking their usual dopaminergic drugs (‘on-medication’) and after all dopaminergic drugs had been withheld for at least 12 hours (‘off-medication’). Due to the timing of their operations, two patients could not be tested ‘on-medication’.
Measures
We recorded three outcome measures from each trial: (1) questionnaire responses (2) proprioceptive drift, and (3) reach movement metrics.
Questionnaire responses
Subjects completed an 11 statement RHI questionnaire used in previous studies6,24, comprising three critical (‘illusion’) statements designed to elicit the subjective strength of the RHI, intermixed with eight control (‘mock’) statements designed to detect response biases (Supplementary Table B). Subjects marked their response on a 14 cm long visual analogue scale with “Strongly disagree” at 0 cm, “Strongly agree” at 14 cm and “Very unsure whether to agree or disagree” in the middle. Thus, higher illusion scores quantitatively capture endorsement of the RHI, while lower illusion scores quantify rejection of the RHI.
Proprioceptive drift
We measured proprioceptive drift as the difference between the subject’s ‘baseline’ and ‘post-stroking’ estimates of middle finger position. We also assessed proprioceptive (in)accuracy as the difference between the ‘baseline’ estimate and the actual position of their middle finger. For both proprioceptive drift and baseline proprioceptive inaccuracy, positive measurements indicate the medial direction (towards the position of the rubber hand), whereas negative measurements indicate the lateral direction.
Reach movement metrics
We recorded reach trajectories using a magnetic 3D motion tracker and a sensor attached to the subject’s thumbnail. We defined the reaching movement as the period starting from when the velocity of the sensor first exceeded 20 mm/s, to when it finally slowed below 20 mm/s. We then calculated seven reach metrics: 1. duration of movement, 2. mean velocity of movement, 3. peak velocity of movement, 4. time to reach peak velocity, 5. peak lateral displacement, 6. initial angle of movement (the angle of instantaneous velocity from the midline when 10% of the movement was completed) and 7. integrated jerk (the time derivative of acceleration integrated over the course of the movement).
Statistical analysis
To compare STN-DBS patients in ‘on-stimulation’ and ‘off-stimulation’ states with controls, we estimated mixed-effects regression models for questionnaire responses and proprioceptive drift using fixed effects for ‘subject group’ (controls vs. PD on-stimulation vs. PD off-stimulation), ‘stroking condition’ (synchronous vs. asynchronous) and their interaction. We estimated random effects for ‘stroking condition’ nested within subjects. Questionnaire data (visual analogue scores) was bound between 0 and 14 and thus treated as the odds of a binary response between strongly disagree and strongly agree. We used logistic regression on averaged responses to mock and illusion statements in each trial, estimating additional fixed and random effects for the statement type (‘mock’ vs. ‘illusion’). Linear regression was appropriate for proprioceptive drift data. We applied Bonferroni corrections for multiple comparisons to posthoc contrasts. We controlled for age, sex, cognition (MoCA), mood (HADS) and apathy (Apathy Scale) as potential covariates in both models. We also controlled for baseline proprioceptive inaccuracy in the proprioceptive drift model.
To evaluate the effect of STN-DBS implantation surgery on the RHI, in the subset of patients who had preoperative testing, we compared their outcome measures in four ‘treatment states’ (pre-op off-medication, pre-op on-medication, post-op off-stimulation, post-op on-stimulation). We estimated additional mixed effects models of questionnaire responses and proprioceptive drift, using the fixed effects of ‘treatment state’, ‘stroking condition’ and their interaction. Random effects for questionnaire response and proprioceptive drift models were as described above. We controlled for the duration of disease, motor severity (UPDRS) and LEDD in both models, as well as baseline proprioceptive inaccuracy in the proprioceptive drift model.
To evaluate the effect of the RHI on movement in the STN-DBS patients, we estimated linear mixed effects models for each of the seven reach movement metrics, using fixed effects of side-of-test, ‘stroking condition’, ‘stimulation state’ (on vs. off), and their interactions. Random effects were estimated for stimulation state nested within subjects. We controlled for motor severity (UPDRS) as a potential covariate. Statistical analysis was performed in R29, using packages ‘lme4’30, ‘nlme’31, ‘Effects’32,33, ‘Phia’34, and ‘Stargazer’35.
The datasets for the current study are available in the figshare repository, https://doi.org/10.6084/m9.figshare.5662219.v1.
Results
Subject excluded
One PD patient (who was studied preoperatively) had a unique postoperative RHI response wherein he strongly endorsed illusion statements in the asynchronous condition but not in the synchronous condition. When questioned at the conclusion of testing, he stated that in the synchronous trials he was watching his hand being stroked rather than a rubber hand! This response was not seen in any other subjects in our study, and to our knowledge, has not been reported in the RHI literature. Because of doubt over the validity of his questionnaire responses, we excluded him from all analysis.
Questionnaire data
All subjects
As expected, all subject groups reported a stronger RHI in the synchronous condition compared with the asynchronous condition, which was reflected in the illusion statements but not the mock statements. Regression analysis of questionnaire responses showed a three-way interaction between ‘statement type’, ‘stroking condition’ and ‘subject group’ (χ2 = 37.35, p < 0.0001). Examination of contrasts revealed that all subject groups were more likely to agree with illusion statements in the synchronous condition than in the asynchronous condition (controls: χ2 = 156.67, p < 0.0001; PD off-stimulation: χ2 = 33.49, p < 0.0001; PD on-stimulation: χ2 = 68.38, p < 0.0001). By contrast, agreement with mock statements did not differ between stroking conditions or amongst subject groups (all p > 0.05).
Focusing on the illusion statements (Fig. 2A), PD patients did not reject the RHI in the asynchronous condition as strongly as controls (PD off-stimulation: χ2 = 34.18, p < 0.0001; PD on-stimulation: χ2 = 12.26, p < 0.006), which is consistent with our first hypothesis. In the asynchronous condition, patients had lower illusion scores in the on-stimulation state compared with the off-stimulation state (χ2 = 20.02, p < 0.0001), which is consistent with our third hypothesis that switching on STN-DBS strengthens rejection of the RHI experience in the asynchonous condition. By contrast, in the synchronous condition, endorsement of the RHI was similar between controls and patient on- or off-stimulation (all p > 0.05). Age, sex, cognition, mood and apathy were not significant covariates and thus excluded from the final model (Supplementary Table C).
Scoring of critical Rubber Hand Illusion statements in (A) 23 Parkinson’s disease patients treated with deep brain stimulation of the subthalamic nucleus (in on-stimulation and off-stimulation states) and 21 healthy controls and (B) a subset of 11 Parkinson’s disease patients studied prior to STN-DBS implantation surgery (pre-op) whilst taking their usual anti-Parkinson drugs (‘on-medication’, n = 9), and after withholding all anti-Parkinson drugs (‘off-medication’, n = 11). Thick line: median, hinge: IQR, whisker: hinge ± 1.5*IQR, ◦: outlier, async: asynchronous, sync: synchronous. P-values are based on the regression model, ****p < 0.0001, **p < 0.01, *p < 0.05. All unlabelled contrasts were not significant.
Subset of PD patients studied preoperatively
The preoperative subset of 11 patients had similar results to the larger patient cohort; regression analysis of questionnaire responses showed a three-way interaction between ‘statement type’, ‘stroking condition’ and ‘treatment state’ (χ2 = 19.06, p = 0.025). In all treatment states, patients had higher illusion scores in the synchronous stroking condition than in the asynchronous condition (pre-op off-medication: χ2 = 36.21, p < 0.0001; pre-op on-medication: χ2 = 32.83, p < 0.0001; post-op off-stimulation: χ2 = 36.17, p < 0.0001); post-op on-stimulation: χ2 = 77.44, p < 0.0001). By contrast, agreement with mock statements did not differ between stroking conditions in any treatment state (all p > 0.05).
Focussing on illusion statements (Fig. 2B), illusion scores did not differ between pre-op and post-op treatment states (all p > 0.05). As with the 23 STN-DBS patients, postoperative illusion scores in the asynchronous condition in this subset were lower when their stimulators were switched on (χ2 = 10.89, p = 0.023). Preoperative illusion scores in the asynchronous condition were similar between on- and off-medication states (p > 0.05). Illusion scores in the synchronous condition were similar between all four treatment states (all p > 0.05). LEDD was not a significant covariate and although duration of disease was significant (log(OR) = 0.07, 95% CI [0.02,0.13], p = 0.004), it did not affect the fixed effects of interest (Supplementary Table D).
Proprioceptive drift
All subjects
Proprioceptive drift was larger in the synchronous condition than in the asynchronous condition and in PD patients compared with controls (Fig. 3A), which is consistent with our second hypothesis. However, in STN-DBS treated patients, proprioceptive drift depended on baseline proprioceptive inaccuracy (the difference between the baseline estimate and the actual position of their middle finger); regression analysis showed an effect of stroking condition and an interaction between group and baseline proprioceptive inaccuracy (χ2 = 15.75, p < 0.001). Although baseline proprioceptive inaccuracy did not differ between controls and patients on- or off-stimulation (all p > 0.05), patients had less proprioceptive drift when their baseline estimates were closer to the position of rubber hand (interaction plot Fig. 3C). Examination of contrasts showed all subject groups had greater proprioceptive drift in the synchronous condition than in the asynchronous condition (χ2 = 44.91, p < 0.0001). Patients in the off-stimulation state had greater proprioceptive drift than controls in both stroking conditions (both χ2 = 7.95, p = 0.029). The difference between patients in the on-stimulation state and controls was not significant in either stroking condition (both p > 0.05). Contrary to our fourth hypothesis that switching STN-DBS on improves the precision of somatosensory perception and thus decreases proprioceptive drift, proprioceptive drift did not differ between on- and off-stimulation states in either stroking condition (both p > 0.05). Age, sex, cognition, mood and apathy were not significant covariates and thus excluded from the final model (Supplementary Table E).
Mean and 95% CI (error bars) of proprioceptive drift in (A) 23 Parkinson’s disease patients treated with deep brain stimulation of the subthalamic nucleus (STN-DBS) and 21 controls, and (B) a subset of 11 Parkinson’s disease patients tested prior to STN-DBS implantation surgery (pre-op) whilst taking their usual anti-Parkinson drugs (‘on-medication’, n = 9), and after withholding all anti-Parkinson drugs (‘off-medication’, n = 11). Patients had greater proprioceptive drift than controls only in the off-stimulation state. Amongst the subset of patients studied preoperatively, there was no difference in proprioceptive drift between the four treatment states. P-values are based on the regression model, ****p < 0.0001, *p < 0.05. All unlabelled contrasts were not significant. (C) Interaction plot showing that in the 23 patients, proprioceptive drift (i.e. the difference between their baseline and post-stroking estimates of finger position) decreased as baseline estimates became more inaccurate towards the direction of the rubber hand. Shading: 95% CI.
Subset of PD patients studied preoperatively
The preoperative subset of 11 patients had similar results to the larger patient cohort (Fig. 3B). Proprioceptive drift was greater in the synchronous condition than in the asynchronous condition, and patients had less proprioceptive drift when their baseline estimates were closer to the position of rubber hand; regression analysis showed an effect of stroking condition (b = 20.93, 95% CI [0.53, 24.36], p = 0.016) and baseline proprioceptive inaccuracy (b = −0.17, 95% CI [−0.27, −0.07], p = 0.004). Examination of contrasts revealed no differences amongst the preoperative and postoperative treatments states (all p > 0.05). Duration of disease, motor severity and LEDD were not significant covariates and thus were not included in the final model (Supplementary Table F).
Reach movement metrics
Patients had a larger peak lateral displacement in the synchronous condition than in the asynchronous condition; regression analysis showed an effect of stroking condition (b = 11.32, 95% CI [3.60, 17.87], p = 0.004) and side-of-test (b = −45.91, 95% CI [−69.90, −13.08], p = 0.005) but not stimulation state (b = 0.59, 95% CI [−15.06, 15.80], p = 0.944). The effect of stroking condition was explained only by proprioceptive drift (b = 2.15, 95% CI [0.17, 4.31], p = 0.053), not post-stroking estimate (b = −0.47, 95% CI [−2.01, 1.21], p = 0.571) or motor severity (b = 0.03, 95% CI [−0.89, 1.07], p = 0.951). That is to say, when patients tried to touch the laterally positioned target, they made larger lateral movements in the synchronous condition than in the asynchronous condition because proprioceptive drift towards the medially placed rubber hand was greater in the synchronous condition.
No other movement metric was affected by stroking condition (Supplementary Tables H–J). As expected, patients were faster in the on-stimulation state than in the off-stimulation state (mean velocity: b = 42.47, 95% CI [23.54, 69.22], p = 0.001; movement duration: b = −0.56, 95% CI [−0.91, −0.23], p = 0.006) The effects of stimulation on mean velocity and movement duration were explained by the improvement in motor severity (UPDRS) (Supplementary Tables H–I).
Discussion
PD and STN-DBS affect illusory perception in multisensory bodily awareness. Firstly, we replicated our previous findings6—that people with PD do not reject the RHI as strongly as controls in the asynchronous condition, and have greater proprioceptive drift in both synchronous and asynchronous conditions—in a cohort of STN-DBS treated patients with more advanced disease. In the present study, we also modulated cortico-basal ganglia-thalamic circuitry by switching STN-DBS on and off. We found that switching STN-DBS on strengthens patients’ rejection of the RHI in the asynchronous condition, although it remains weaker than that of controls. By contrast, STN-DBS had no effect on proprioceptive drift. Surgical implantation of the STN-DBS electrodes alone, in the absence of electrical stimulation, had no effect on the RHI.
RHI responses in PD are novel in two ways: 1) dissociation between the subjective RHI questionnaire and proprioceptive drift, and 2) in the RHI questionnaire, dissociation between the asynchronous condition and synchronous conditions. These findings suggest that the two RHI phenomena measure different things with different neural substrates that are affected in different ways by PD.
Our finding that PD increases proprioceptive drift independent of visuo-tactile synchrony is consistent with the ‘visual capture’ model of proprioceptive drift, where visuo-proprioceptive integration is sufficient for spatial recalibration and asynchronous stroking attenuates visual capture15. PD patients have well-characterised proprioceptive deficits16,17 and increased visual dependence18,19 that may make them more susceptible to visual capture and thus proprioceptive drift. We hypothesised that STN-DBS would reduce proprioceptive drift because it has been reported to improve proprioceptive deficits in kinesthesia20 and haptic perception21 in PD. However, in our study, STN-DBS did not improve baseline or post-stroking estimates of finger position. Neuroanatomically, proprioceptive drift has been attributed to the inferior parietal lobe because disrupting its function in healthy subjects using repetitive transcranial magnetic stimulation attenuates proprioceptive drift whilst preserving illusional experience36. STN-DBS did not affect this network.
The mechanisms for feeling touch on a rubber hand as if it were your own hand is theorized to involve multisensory integration of visual, tactile and proprioceptive cues with an internal sense of time and body image1. The temporal and spatial coherence of the visuo-tactile cues is critical, as synchronicity typically evokes the RHI whereas asynchronous cues do not2,9. So why do PD patients have consistently higher illusion scores than controls in the asynchronous but not the synchronous condition? We cannot exclude a ceiling effect because the distribution of illusion scores in the synchronous condition skews towards the upper limit of 14; however, the finding that STN-DBS alters illusion scores only in the asynchronous conditions adds weight to the idea that PD (and STN-DBS) differentially affect the asynchronous condition. By extension, PD must either affect temporal processing of multisensory cues or the evaluation of temporally incongruent multisensory cues.
PD patients have well-characterised temporal deficits including increased somatosensory temporal discrimination thresholds10 and inaccurate estimation of temporal inter-stimulus intervals11,12. STN-DBS improves discrimination of auditory inter-stimulus intervals in PD13. It is possible that STN-DBS improves temporal discrimination in the RHI to strengthen perception of the asynchronous signal and hence rejection of RHI in the asynchronous condition. In this study, STN-DBS affected only the subjective questionnaire responses and did not strengthen the asynchronous signal sufficiently to reduce proprioceptive drift. Precise temporal processing may be of greater importance in RHI judgements than in proprioceptive drift. However, dopaminergic drugs, like STN-DBS, also improve estimation of auditory inter-stimulus intervals11,13 but do not affect the RHI6.
STN-DBS, but not dopaminergic medication, is known to affect complex cognitive37 and perceptual decisions38; simple cognitive and perceptual decisions are not affected by STN-DBS. For example, Green et al. (2013) found that as visual stimulus complexity increased, the reaction times of STN-DBS treated patients did not increase as much as that of controls. This is also consistent with our study in (onset) binocular rivalry in which DBS, but not dopaminergic drugs, facilitated perceptual decisions39. Moreover, this facilitation effect was seen in perceptual decisions during complex binocular rivalry, but not in simpler non-rivalrous situations39. In some perceptual tasks, this facilitation effect lead to increased errors38, however in the current study, switching stimulation on made perceptual inference in the asynchronous condition closer to that of controls. There are similarities between our results and olfactory studies in PD, where STN-DBS improves odour recognition but not odour detection thresholds—suggesting that STN-DBS enhances higher-order perceptual inference by modulating connectivity between the basal ganglia and sensory association cortices40,41. Body perception is a complex task requiring integration of possibly incongruent multisensory cues with an internal sense of time to generate a momentary perception of the body that is then evaluated against higher-order notions of body image1. Estimating auditory intervals (temporal processing of a single sensory modality) and limb localisation (visuo-proprioceptive integration without decisions about body image) may be comparatively simpler and thus not affected by STN-DBS.
Anatomically, the subjective RHI experience is attributed to ventral premotor3,4, ventromedial prefrontal and lateral occipitotemporal cortices5 because fMRI activity in these areas correlate with illusion scores. Activity in ipsilateral premotor cortices also correlates with the temporal synchronicity of the stimuli42. The medial prefrontal cortex is part of the associative loop of the STN7, and this connection is one potential pathway for STN-DBS to affect illusion scores. The STN is hypothesised to act as a brake in the cortical-striatal network, increasing the threshold of evidence required to make a complex decision when there are conflicting cues—a process thought to be disrupted by STN-DBS37. How STN-DBS does this is not fully understood. Evidence from experiments in cognitive tasks points to modulation of brain network activity: EEG studies show that increased theta power in the medial prefrontal cortex associated with high-conflict tasks predicts increased time to decision, while STN-DBS reverses mediofrontal influence on decision thresholds14. Furthermore, intraoperative intracranial recordings in the STN show corresponding changes in low-frequency power during high-conflict tasks, suggesting communication with the medial prefrontal cortex14.
Consistent with our final hypothesis that certain movement metrics would be affected by stroking condition, we found that when PD patients tried to touch the laterally positioned target, they made larger lateral movements in the synchronous condition than in the asynchronous condition. It is important to note that this was explained by proprioceptive drift (away from the reach target) rather than by their post-stroking estimate (i.e., how far away they perceived their hand to be from the target) or their motor severity (UPDRS). This was not found in our previous study of PD patients without STN-DBS6, but they may have been too mildly affected for such an effect to manifest. The STN-DBS treated patients in this study had longer disease durations, greater motor severity (UPDRS off-treatment) and more proprioceptive drift. Taken together, our findings indicate that illusional misperception of hand position can alter subsequent bodily movement in people with advanced PD. Our results are consistent with other studies that found somatic misperceptions influenced actions in PD. In these studies, sensory scaling errors reduced movement amplitude (hypokinesia)43, and teaching patients to make actions “too big” and vocalisations “too loud” overcame hypokinetic movement and speech that they perceive to be normal43,44.
The duration of stimulation “washout” in this study (30 min) was sufficient to invoke the effects that we report here and to elicit a significant change in UPDRS scores (Table 1), which is consistent with reports that 75% of the effect of STN-DBS on the signs of PD wears off within 15–30 minutes of discontinuation45. Moreover, questionnaire responses and proprioceptive drift in the postoperative off-stimulation state did not differ from preoperative values, which suggests no chronic effects of stimulation on the RHI.
Despite postoperative confirmation that the DBS electrodes were situated in the STN, the precise location and active terminals were not uniform across patients, and we cannot predict individual differences in the spread of local electrical current. Hence we are unable to discern whether the effect of stimulation results from modulation of the STN or more wider cortico-basal ganglia-thalamic circuitry. Another effective target for DBS in PD is the GPi. In the future, it may be informative to compare the effects of DBS on the RHI in PD based on the exact site of stimulation (e.g., STN vs GPi).
In conclusion, illusory misperception of arm position was greater in patients with severe PD than healthy subjects, and altered their subsequent movements. Stimulation of the STN had no effect on proprioceptive drift. However, not only have we confirmed that PD patients reject illusory experience less than healthy subjects in a typically unambiguous condition, we discovered that STN-DBS strengthens their rejection of the RHI, although it remains weaker than in those without the disease. Our findings implicate the STN and subcortical connections in the network for multisensory integration in bodily awareness and pave the way for future research into the perceptual effects of DBS.
References
Ehrsson, H. H. The concept of body ownership and its relation to multi-sensory integration. In The new handbook of multisensory processing. (ed. Stein, B. E.) 775–792 (MIT Press, 2012).
Botvinick, M. & Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 391, 756–756 (1998).
Ehrsson, H. H., Spence, C. & Passingham, R. E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305, 875–877 (2004).
Ehrsson, H. H., Holmes, N. P. & Passingham, R. E. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 25, 10564–10573 (2005).
Limanowski, J. & Blankenburg, F. That’s not quite me: limb ownership encoding in the brain. Soc. Cogn. Affect. Neurosci. 11, 1130–1140 (2015).
Ding, C. et al. Parkinson’s disease alters multisensory perception: Insights from the Rubber Hand Illusion. Neuropsychologia 97, 38–45 (2017).
DeLong, M. R. & Wichmann, T. Circuits and Circuit Disorders of the Basal Ganglia. Arch. Neurol. 64, 20 (2007).
Herrington, T. M., Cheng, J. J. & Eskandar, E. N. Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38 (2016).
Shimada, S., Fukuda, K. & Hiraki, K. Rubber hand illusion under delayed visual feedback. PLoS One 4, e6185 (2009).
Artieda, J., Pastor, M. A., Lacruz, F. & Obeso, J. A. Temporal discrimination is abnormal in Parkinson’s disease. Brain 115(Pt 1), 199–210 (1992).
Pastor, M. A., Artieda, J., Jahanshahi, M. & Obeso, J. A. Time estimation and reproduction is abnormal in Parkinson’s disease. Brain 115(Pt 1), 211–225 (1992).
O’Boyle, D. J., Freeman, J. S. & Cody, F. W. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain 119(Pt 1), 51–70 (1996).
Koch, G. et al. Subthalamic deep brain stimulation improves time perception in Parkinson’s disease. Neuroreport 15, 1071–1073 (2004).
Gulberti, A. et al. Predictive timing functions of cortical beta oscillations are impaired in Parkinson’s disease and influenced by L-DOPA and deep brain stimulation of the subthalamic nucleus. Neuroimage Clin 9, 436–449 (2015).
Rohde, M., Di Luca, M. & Ernst, M. O. The Rubber Hand Illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One 6, e21659 (2011).
Schneider, J. S., Diamond, S. G. & Markham, C. H. Parkinson’s disease: sensory and motor problems in arms and hands. Neurology 37, 951–956 (1987).
Maschke, M., Gomez, C. M., Tuite, P. J. & Konczak, J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126, 2312–2322 (2003).
Adamovich, S. V., Berkinblit, M. B., Hening, W., Sage, J. & Poizner, H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 104, 1027–1041 (2001).
Sacrey, L.-A. R., Travis, S. G. & Whishaw, I. Q. Drug treatment and familiar music aids an attention shift from vision to somatosensation in Parkinson’s disease on the reach-to-eat task. Behav. Brain Res. 217, 391–398 (2011).
Maschke, M., Tuite, P. J., Pickett, K., Wächter, T. & Konczak, J. The effect of subthalamic nucleus stimulation on kinaesthesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 76, 569–571 (2005).
Aman, J. E., Abosch, A., Bebler, M., Lu, C.-H. & Konczak, J. Subthalamic nucleus deep brain stimulation improves somatosensory function in Parkinson’s disease. Mov. Disord. 29, 221–228 (2014).
Heed, T. et al. Visual information and rubber hand embodiment differentially affect reach-to-grasp actions. Acta Psychol. 138, 263–271 (2011).
Kammers, M. P. M., Kootker, J. A., Hogendoorn, H. & Dijkerman, H. C. How many motoric body representations can we grasp? Exp. Brain Res. 202, 203–212 (2010).
Palmer, C. J., Paton, B., Hohwy, J. & Enticott, P. G. Movement under uncertainty: the effects of the rubber-hand illusion vary along the nonclinical autism spectrum. Neuropsychologia 51, 1942–1951 (2013).
Zopf, R., Truong, S., Finkbeiner, M., Friedman, J. & Williams, M. A. Viewing and feeling touch modulates hand position for reaching. Neuropsychologia 49, 1287–1293 (2011).
Kammers, M. P. M., de Vignemont, F., Verhagen, L. & Dijkerman, H. C. The rubber hand illusion in action. Neuropsychologia 47, 204–211 (2009).
Mongeon, D., Blanchet, P., Bergeron, S. & Messier, J. Impact of Parkinson’s disease on proprioceptively based on-line movement control. Exp. Brain Res. 233, 2707–2721 (2015).
Lee, D., Henriques, D. Y., Snider, J., Song, D. & Poizner, H. Reaching to proprioceptively defined targets in Parkinson’s disease: Effects of deep brain stimulation therapy. Neuroscience 244, 99–112 (2013).
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2013).
Bates, D., Maechler, M., Bolker, B. & Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. http://CRAN.R-project.org/package=lme4 (2014).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-117. http://CRAN.R-project.org/package=nlme (2014).
Fox, J. & Hong, J. Effect Displays in R for Multinomial and Proportional-Odds Logit Models: Extensions to the effects Package. J. Stat. Softw. 32(1), 1–24. http://www.jstatsoft.org/v32/i01/ (2009).
Fox, J. Effect Displays in R for Generalised Linear Models. J. Stat. Softw. 8.(15), 1–27. http://www.jstatsoft.org/v08/i15/ (2003).
De Rosario-Martinez, H. phia: Post-Hoc Interaction Analysis. R package version 0.2-1. http://CRAN.R-project.org/package=phia. (2015).
Hlavac, M. stargazer: Well-Formatted Regression and Summary Statistics Tables. R package version 5.2. http://CRAN.R-project.org/package=stargazer. (2015).
Kammers, M. P. M. et al. Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. J. Cogn. Neurosci. 21, 1311–1320 (2009).
Frank, M. J., Samanta, J., Moustafa, A. A. & Sherman, S. J. Hold Your Horses: Impulsivity, Deep Brain Stimulation, and Medication in Parkinsonism. Science 318, 1309–1312 (2007).
Green, N. et al. Reduction of influence of task difficulty on perceptual decision making by STN deep brain stimulation. Curr. Biol. 23, 1681–1684 (2013).
Fujiwara, M. et al. Optokinetic nystagmus reflects perceptual directions in the onset binocular rivalry in Parkinson’s disease. PLoS One 12, e0173707 (2017).
Guo, X. et al. Effects of Bilateral Deep Brain Stimulation of the Subthalamic Nucleus on Olfactory Function in Parkinson’s Disease Patients. Stereotact. Funct. Neurosurg. 86, 237–244 (2008).
Hummel, T., Jahnke, U., Sommer, U., Reichmann, H. & Müller, A. Olfactory function in patients with idiopathic Parkinson’s disease: effects of deep brain stimulation in the subthalamic nucleus. J. Neural Transm. 112, 669–676 (2005).
Bekrater-Bodmann, R. et al. The Importance of Synchrony and Temporal Order of Visual and Tactile Input for Illusory Limb Ownership Experiences – An fMRI Study Applying Virtual Reality. PLoS One 9, e87013 (2014).
Berardelli, A., Rothwell, J. C., Thompson, P. D. & Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124, 2131–2146 (2001).
Fox, C., Ebersbach, G., Ramig, L. & Sapir, S. LSVT LOUD and LSVT BIG: Behavioral Treatment Programs for Speech and Body Movement in Parkinson Disease. Parkinsons Dis. 2012, 391946 (2012).
Temperli, P. et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60, 78–81 (2003).
Acknowledgements
The authors wish to thank Richard Beare for help with R statistical software. C.D’s salary is supported by the Monash Institute of Neurological Sciences and an Australian Postgraduate Award from Monash University. N.T. was funded by the Australian Research Council Future Fellowship FT120100619 and the Discovery Project DP130100194.
Author information
Authors and Affiliations
Contributions
Catherine Ding contributed to the design of the project, collected and analysed the data, wrote the first draft, reviewed and edited the manuscript, and prepared the figures. Colin J. Palmer contributed to the design of the project, reviewed and edited the manuscript. Jakob Hohwy contributed to the design of the project, reviewed and edited the manuscript. George J. Youssef contributed to the design of the project, reviewed and edited the manuscript. Bryan Paton contributed to the design of the project, reviewed and edited the manuscript. Naotsugu Tsuchiya contributed to the design of the project, reviewed and edited the manuscript. Julie C. Stout contributed to the design of the project, reviewed and edited the manuscript. Dominic Thyagarajan contributed to the design of the project, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, C., Palmer, C.J., Hohwy, J. et al. Deep Brain Stimulation for Parkinson’s disease changes perception in the Rubber Hand Illusion. Sci Rep 8, 13842 (2018). https://doi.org/10.1038/s41598-018-31867-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31867-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.