Abstract
Parasites exhibiting a high degree of host specificity are expected to be intimately associated with their hosts. Therefore, the evolution of host-specific parasites is at least partially shaped by the evolutionary history and distribution of such hosts. Gill ectoparasites of Dactylogyrus (Monogenea) are specific to cyprinid fish. In the present study, we investigated the evolutionary history of 47 Dactylogyrus species from the Balkan Peninsula, the Mediteranean region exhibiting the highest cyprinid diversity in Europe, and from central European cyprinids. Phylogenetic analyses revealed four well-supported clades of endemic and non-endemic Dactylogyrus spp. with four basal taxa. Endemic cyprinids with a limited distribution range were parasitized by endemic Dactylogyrus species, but some of them shared several Dactylogyrus species with central European cyprinids. Species delimitation analyses based on molecular data suggest that Dactylogyrus diversity is higher than that defined from morphology. Some endemic cyprinid species harboured Dactylogyrus species of different origins, this probably resulting from multiple host switching. Our results support the view that the evolution of Dactylogyrus in the Balkans has been influenced not only by the historical dispersion and distribution of their cyprinid hosts, but also by recent contacts of non-native cyprinid species with endemic cyprinid fauna in this region.
Similar content being viewed by others
Introduction
The species richness of parasitic taxa and their distribution in host species is usually closely related to the history, dispersion and diversity of their hosts1,2,3. The parasitic genus Dactylogyrus (Monogenea), known for its wide species richness (over 900 nominal species according to Gibson et al.4), is restricted mainly to fish species of Cyprinidae, a highly diversified group of primarily freshwater fish5. Dactylogyrus species exhibit a high degree of host specificity within the multitude of their host species6.
Previous studies suggest that each cyprinid species can host at least one Dactylogyrus species7,8,9. Within one host species the distribution of Dactylogyrus species is restricted to specific microhabitats, i.e. different Dactylogyrus species occupy distinct niches within host gills10,11,12. The evolution of niche preference is linked with changes of at least one parameter determining niche position on fish gills (e.g. the changes in the positions among the different gill arches or different segments of a given gill arch)6. It has been hypothesized that, over evolutionary time, monogeneans developed copulatory organs of different shapes and sizes, which resulted in reproductive isolation within overlapping microhabitats13. This was previously documented in Dactylogyrus species as well14.
Unlike central and northern Europe, where the cyprinid fauna is relatively uniform, southern European peninsulas are extremely rich in endemic cyprinid species15. The endemic cyprinid fauna of Mediterranean regions consists of several highly diversified genera whose origin and historical biogeography are still poorly known in spite of several recent studies16,17,18,19,20. Zardoya et al.21 investigated 15 lineages (52 species) of Greek cyprinids and proposed that species related to Danubian cyprinid fauna colonized the Balkan Peninsula during two different time periods. The first one occurred during the Miocene, when fish species such are Barbus cyclolepis22, Alburnoides strymonicus19, Telestes beoticus, T. pleurobipunctatus20, and Squalius peloponensis18 diverged. These species show relatively high molecular divergence in comparison to central European sister group taxa. The second period is related to the Plio-Pleistocene connection of the Balkan Peninsula and the River Danube via river captures23,24. This dispersion event included species such are Barbus balcanicus25, Squalius vardarensis and species of Chondrostoma and Alburnus genera26, which exhibit a much lower degree of molecular divergence with respect to Danubian-related taxa. Previous studies on the phylogeny of Balkan cyprinids are focused on Squalius18,26,27,28,29,30, which is one of two genera (with Barbus) inhabiting all three southern European peninsulas. According to the above-cited study by Sanjur et al.30, based on analysis of the mitochondrial cytochrome b gene, Balkan Squalius species are grouped into three major clades. Several studies, based on different molecular markers and the analysis of several morphological traits, suggested that the Balkan Squalius species with the greatest ancestral diversification is Squalius keadicus, which split from other Squalius lineages approximately 9 Mya24,26. The Balkan ancient lake system, known as Dessaretes, emerged in the Pliocene, and was suggested to have play an important role in freshwater biota speciation processes. For this reason, it is considered to have been a hotspot of endemic Balkan biodiversity31,32,33,34,35. The Dessaretes lake system formerly included Lake Ohrid (located in Albania and F.Y.R.O.M.), Lake Prespa (Albania, Greece, F.Y.R.O.M.), Lake Mikri Prespa (Albania, Greece) and Lake Maliq (Albania). Recently, the current distribution of many cyprinid species from the “Dessaretes” region was reevaluated. For example, Barbus prespensis, initially known as an endemic species from Lake Prespa, was recently shown to be widespread in the south-eastern Adriatic basin, together with other presumably endemic species from Lake Prespa, namely Alburnoides prespensis and Squalius prespensis19,25,36. This basin is a part of the evaporated Lake Maliq, historically connected to Lake Prespa and drained after the Second World War33.
Gregory37 suggested that hosts with a larger area of distribution are infected by more parasitic species. Concerning cyprinids, widely distributed species across Europe such as Rutilus rutilus and Squalius cephalus harbour up to 9 Dactylogyrus species11,38. In contrast, Dupont and Lambert7 found only 5 Dactylogyrus species on Rutilus rubilio, an endemic cyprinid species in the Apennine Peninsula. A phylogenetic reconstruction including 51 Dactylogyrus species and based on molecular data suggested that species parasitizing central European cyprinids form three monophyletic groups11 and are associated with different phylogenetic lineages of cyprinid species representing subfamilies with different origins, histories, and biogeographical distributions. Since studies of endemic and non-endemic Dactylogyrus from Balkan cyprinids are scarce and mainly based on morphological data7,39,40,41, the evolutionary histories and patterns of endemism of these host-specific species are still unresolved. Several previous studies concerning different regions of the northern Mediterranean Sea suggested that endemic cyprinids harbour endemic Dactylogyrus species7,9,42. Some phylogenetic studies were focused on Dactylogyrus species from selected cyprinid genera, such as Dactylogyrus spp. parasitizing Barbus species43. According to the authors, such Dactylogyrus species are supposed to exhibit both genetic and morphological variabilities between different host species. Dupont44 investigated the historical biogeography of Dactylogyrus species of endemic Rutilus, Luciobarbus, and Pachychilon hosts from the Balkan Peninsula and suggested that the endemism of Dactylogyrus can be explained by the formation of landmass and freshwater streams during the Neogene and Pleistocene eras.
The aim of the present study was to investigate the diversity, evolutionary history, and phylogenetic relationships of Dactylogyrus spp. parasitizing endemic cyprinids of the Balkan Peninsula. First, we analyzed the degree of endemism in Dactylogyrus species parasitizing these cyprinids. Next, we focused on the phylogenetic relationships between endemic Dactylogyrus and commonly distributed Dactylogyrus (species shared between central European and endemic Balkan cyprinid species) in order to infer potential scenarios of historical contact between different cyprinids. Concerning Dactylogyrus species with a wide host range, we also searched for genetic structuration by analyzing the level of genetic diversity and its correlation with the geographical distances between their hosts. Finally, we assessed the species status of generalist Dactylogyrus on the basis of molecular data in order to test whether the degree of genetic variability was in concordance with the current species status based on a classical taxonomical approach.
Results
Dactylogyrus species richness
A total of 53 Dactylogyrus species were identified from cyprinid hosts from the Balkans (Table 1) and central Europe. 47 species were collected from endemic Balkan cyprinids. Six additional species were collected from the Czech Republic and included in analyses. Balkan cyprinids were parasitized by 1 to 5 Dactylogyrus species with an average of 2 species per host species. The highest Dactylogyrus species diversity was reported on representatives of the genera Pachychilon – P. pictum (5); Squalius – S. squalus (4) and S. prespensis (4); Barbus – B. prespensis (4); and Rutilus – R. basak (4), R. lacustris (4), and R. ohridanus (4). Eight Dactylogyrus species were unidentified and are expected to be new to science. These potentially new species were collected from the following host species: Delminichthys adspersus, Chondrostoma knerii, Squalius tenellus, Luciobarbus albanicus, L. graecus, Tropidophoxinellus spartiaticus, Telestes karsticus and Pachychilon macedonicum.
Phylogenetic analyses and genetic distances
The concatenated sequence alignment of partial 18S and partial 28S rDNA from representatives of 54 Dactylogyrus species from the Balkan Peninsula and central Europe contained 1158 unambiguous nucleotide positions. The data were treated as partitioned and GTR+I was selected as the most optimal evolutionary model for the 446 bp-long partial 18S rDNA sequences, and GTR+I+G for the 712 bp-long partial 28S rDNA sequences. BI (Bayesian inference) and ML (Maximum Likelihood) analyses produced trees with identical topologies which varied in node support values (Fig. 1). The resulting phylogram divided most of the species into 4 strongly-to-moderately supported clades. Four Dactylogyrus species (D. erhardovae, D. caballeroi, D. crucifer and D. rarissimus) were placed in an external position to these four clades. The first clade (clade 1), weakly supported by BI and well supported by ML analyses, included the species D. sekulovici from Pachychilon pictum and Dactylogyrus sp. 4 from Delminichthys adspersus. The second clade (clade 2), highly supported by BI and weakly supported by ML analyses, was the largest and included all species parasitizing Barbus and Luciobarbus. Dactylogyrus species endemic for the Balkan Peninsula and also widely distributed Dactylogyrus species clustered in this second clade. Generally, species with similarly shaped haptoral hard parts clustered together and such clusters were well or moderately supported by at least BI analysis (PP, posterior probability > 0.81). For example, D. petkovici, D. martinovici and Dactylogyrus sp. 5, representing a monophyletic group, share a similar type of thin anchor hooks and a ventral bar with five extremities, while Dactylogyrus sp. 2 and Dactylogyrus sp. 3, representing another monophyletic group, display hard parts of the haptor that are almost indistinguishable in shape. Three Dactylogyrus species from Barbus (i.e. D. petenyi, D. malleus and D. prespensis, which also share a similar shape of their haptoral hard parts) were clustered with D. omenti from Aulopyge huegelii. The third clade was strongly supported by both BI and ML analyses and included D. alatus, D. sphyrna and D. vistulae, which are large worms with large haptoral anchor hooks. The last well-supported clade (PP = 1, BS, bootstrap value = 100) included D. auriculatus from Abramis brama and D. ivanovichi from P. pictum (clade 4), which exhibited identically shaped MCO (male copulatory organ) hard parts but VA (vaginal armament) of slightly different shape. All species from clades 3 and 4, except D. alatus, had no connective ventral bar. Dactylogyrus zandti appeared to be a sister species to clades 3 and 4, but its position was not supported.
Phylogram of 54 Dactylogyrus species from the Balkans and Central Europe reconstructed by Bayesian inference. The tree is based on concatenated data of partial 18S rDNA and partial 28S rDNA sequences. Values along branches indicate posterior probabilities and boostrap values resulting from Bayesian inference and Maximum likelihood analyses, respectively. Values <0.80 for BI and <50% for ML are indicated by dashes (-). Branch lengths correspond to the expected number of substitutions per site. Labels 1–4 refer to different Dactylogyrus lineages. The phylogenetic tree was rooted using Dactylogyrus species parasitising Carassius gibelio and Cyprinus carpio (following Šimková et al.12).
To resolve the phylogenetic relationships among groups within the second clade, we used a concatenated alignment of partial 18S, 28S rDNA, and the highly variable ITS1 (Internal Transcribe Spacer 1) region. The alignment of 86 sequences comprised 1503 unambiguously aligned nucleotide positions.The most optimal evolutionary models were TrNef+I for the alignment of 446 bp-long partial 18S rDNA sequences, SYM+G for the alignment of 344 bp-long ITS1 sequences, and TVMef+I+G for the alignment of 713 bp-long partial 28S rDNA sequences. BI and ML analyses generated trees with the same topologies (Fig. 2). The resulting trees were rooted using clade 1 from the first phylogenetic reconstruction (Fig. 1).
Phylogram of selected Dactylogyrus species from the Balkans and Central Europe constructed by Bayesian inference. The tree is based on concatenated data of partial 18S rDNA, ITS1 region and partial 28S rDNA sequences. Values along branches indicate posterior probabilities and boostrap values resulting from Bayesian inference and maximum likelihood analyses, respectively. Values <0.80 for BI and <50% for ML are indicated by dashes (-). Branch lengths correspond to number of substitutions per site. Labels A–L refer to different, well supported, Dactylogyrus clades.
The phylogenetic analyses divided clade 2 into several strongly-to-moderately supported groups. Group A included species parasitizing Pachychilon, these sharing the same type of haptoral ventral bar with five radii, similar to the ‘cornu’ type45. This monophyletic group of Dactylogyrus spp. from Pachychilon was highly supported by both BI and ML analyses. All Dactylogyrus species of Scardinius (D. difformis, D. difformoides and D. izjumovae) formed a highly supported monophyletic group (group C). The group of two Dactylogyrus species from Alburnus (group B) formed a sister clade to the abovementioned species from Scardinius. Dactylogyrus prostae, D. nanoides, and D. folkmanovae from Squalius formed three very strongly supported monophyletic groups (groups D, E, and F, respectively). Group E also clustered with D. rysavyi from A. thessalicus, Dactylogyrus sp. 7 from C. knerii, and Dactylogyrus sp. 1 from S. tenellus, with strong support from both analyses. All three species exhibit a similarly shaped MCO and parasitize phylogenetically closely related cyprinid lineages26,45.
The phylogenetic relationships between Dactylogyrus spp. of Barbus and those of Luciobarbus were unresolved. However, Dactylogyrus spp. of these cyprinids formed three well supported groups (G, H and I). All specimens of D. crivellius, collected from six Barbus species in the Balkans, formed a strongly supported clade. This species clustered with D. carpathicus from B. barbus. The group of D. crivellius and D. carpathicus was sister to the group including two Dactylogyrus species (sp. 2 and sp. 3) of Balkan Luciobarbus spp. (within group I). While Dactylogyrus sp. 2 and Dactylogyrus sp. 3 were found to be almost identical on the basis of morphological characters, they differed at the molecular level (concatenated partial 18S rDNA and ITS1 region, p-distance = 0.041). Our results did not support the monophyly of D. petenyi, as this species clustered with D. malleus and D. prespensis (group G). Dactylogyrus omenti from Aulopyge huegelii appears also to be phylogenetically closely related to the species parasitizing Barbus and Luciobarbus, but its position was only moderately supported by BI analysis. The position of D. rosickyi of P. pictum was also uncertain; however, BI analysis strongly supported its position within the clade including groups C–I. Dactylogyrus rutili from Rutilus formed a well-supported group (group J) and, according to our results, appears to be phylogenetically closely related to D. suecicus (whose monophyly was not supported) and Dactylogyrus sp. 8 from T. karsticus. Surprisingly, D. ergensi collected from three host species formed a paraphyletic group. Dactylogyrus ergensi from C. ohridana was phylogenetically related to D. caucasicus, parasitizing on Alburnoides species (group L), in contrast to other D. ergensi specimens collected from C. knerii and C. vardarensis. Nonetheless, D. caucasicus, D. dirigerus and D. ergensi (included in groups K and L) share a similarly shaped MCO.
The computation of genetic distances between specimens of generalist Dactylogyrus species revealed moderate-to-high interpopulation genetic variability. Pairwise genetic distances were calculated for D. vistulae, D. rarissimus, and D. folkmanovae after eliminating all positions containing gaps and missing data. The selected species are representatives of Dactylogyrus with a wide distribution range in Europe. While D. folkmanovae is a parasite only of Squalius spp., D. vistulae and D. rarissimus are real generalists parasitizing on species of different cyprinid genera. An alignment of 994 nucleotide positions was used for D. vistulae collected from 24 cyprinid species of six genera at 20 localities across the Balkan Peninsula and the Czech Republic. Pairwise sequence diversities varied from 0.000 to 0.020 (Table 2). Generally, geographically adjacent populations were more similar at the molecular level, a finding supported by the Mantel test (P = 0.015). Dactylogyrus vistulae from S. tenellus, S. svallize, S. illyricus, Phoxinellus pseudalepidotus, P. alepidotus, and T. metohiensis were genetically identical and all their host species were from the Dalmatian ichthyogeographical district. The same pattern was observed for D. vistulae specimens from C. nasus and Leuciscus idus, both from central Europe: they were similar at the molecular level. One of the few exceptions was D. vistulae from S. cephalus in the Czech Republic, which was genetically more similar to Balkan populations collected from S. squalus and S. vardarensis than to central European populations. Dactylogyrus rarissimus was collected from 11 species including four cyprinid genera – Alburnus, Pelasgus, Rutilus and Telestes. After removing gaps and missing data, the final alignment contained a total of 978 nucleotide positions. The interpopulation genetic variability ranged from 0.001 to 0.030 (Table 3). The pairwise distances revealed that D. rarissimus from R. rutilus and R. lacustris were the most similar (p-distance = 0.003). Specimens of D. rarissimus from T. alfiensis were the most genetically dissimilar to all other specimens collected from other host species (p-distance > 0.021). Regarding D. rarissimus, the Mantel test did not reveal any significant spatial genetic structure (P > 0.05). Dactylogyrus folkmanovae specimens were collected from seven Squalius species at nine localities from the Balkans and central Europe. The final alignment contained 977 positions and genetic distances varied from 0.002 to 0.037 (Table 4). Interpopulation genetic variability was found even between specimens collected from two populations of one host species, namely S. prespensis (p-distance = 0.002), where both populations were in the same ichthyogeographical district. Surprisingly, the same genetic distance was observed between D. folkmanovae specimens collected from S. cephalus in Bosnia and Herzegovina and from S. cephalus in the Czech Republic. The Mantel test indicated a positive correlation between genetic and geographical distance for D. folkmanovae populations (P = 0.001).
Species delimitation
The species status of Dactylogyrus parasites exhibiting high interpopulation molecular diversity was investigated on the basis of a statistical analysis of our sequence data using PTP. We examined all specimens from clade 2 (Fig. 2). Results of the maximum likelihood analysis (Fig. 3) supported the original species statuses of specimens identified under the following species: D. dirigerus, D. difformis, D. difformoides, D. izjumovae, D. nanoides, D. prostae, D. folkmanovae, and D. vranoviensis. Specimens of D. rutili, collected from three Rutilus species, were recognized as three different species. Meanwhile, two molecular variants of D. suecicus and the phylogenetically closely related Dactylogyrus sp. 8 from T. karsticus were also recognized by our analyses as three different species. With respect to D. dyki, our analyses suggested six different species. Dactylogyrus ergensi specimens from C. vardarensis, C. knerii, and S. squalus were suggested to be three different species. Dactylogyrus ergensi from C. ohridana was suggested to be the same species as D. caucasicus from Alburnoides. Finally, D. petenyi, D. prespensis and D. malleus were identified as a single species on the basis of clustering methods. The strongest Bayesian supported solution was in congruence with the results of the maximum likelihood solution.
Results of species PTP delimitation analysis based on the phylogram in Fig. 2. Vertical bars at terminal branches indicate different species. Values along brackets indicate support values from both maximum likelihood partition and heuristic bayesian search. Species are the same as in Fig. 2 but several branches are rotated.
Discussion
The present study suggests that the diversity of Dactylogyrus species parasitizing endemic cyprinids in the Balkans is poorer when compared to the diversity of Dactylogyrus from central European cyprinids and from cyprinids with a large distribution range (e.g. Šimková et al.11 documented up to 9 different Dactylogyrus species from widely distributed Rutilus rutilus in the Czech Republic). High numbers of Dactylogyrus species were also observed on African cyprinids from the genus Labeo, such as L. coubie with 9 Dactylogyrus species46. In contrast, we observed a maximum of 5 Dactylogyrus species on a single cyprinid species. These numbers are consistent with previous observations of southern European Dactylogyrus fauna, where no more than 5 species were collected from one cyprinid host species7,44,45. Such low Dactylogyrus species diversity probably has several causes. The distribution range of host species highly influences parasite diversity47. Our observations support Gregory’s hypothesis37, i.e. fish species with a wide distribution range are exposed to more parasite species; therefore, they exhibit high parasite diversity. Another potential explanation could be the following: host species with a wide distribution range include a much higher number of populations in comparison to endemic species, which favours parasite speciation. This is illustrated in the present study by R. rutilus and R. aula. While R. rutilus, referred to above as a species with a high Dactylogyrus species richness, is the cyprinid species with the widest distribution range in Europe, the distribution area of R. aula is limited to the Adriatic basin in Italy and the northwestern Balkans (the Northern Adriatic ichthyogeographical district15). R. aula is parasitized by a single Dactylogyrus species – namely, D. erhardovae – in contrast to the aforementioned R. rutilus11. A similar example concerns the Balkan endemic species S. illyricus or S. peloponensis, which exhibit very low Dactylogyrus species richness (i.e. single species) in comparison to Squalius cephalus, from which Seifertová et al.38 documented 9 different Dactylogyrus species (up to 14 Dactylogyrus species according to the checklist by Moravec8). Time of the year when the sampling is performed and the number of investigated populations are known to impact parasite diversity47,48. Data on Dactylogyrus diversity in cyprinids in central Europe are compiled from numerous studies (i.e. the checklist compiled by Moravec8) and include several sampling periods from different river basins, while the present study is focused on a single sampling period in a specific region. The investigated cyprinid hosts endemic to the Balkans are generally distributed in a restricted region where the number of populations potentially harbouring different parasites is expected to be rather lower than in central Europe. Therefore, also following Gregory’s hypothesis, we expected lower parasite diversity in endemic cyprinids with a restricted distribution range. Only a few host species, such as S. squalus, were collected from several distinct localities; however, the different host populations did not differ in their numbers of Dactylogyrus species. It was also shown that the composition of monogenean communities is influenced by environmental factors, especially water temperature. In such cases, shifts in the species compositions of monogenean communities within host species were observed throughout the year49,50,51,52,53.
The present phylogenetic analyses revealed four well-to-moderately supported clades including both endemic and non-endemic Dactylogyrus species, while four species – namely, D. erhardovae, D. crucifer, D. caballeroi, and D. rarissimus (all parasites of Rutilus spp.) – had external positions to these clades. Dactylogyrus erhardovae is considered to be a genus specific parasite of Rutilus, the first description of this species originating from R. rubilio54, an endemic species of the Apennine Peninsula55,56. In the Balkans, Dactylogyrus erhardovae was also found on R. aula and R. basak, phylogenetically closely related species26,57 distributed in the rivers of the Adriatic Sea basin, which is the proximal ichthyogeographic district to the Tyrrhenian Sea basin, where R. rubilio occurs. Dactylogyrus crucifer was originally described from Rutilus rutilus, but Šimková et al.12 collected this species also from Leuciscus idus and Scardinius erythrophthalmus and therefore suggested that D. crucifer represents a generalist species. In our study, D. crucifer was only collected from Rutilus species (R. rutilus from the Czech Republic and R. lacustris from the Ponto-Caspian area), which supports the association between Rutilus hosts and D. crucifer and even indicates that the occurrence of this parasite on other cyprinid species may be the result of accidental infection. Both Rutilus species parasitized by D. crucifer originated and live in sympatry in the Black Sea and Caspian Sea basins58, which may promote the host switching of D. crucifer between these two sister Rutilus lineages.
Interestingly, we showed that Dactylogyrus sp. 4 from D. adspersus and D. sekulovici from P. pictum clustered together (group 1). Both Dactylogyrus species seem to be host specific - at least, there are no previous records of these two species from other cyprinid species. Regarding the morphology of the hard parts, these two Dactylogyrus species differ in the shape of their MCOs. While Dactylogyrus sp. 4 has hard parts morphologically similar to those of D. erhardovae from Rutilus, it shares with D. sekulovici only the shape of the haptoral connective bars (see Pugachev et al.45 for morphology of D. sekulovici). Two cyprinid species – namely, D. adspersus and P. pictum – are representatives of two phylogenetically unrelated ancient lineages26, but have a similar geographical distribution, i.e. they are restricted to the rivers of the Adriatic Sea Basin. Pachychilon pictum occurs only in the Albanian ichthyogeographical district59; D. adspersus inhabits the central Adriatic (Dalmatian) district, which shares only two species with the Danubian basin59,60,61, and is probably linked to the Adriatic district by underground connections16. The paraphyly of the Dactylogyrus species from P. pictum suggests their multiple origin on this host. The phylogenetic proximity of D. sekulovici to Dactylogyrus sp. 4 suggests a host switch between two cyprinid species living in the same area of the central Adriatic region. The second host-specific parasite of P. pictum is D. ivanovichi44,45. Its phylogenetic position suggests a different origin (when compared to D. sekulovici), likely also resulting from a host switch. Dactylogyrus ivanovichi is phylogenetically closely related to D. auriculatus from Abramis brama. The two species exhibit MCOs with an identical structure and differ only in the positioning of the VA and in the root lengths of haptoral anchor hooks45. These two species, like the two species of the sister clade (clade 3), secondarily lost their connective haptoral ventral bar45. The phylogenetic proximity of D. ivanovichi and D. auriculatus and the morphological similarities in copulatory organs between D. ivanovichi and Dactylogyrus spp. of A. brama suggest that D. ivanovichi originated from a recent host switch from the widely distributed A. brama, and then adapted its attachment organ to new host species. Other Dactylogyrus species from P. pictum, namely D. martinovici and D. petkovici, are phylogenetically closely related to Dactylogyrus sp. 5 of P. macedonicum. Dactylogyrus martinovici, D. petkovici, and Dactylogyrus sp. 5 exhibit haptoral hard parts with an almost identical shape but differ in the shapes of their copulatory organs. This is in congruence with Šimková et al.6, suggesting similar adaptations of the haptor among Dactylogyrus species parasitizing phylogenetically related hosts. We can hypothesize that these three species evolving from the same ancestor have for a long time been associated with Pachychilon and that D. martinovici and D. petkovici emerged as a result of more recent intra-host duplication followed by reproductive isolation. In contrast, D. ivanovichi and D. sekulovici are the result of earlier host switching between cyprinid species of different genera living in contact zones and of subsequent speciation. Finally, another Dactylogyrus species from P. pictum, D. rosickyi, exhibits a different phylogenetic position when compared to the aforementioned Dactylogyrus of Pachychilon spp., which suggests a different origin for this species.
Regarding Dactylogyrus from Barbus spp., our analyses did not fully resolve the phylogenetic relationships between these species, but in general all species are clustered in three well or moderately supported groups (G–I). In total, we collected 5 different Dactylogyrus species from 10 Barbus hosts. The most common was D. dyki, parasitizing 8 Barbus species and representing one clade in our phylogenetic analysis. Šimková et al.43 observed significant interpopulational phenotypic plasticity and molecular variability among D. dyki isolated from 3 Barbus species, which is in accordance with the present study. The monophyly of the group including D. dyki specimens was supported. However, low support for D. dyki from B. strumicae was found and these specimens were recognized as a different species by species delimitation analysis. Following the suggestion of Šimková et al.43, D. dyki from Barbus spp. could represent a species complex of several morphologically similar species. The confirmation of this hypothesis requires further morphological reevaluation of Dactylogyrus representatives from all Barbus hosts, including those from B. meridionalis in Western Europe and B. tyberinus from the Apennines. We inferred some paraphyly concerning D. balkanicus. Whilst Dactylogyrus specimens of B. prespensis and B. rebeli were clustered together, specimens from B. plebejus appeared to be phylogenetically related to D. dyki. The sister status of these two species is supported by the similar shape of the sclerotized parts of their haptors (both species share a small triangular connective ventral bar), and also the remarkably similar shape of their MCOs45. Both species were collected from B. rebeli and B. prespensis, phylogenetically closely related Barbus species25,62, suggesting (1) historical intra-host speciation, i.e. parasite duplication on their common ancestor and a later host switch to another endemic Barbus, or (2) parasite duplication on recent Barbus species in this region and a host switch to the phylogenetically and geographically closest Barbus species. According to our phylogenetic analyses, D. petenyi, D. malleus, and D. prespensis form a well-supported group, namely group G. These three Dactylogyrus species parasitizing Barbus species share similar morphologies of the copulatory organs and haptoral hard parts. Surprisingly, specimens of D. petenyi do not form a monophyletic group. Species delimitation analysis suggests that each representative of group G represents a single species.
Specimens of D. crivellius from different host species formed a monophyletic group. Our phylogenetic analyses support a monophyletic group including D. crivellius from Balkan Barbus spp., D. carpathicus from B. barbus, and Dactylogyrus sp. 2 and Dactylogyrus sp. 3. These four species exhibit the same morphology of a ventral bar with 5 extremities, a typical feature of Dactylogyrus spp. from Luciobarbus. Species with this morphology are considered as the ‘carpathicus’42 or ‘cornu’45 type. This supports the hypothesis that haptoral hard parts are more suitable for resolving the phylogeny of monogeneans; that is, haptor morphology is similar between closely related species6,63,64.
The phylogenetic position of D. omenti among Dactylogyrus species parasitizing Barbus and Luciobarbus was already suggested by Benovics et al.65. Even though its exact phylogenetic position is not fully resolved, our result suggests that this species is phylogenetically closer to D. petenyi and D. prespensis than to the aforementioned species which share the ‘cornu’ type of haptoral ventral bar. Adding more Dactylogyrus species from Iberian, North African, and Middle Eastern Barbus and Luciobarbus in a phylogenetic reconstruction and assessing coevolutionary scenarios involving these parasites and their hosts could better resolve the relationships within this group of Dactylogyrus.
Several well-supported phylogenetic groups (J–L) were formed exclusively by Dactylogyrus species of the ‘ergensi’ type of copulatory organ, or, in the case of D. tissensis, the ‘chondrostomi’ type of copulatory organ47. While the MCO and VA among Dactylogyrus spp. belonging to groups J–L are very similar, these species differ in the shapes and sizes of their haptoral hard parts. All Dactylogyrus species of groups K and L parasitize species of the genera Alburnoides and Chondrostoma. The species status of D. caucasicus parasitizing Alburnoides and that of D. dirigerus parasitizing Chondrostoma were supported by species delimitation analysis. Surprisingly, Rutilus-specific D. rutili belonging to the phylogenetically distant group J possesses the same type of copulatory organ as D. caucasicus and D. dirigerus. This suggests that a similar copulatory organ morphotype can emerge independently several times during the evolution of Dactylogyrus species in evolutionarily distant hosts (such are Rutilus, Chondrostoma, and Alburnoides26). Rohde2 hypothesized that the rapid evolution of morphological variation in copulatory organs is considered as a mechanism for avoiding hybridization. In contrast, similar types of copulatory organs in Dactylogyrus species may be recognized in different host lineages, as shown in the present study. Then, species with a similar MCO morphotype could be found within congeneric hosts only if these parasite lineages had diversified recently (e.g. D. ergensi and D. dirigerus of Chondrostoma).
High numbers of southern European endemic Dactylogyrus species were strictly host specific and/or distributed only in one region. However, some of them were collected from a wide range of cyprinid hosts. Dactylogyrus vistulae is the species with the widest host range in the Balkans. In addition to the host range for this parasite revealed in this study, the presence of D. vistulae was also reported from R. rutilus in Finland66 and from V. vimba in the Czech Republic8. Genetic distances between specimens collected from different host species correlated with geographical distances, suggesting the geographical structure of D. vistulae populations, rather than some association with the phylogenetic relatedness of the host species. For example, D. vistulae from C. phoxinus appears to be genetically more similar to D. vistulae from hosts in the same or close ichthyogeographical region than to D. vistulae collected from geographically separated congeneric Chondrostoma. Since D. vistulae is widely distributed and relatively easily distinguishable from other Dactylogyrus spp. on the same hosts (on the basis of morphological characters and its large body size45), it could potentially represent a suitable model for population studies that could elucidate the origin of this species and the distribution pattern between phylogenetically distant hosts or between two host species from different regions. Another species with a wide distribution range is D. rarissimus. It was originally considered as a specialist of R. rutilus6,12,67; however, we collected this species in the Balkans from phylogenetically well-separated genera: Rutilus, Alburnus, Pelasgus and Telestes. In this case, the Mantel test did not reveal a significant correlation between genetic and geographical distances, even as specimens collected from T. alfiensis and P. laconicus in Peloponnese (the only representatives of D. rarissimus from the Ionian ichthyogeographic district) are genetically the most different from northern populations originating from the Albanian district (such as R. ohridanus). We measured only a very small genetic difference between D. rarissimus from R. rutilus and D. rarissimus from R. lacustris (similarly to that measured for D. crucifer), which supports the recent divergence of these Rutilus species or, alternatively, a more ancient separation followed by recent contact. All these results suggest that D. rarissimus is a true generalist species parasitizing several cyprinid genera. We investigated the correlation between genetic and geographical distances among D. folkmanovae individuals. In contrast to D. vistulae and D. rarissimus, D. folkmanovae was reported as a generalist parasite of S. cephalus and R. rutilus8,67; however, it is generally reported in Squalius species12 and, in the Balkans, D. folkmanovae occurs strictly on Squalius spp. Dactylogyrus folkmanovae from S. squalus appeared to be the most genetically different from individuals parasitizing other host species. Of the southern European endemic Squalius species, Squalius squalus exhibits the largest distribution range, i.e. it covers the whole peri-Adriatic region15, and is phylogenetically closely related to S. prespensis26. This is in congruence with measurements of genetic distance, according to which D. folkmanovae of S. squalus and S. prespensis are the most similar. These results suggest that D. folkmanovae of S. squalus is the oldest lineage within this species in the Balkans. In contrast, representatives of D. folkmanovae from S. cephalus in the Czech Republic and D. folkmanovae from S. cephalus in Bosnia and Herzegovina are genetically very similar. These small genetic distances (in the case of both D. vistulae and D. folkmanovae) could be the result of more recent contact between hosts from these two distant regions via underground connections, as proposed by Palandačić et al.16, or through the introduction of non-native species/populations into the Balkan region. Fish introduction has been a very common occurence in the Balkans and includes both exotic, and native species from geographically near localities68,69. River drainages70,71 and also isolated karstic drainages are affected, where non-native species such as S. cephalus and R. rutilus have been introduced72. Low molecular variability between Czech and Bosnian-Herzegovinian populations of D. folkmanovae may favour the hypothesis of the natural dispersion of the fish via river connections. However, the investigation of other European populations and the use of other genetic markers suitable for population genetics of Dactylogyrus are necessary to reveal the distribution patterns of widespread Dactylogyrus species. In addition, the extent of parasite transfer from introduced species to endemic species needs to be studied further to reduce the possible risk of parasite introduction to already threatened native species.
In this study, we revealed interpopulation genetic variability within endemic Balkan Dactylogyrus species. The intraspecific genetic distances could also be linked to the morphological variability which was suggested for other monogenean taxa73,74,75. Concerning Dactylogyrus, morphological variability among the haptoral hard parts of a given Dactylogyrus species was recorded even within a single host specimen of L. maghrebensis71, but without any molecular variability, suggesting phenotypic plasticity and/or selection within a specific microhabitat. On the other hand, as documented above, our molecular data also revealed potential complexes of cryptic species, formerly considered to be a single species solely on the basis of a morphological approach. According to species delimitation analysis, the 38 Dactylogyrus species included in the analysis may in fact represent 47 species. This finding is in accordance with previous studies, in which delimitation analyses were incongruent with classical taxonomy76,77. In our study, Dactylogyrus sp. 2 and Dactylogyrus sp. 3 from L. graecus and L. albanicus, respectively, were shown to be morphologically indistinguishable species; however, molecular data suggest that they are actually two different species (which is also supported by species delimitation analysis). A similar result was revealed for other Dactylogyrus species, such as D. rutili, which seems, on the basis of delimitation analysis, to represent three species parasitizing three host species, and D. dyki, which seems to represent six potential species on 10 Barbus host species. Our future aim will be to undertake the morphometrical reevaluation of taxonomically important traits in combination with the use of molecular data in order to resolve the potential species complexes previously recognized within Dactylogyrus76.
Material and Methods
Parasite sampling
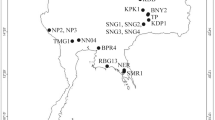
From 2014 to 2017, individuals from 63 cyprinid fish species were sampled from 47 different localities in the Balkan Peninsula and the Czech Republic (Table 5, Fig. 4). Approximately 90% of all endemic cyprinid species in the Balkans were processed in this study15. Fish were dissected using the standard methods described by Ergens and Lom78 and their Dactylogyrus species were collected. More precisely, Dactylogyrus specimens were removed from the gills, mounted on slides, and covered in a mixture of glycerine and ammonium picrate (GAP79) for further determination. All applicable institutional, national and international guidelines for the care and use of animals were followed and approved by the Animal Care and Use Committee of the Faculty of Science, Masaryk University in Brno (Czech Republic). Identification at the species level was performed using an Olympus BX51 microscope equipped with phase contrast optics. Dactylogyrus species were determined using Pugachev et al.45 on the basis of the size and shape of the hard parts of the attachment organ (the haptor) and the reproductive organs (MCO and VA). Some Dactylogyrus specimens from each cyprinid species investigated were bisected using fine needles under a dissecting microscope, and the body part with the haptor was individually preserved in 96% ethanol for further DNA extraction. The remaining body part, i.e. that including the hard parts of the respective reproductive organ, was mounted on a slide for species determination.
Map of collection localities in the Balkans. The sames codes for localities are used in tables under the label LocID. The map was generated in QGIS 3.0.394.
DNA extraction, amplification, and sequencing
Individual parasites were removed from the ethanol and dried using a vacuum centrifuge. DNA was extracted using the standard protocol (DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany). Partial 18S rDNA and the the entire ITS1 region were amplified using the primers S1 (5′-ATTCCGATAACGAACGAGACT-3′) and IR8 (5′-GCTAGCTGCGTTCTTCATCGA-3′)80, which anneal to the 18S and 5.8S rDNA respectively. Partial 28S rDNA was amplified using the following primers: forward C1 (5′-ACCCGCTGAATTTAAGCA-3′) and reverse D2 (5′-TGGTCCGTGTTTCAAGAC-3′)81. Each amplification reaction for partial 18S rDNA and the ITS1 region was performed in a final volume of 15 µl, containing 1.5 units of Taq polymerase, 1X buffer, 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.5 µM of each primer, and 2.5 µl of DNA (20 ng/µl). PCR was carried out using the following steps: 2 min at 94 °C, followed by 40 cycles of 1 min at 94 °C, 1 min at 53 °C, and 1 min 30s at 72 °C, and 10 minutes of final elongation at 72°C. The PCR for partial 28S was performed using the same conditions as described in Šimková et al.82. The PCR products were checked on 1% agarose gel and purified using ExoSAP-IT kit (Ecoli, Bratislava, SK) following the standard protocol. Purified products were directly sequenced using the PCR primers and BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA). Sequencing was performed on an ABI 3130 Genetic Analyzer (Applied Biosystems). New sequences were deposited in GenBank (their accession numbers are shown with asterisks in Table 5).
Phylogenetic analyses
DNA sequences were aligned using fast Fourier transform in MAFFT83. The sequences were trimmed to concur with Dactylogyrus sequences obtained from GenBank. The sequences for 14 Dactylogyrus species from central European cyprinids were obtained by sequencing in this study or acquired from GenBank (see Table 5 for accession numbers).
Genetic distances between specimens of selected Dactylogyrus species collected from different host species were computed using sequences of partial 18S rDNA combined with ITS1 region. Uncorrected pairwise distances were calculated in MEGA 784.
Gaps and ambiguously aligned regions were removed from the alignment using GBlocks v. 0.9185. Phylogenetic analyses using maximum likelihood were computed with RaxML v8.1.X86, and by means of Bayesian inference with MrBayes 3.287. For each analysis, jModelTest 2.1.10 was employed to select the most appropriate model of DNA evolution88,89 using the Bayesian information criterion (BIC). Trees obtained by ML analyses were validated using 1000 bootstrap iterations. Bayesian inference was performed using the Metropolis-coupled Markov chain Monte Carlo algorithm, with 2 parallel runs of 1 cold and 3 hot chains. This was run for 107 generations and trees were sampled every 102 generations. 30% of all saved trees were discarded as a relative burn-in period according to the standard deviation split frequency value (<0.01).
Phylogenetic reconstruction including all sampled Dactylogyrus species was based on concatenated sequences of partial 18S rDNA and partial 28S rDNA (Fig. 1). The resulting phylogram was rooted using the evolutionarily divergent lineage of Dactylogyrus species parasitising Carassius gibelio and Cyprinus carpio12. To resolve the phylogenetic relationships among specific subgroups, partial subtree analyses were performed using partial 18S rDNA combined with the ITS1 region and partial 28S rDNA. Optimal evolutionary models were selected for each marker using BIC, each model including an alpha parameter for the gamma distribution (G) accounting for rate heterogeneity across sites and/or a proportion of invariable sites (I).
Species delineation in the final trees was carried out using a PTP (Poisson Tree Processes) model90. This approach was applied to the BI tree computed from concatenated partial 18S rDNA, 28S rDNA, and the partial ITS1 region, and run for 5 × 105 generations. 30% of the resulting trees were discarded as burn-in. PTP can give species delimitation hypothesis based on gene trees inferred from molecular sequences, modelling the speciation or branching events in terms of the number of mutations. This method does not require an ultrametric input tree or a sequence similarity threshold as input, but uses only the tree resulting from either phylogenetic reconstruction.
The Mantel test91 to test the correlation between genetic and geographical distances was performed in R92 using the mantel function in the vegan package93.
Data Availability
All new sequences of Dactylogyrus obtained during this study were deposited in NCBI GenBank under accession numbers MG792838–MG793066. Appropriate accession numbers according to Dactylogyrus species and specific rDNA regions are presented in Tables 1–3. Since whole fish specimens were completely processed during parasitological dissection, additional specimens of each analysed host species were collected from the same locality and fish vouchers were deposited in the ichthyological collection of the National Museum in Prague (Czech Republic). Voucher specimens of the sequenced Dactylogyrus species (excluding undescribed species) are deposited in the Finnish Museum of Natural History in Helsinki (available under the accession numbers MZH KN10850–989).
References
Brooks, D. R & McLennan, D. A. Parascript: parasites and the language of evolution 429 pp. (Smithonian Institution Press, 1993).
Rohde, K. Ecology and biogeography of marine parasites. Adv. Mar. Biol. 43, 1–86 (2002).
Poulin, R. Evolutionary ecology of parasites, 2nd edition. 214 pp. (Princeton University Press, 2007).
Gibson, D. I., Timofeeva, T. A. & Gerasev, P. I. Catalogue of the nominal species of the monogeneans of genus Dactylogyrus Diesing, 1850 and their host genera. Syst. Parasitol. 35, 3–48 (1996).
Stout, C. C., Tan, M., Lemmon, A. R., Lemmon, E. M. & Armbuster, J. W. Resolving Cypriniformes relationships using anchored enrichment approach. BMC Evol. Biol. 16, 244 (2016).
Šimková, A., Verneau, O., Gelnar, M. & Morand, S. Specificity and specialization of congeneric monogeneans parasitizing cyprinid fish. Evolution 60, 1023–1037 (2006).
Dupont, F. & Lambert, A. Study of the parasitic communities of Monogenea Dactylogyridae from Cyprinidae in Lake Mikri Prespa (Northern Greece). Description of three new species from endemic Barbus: Barbus cyclolepis prespensis Karaman. 1924. Ann. Parasit. Hum. Comp. 6, 597–616 (1986).
Moravec, F. Checklist of the metazoan parasites of fishes of Czech Republic and Slovak Republic (1873–2000) 168 pp. (Academia, 2001).
Galli, P., Stefani, F., Zaccara, S. & Crosa, G. Occurrence of Monogenea in Italian freshwater fish (Po river basin). Parassitologia 44, 189–197 (2002).
Kadlec, D., Šimková, A. & Gelnar, M. The microhabitat distribution of two Dactylogyrus species parasitizing the gills of the barbel, Barbus barbus. J. Helminthol. 77, 317–325 (2003).
Šimková, A., Desdevises, Y., Gelnar, M. & Morand, S. Co-existence of nine gill ectoparasites (Dactylogyrus: Monogenea) parasitising the roach (Rutilus rutilus L.): history and present ecology. Int. J. Parasitol. 30, 1077–1088 (2000).
Šimková, A., Morand, S., Jobet, E., Gelnar, M. & Verneau, O. Molacular phylogeny of congeneric monogenean parasites (Dactylogyrus): a case of intrahost speciation. Evolution 58, 1001–1018 (2004).
Rohde, K. Simple ecological systems, simple solution to complex problems? Evol. Theor. 8, 305–350 (1989).
Šimková, A. & Morand, S. Co-evolutionary patterns in congeneric monogeneans: a review of Dactylogyrus species and their cyprinid hosts. J. Fish Biol. 73, 2210–2227 (2008).
Kottelat, M. & Freyhof, J. Handbook of European freshwater fishes. 646 pp. (Publications Kottelat, 2007).
Palandačić, A., Bravničar, J., Zupančič, P., Šanda, R. & Snoj, A. Molecular data suggest multispecies complex of Phoxinus (Cyprindae) in the Western Balkan Peninsula. Mol. Phylogenet. Evol. 92, 118–123 (2015).
Palandačić, A., Naseka, A., Ramler, D. & Anhelt, H. Contrasting morphology with molecular data: an approach to revision of species complexes based on the example of European Cyprinidae. BMC Evol. Biol. 17, 184 (2017).
Perea, S., Vukić, J., Šanda, R. & Doadrio, I. Ancient mitochondrial capture as factor of promoting mitonuclear discordance in freshwater fishes: a case study in the genus Squalius (Actinopterygii, Cyprinidae) in Greece. PloS ONE 11, e0166292, https://doi.org/10.1371/journal.pone.0166292 (2016).
Stierandová, S. et al. A multilocus assessment of nuclear and mitochondrial sequence data elucidates phylogenetic relationships among European spirlins (Alburnoides, Cyprinidae). Mol. Phylogenet. Evol. 94, 479–491 (2016).
Buj, I. et al. Ancient connections among European rivers and watersheds revealed from the evolutionary history of the genus Telestes (Actinopterygii; Cypriniformes). PloS ONE 12, e0187366 (2017).
Zardoya, R., Economidis, P. S. & Doadrio, I. Phylogenetic relationships of Greek Cyprinidae: molecular evidence for at least two origins of the Greek cyprinid fauna. Mol. Phylogenet. Evol. 13, 122–131 (1999).
Gante, H. F. Diversification of Circum-Mediterranean Barbels In Changing Biodiversity in Changing Environment (eds Grillo O. & Venora G.) 283-298 (Intech. Rijeka, 2011).
Banarescu, P. Zoogeography of Fresh Waters. General distribution and dispersal of freshwater animals. 1, 511 (1991). AULA-Verlag.
Doadrio, I. & Carmona, J. A. Genetic divergence in Greek populations of the genus Leuciscus and its evolutionary and biogeographical implications. J. Fish Biol. 53, 591–613 (1998).
Marková, S. et al. Nuclear and mitochondrial DNA sequence data reveal the evolutionary history of Barbus (Cyprinidae) in the ancient lake systems of the Balkans. Mol. Phylogenet. Evol. 55, 488–500 (2010).
Perea, S. et al. Phylogenetic relationships and biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both mitochondrial and nuclear data. BMC Evol. Biol. 10, 265 (2010).
Imsiridou, A. et al. Genetic differentiation and phylogenetic relationships among Greek Chub Leuciscus cephalus L. (Pisces, Cyprinidae) populations as revealed by RFLP analysis of mitochondrial DNA. Biochem. Syst. Ecol. 26, 415–429 (1998).
Durand, J. D., Templeton, A. R., Guinand, B., Imsiridou, A. & Bouvett, Y. Nested clade and phylogenetic analyses of the chub Leuciscus cephalus (Teleostei, Cyprinidae), in Greece: implications for Balkan Peninsula biogeography. Mol. Phylogenet. Evol. 13, 566–580 (1999).
Doadrio, I. & Carmona, J. A. Testing freshwater Lago Mare dispersal theory on the phylogeny relationships of Iberian cyprinid genera Chondrostoma and Squalius (Cypriniformes, Cyprinidae). Graellsia. 59, 457–473 (2003).
Sanjur, O. I., Carmona, J. A. & Doadrio, I. Evolutionary and biogeographical patterns within Iberian populations of the genus Squalius inferred from molecular data. Mol. Phylogenet. Evol. 29, 20–30 (2003).
Sušnik, S., Snoj, A., Wilson, I. F., Mrdak, D. & Weiss, S. Historical demography of brown trout (Salmo trutta) in the Adriatic drainage including the putative S. letnica endemic to lake Ohrid. Mol. Phylogenet. Evol. 44, 63–76 (2007).
Abell, R. et al. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience. 58, 403–414 (2008).
Albrecht, C. & Wilke, T. Ancient Lake Ohrid: biodiversity and evolution. Hydrobiologia. 615, 103–140.
Schultheiss, R., Albrecht, C., Bössneck, U. & Wilke, T. The neglected side of speciation in ancient lakes: phylogeography of an inconspicuous mollusc taxon in lakes Ohrid and Prespa. Hydrobiologia. 615, 462–467 (2008).
Wagner, B. & Wilke, T. Evolutionary and geological history of the Balkan lakes Ohrid and Prespa. Biogeosciences. 8, 995–998 (2011).
Geiger, M. F. et al. Spatial heterogeneity in the Mediterranean biodiversity hotspot affect barcoding accuracy of its freshwater fishes. Mol. Ecol. Resour. 14, 1210–1221 (2014).
Gregory, R. D. Parasites and host geographic range is illustrated by waterflow. Funct. Ecol. 4, 645–654 (1990).
Seifertová, M., Vyskočilová, M., Morand, S. & Šimková, A. Metazoan parasites of freshwater cyprinid fish (Leuciscus cephalus): testing biogeography hypotheses of species diversity. Parasitology. 135, 1417–1435 (2008).
Stojanovski, S. et al. Fauna of Monogenean Trematods – parasites of some cyprinid fishes from Lake Prespa (Macedonia). Acta Vet. 54, 73–82 (2004).
Stojanovski, S., Hristovski, N., Cakic, P. & Hristovski, M. Fauna of Monogenean Trematods – parasites of some cyprinid fishes from Lake Ohrid (Macedonia). Nat. Monteneg. 4, 61–70 (2005).
Stojanovski, S., Hristovski, N., Velkova-Jordanoska, L., Blazekevic-Dimovska, D. & Atanosov, G. Parasite fauna of Chub (Squalius squalus Bonaparte, 1837) from Lake Ohrid (Fyrmacedonia). Acta Zool. Bulgar. 4, 119–122 (2012).
El Gharbi, S., Renaud, F. & Lambert, A. Dactylogyrids (Platyhelminthes: Monogenea) of Barbus spp. (Teleostei: Cyprinidae) from Iberian Peninsula. Res. Rev. Parasitol. 52, 103–116 (1992).
Šimková, A., Pečínková, M., Řehulková, E., Vyskočilová, M. & Ondráčková, M. Dactylogyrus species parasitizing European Barbus species: morphometric and molecular variability. Parasitology. 134, 1751–1765 (2007).
Dupont, F. Biogeographie historique des Dactylogyrus, monogènes parasites de poisons Cyprinidae dans la peninsula Balkanique. Biol. Gallo-hellenica. 13, 145–152 (1989).
Pugachev, O. N., Gerasev, P. I., Gussev, A. V., Ergens, R. & Khotenowsky, I. Guide to Monogenoidea of freshwater fish of Palearctic and Amur Regions. 564 pp. (Ledizione-Ledi Publishing, 2009).
Musilová, N., Řehulková, E. & Gelnar, M. Dactylogyrids (Platyhelminthes: Monogenea) from the gills of the african carp, Labeo coubie Rüppel (Cyprinidae), from Senegal, with description of three new species of Dactylogyrus and the redescription of Dactylogyrus cyclocirrus Paperna, 1973. Zootaxa. 2241, 47–68 (2009).
Poulin, R. & Morand, S. Parasite biodiversity. 216 pp. (Smithonians Book, 2004).
Sinaré, Y., Boungou, M., Ouéda, A., Gnémé, A. & Kabré, G. B. Diversity and seasonal distribution of parasites of Oreochromis niloticus in semi-arid reservoirs (West Africa, Burkina Faso). Afr. J. Agr. Res. 11, 1164–1170 (2016).
González-Lanza, C. & Alvarez-Pellitero. Description and population dynamics of Dactylogyrus legionensis n.sp. From Barbus barbus bocagei Steind. J. Helminthol. 56, 263–273 (1982).
Lux, E. Population dynamics and interrelationships of some Dactylogyrus and Gyrodactylus species on Cyprinus carpio. Angew. Parasitol. 31, 143–149 (1990).
Appleby, C. & Mo, T. A. Population dynamics of Gyrodactylus salaris (Monogenea) infecting Atlantic salmon, Salmo salar, Parr in the river Batnfjordselva, Norway. J. Parasitol. 83, 23–30 (1997).
Šimková, A., Sasal, P., Kadlec, D. & Gelnar, M. Water temperature influencing Dactylogyrus species communities in roach, Rutilus rutilus, in Czech Republic. J. Helminthol. 75, 373–383 (2001).
Zhang, G., Yan, S., Wang, M., Gibson, D. I. & Yang, T. Population and community dynamics of four species of Pseudodactylogyrus (Monogenea, Dactylogyridae) on Japanese eel, Anguilla japonica (Temminck and Schlegel, 1846) cultured in two Chinese fish farms. Turk. J. Fish Aquat. S. 15, 887–897 (2015).
Ergens, R. The parasite fauna of fishes from Montenegro. I. Polyonchoinea (Monogenoidea) of some fishes of the Lakes Skadar and Veliko Crno. Pol’Oprivreda i Shumarstvo 16, 1–44 (1970).
Bianco, P. G. Mediterraneanmisc endemic freshwater fishes of Italy. Biol. Conserv. 72, 159–170 (1995).
Crivelli, A. J. Rutilus rubilio. The IUCN Red List of Threatened Species 2006: e. T19786A9014268, https://doi.org/10.2305/IUCN.UK.2006.RLTS.T19786A9014268.en (2006)
Ketmaier, V., Bianco, P. G. & Durand, J.-D. Molecular systematics, phylogeny and biogeography of roaches (Rutilus, Teleostei, Cyprinidae). Mol. Phylogenet. Evol. 49, 362–367 (2008).
Levin, B. A. et al. Phylogeny and phylogeography of the roaches, genus Rutilus (Cyprinidae), at the Eastern part of its range as inferred from mtDNA analysis. Hydrobiologia. 788, 33–46 (2017).
Bianco, P. G. Potentional role of the paleohistory of the Mediterranean and Parathethys basins on the early dispersal of Euro-Mediterranean freshwater fishes. Ichthyol. Explor. Fres. 1, 167–184 (1990).
Bianco, P. G. The zoogeographic units of Italy and western Balkans based on cyprinid species ranges (Pisces). Biol. Galleo-Hellenica. 12, 291–299 (1986).
Ivanovic, B. M. Ichthyofauna of Skadar Lake. 146pp. (Biological Station, 1973).
Yang, L. et al. Phylogeny and polyploidy: resolving classification of cyprinine fishes (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 85, 97–116 (2015).
Mandeng, F. D. M. et al. Phylogeny of Cichlidogyrus spp. (Monogenea: Dactylogyridea) clarifies a host-switch between fish families and reveals an adaptive component to attachment organ morphology of this parasite genus. Parasit. Vectors 8, 582 (2015).
Vignon, M., Pariselle, A. & Vanhove, M. P. M. Modularity attachment organs of African Cichlidogyrus (Platyhelminthes: Monogenea: Ancyrocephalidae) reflects phylogeny rather than host specificity. Biol. J. Linn. Soc. 102, 694–706 (2011).
Benovics, M., Kičinjaová, M. L. & Šimková, A. The phylogenetic position of the enigmatic Balkan Aulopyge huegelii (Teleostei: Cyprinidae) from the perspective of host-specific Dactylogyrus parasites (Monogenea), with a description of Dactylogyrus omenti n. sp. Parasit. Vectors. 10, 547 (2017).
Koskivaara, M. & Valtonen, E. T. Dactylogyrus (Monogenea) communities on the gills of roach in three lakes in Central Finland. Parasitology 104, 263–272 (1992).
Jarkovský, J., Morand, S. & Šimková, A. Reproductive barriers between congeneric monogenean parasites (Dactylogyrus: Monogenea): attachment apparatus morphology or copulatory organ incompatibility? Parasitol. Res. 92, 95–105 (2004).
Piria, M. et al. Alien freshwater fish species in the Balkans - Vectors and pathways of introduction. Fish Fish. 2017, 1–32 (2017).
Koutsikos, N. et al. Recent contributions to the distribution of the freshwater ichthyofauna in Greece. Mediterr. Mar. Sci. 13, 268–277 (2012).
Economou, A. N. et al. The freshwater ichthyofauna of Greece – an update based on a hydrographic basin survey. Mediterr. Mar. Sci. 8, 91–166 (2007).
Glamuzina, B. et al. Comparison of taxon-specific and taxon-generic risk screening tools to identify potentially invasive non-native fishes in the river Neretva catchment (Bosnia and Herzegovina and Croatia). River Res. Applic. 33, 670–679 (2017).
Jelić, D., Špelić, I. & Žutinić, P. Introduced species community over-dominates endemic ichthyofauna of High Lika Plateau (central Croatia) over a 100 year period. Acta Zool. Acad. Sci. H. 62, 191–216 (2016).
Rohde, R. & Watson, N. Morphology, microhabitats and geographical variation of Kuhnia spp. (Monogenea: Polyopisthicotylea). Int. J. Parasitol. 15, 569–586 (1985).
Boeger, W. A. & Kritsky, D. C. Neotropical Monogenea. 12. Dactylogyridae from Serrasalmus natteri (Cypriniformes, Serrasalmidae) and aspects of their morphologic variation and distribution in Brazilian Amazon. P. Helm. Soc. Washi. 55, 188–213 (1988).
Vignon, M. & Sasal, P. The use of geometric morphometrics in understanding shape variability of sclerotized haptoral structures of monogeneans (Platyhelminthes) with insights into biogeographic variability. Parasitol. Int. 59, 183–191 (2010).
Rahmouni, I., Řehulková, E., Pariselle, A., Rkhami, O. B. & Šimková, A. Four new species of Dactylogyrus Diesing, 1850 (Monogenea: Dactylogyridae) parasitising the gills of northern Morrocan Luciobarbus Heckel (Cyprinidae): morphological and molecular characterisation. Syst. Parasitol. 94, 575–591 (2017).
Jousselin, E., Desdevises, Y. & Coeur d’acier, A. Fine-scale cospeciation between Brachycaudus and Buchnera aphidicola: bacterial genome helps define species and evolutionary relationships in aphids. P. Roy. Soc. B 276, 187–196 (2009).
Ergens, R. & Lom, J. Causative agents of fish diseases. 384 pp. (Academia, 1970).
Malmberg, G. Om forekomsten av Gyrodactylus pa svenska fiskar. Skrifter Utgivna av Sodra Sveriges Fiskeriforening. Arsskift 1956, 19–76 (1957).
Šimková, A., Plaisance, L., Matějusová, I., Morand, S. & Verneau, O. Phylogenetic relationships of the Dactylogiridae Bychowsky, 1933 (Monogenea: Dactylogyridae): the need for the systematic revision of the Ancyrophalinae Bychowsky, 1937. Sys. Parasitol. 54, 1–11 (2003).
Hassouna, N., Michot, B. & Bachellerie, J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large suburnit rRNA in higher eukaryotes. Nucleic Acids Res. 12, 3563–3583 (1984).
Šimková, A., Matějusová, I. & Cunningham, C. O. A molecular phylogeny of the Dactylogyridae sensu Kritsky & Boeger (1989) (Monogenea) based on the D1-D3 domains of large subunit rDNA. Parasitology 133, 43–53 (2006).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2015).
Talavera, G. & Castresana, J. Improvement of phylogenies after removing divergent and amigously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577 (2007).
Stamatakis, A. RaxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across large model space. Syst. Biol. 61, 539–542 (2012).
Guindon, S. & Gascuel, O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 27, 1759–1767 (2003).
Darriba, D., Taboala, G. L., Doallo, R. & Posada, D. JModelTest2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012).
Zhang, J., Kapli, P., Pavlidis, P. & Stamakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876 (2013).
Mantel, N. The detection of desease clustering and generalized regression approach. Cancer Res. 27, 209–220 (1967).
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/ (2017).
Oksanen, J. et al. Vegan: community ecology packageR package version 2.4-2. https://CRAN.R-project.org/package=vegan (2017).
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation. http://www.qgis.org/ (2018).
Acknowledgements
We are grateful to Jaroslav Červenka, Milan Gelnar, Maria Lujza Červenka Kičinja, Kristýna Koukalová, Tomáš Pakosta, Eva Řehulková, Kateřina Vyčítalová and Petra Zahradníčková for their help with fish dissection and parasite collection and to Ivan Bogut, Dario Marić, Spase Shumka, Denik Ulqini, Ivana Buj, Zoran Marčić, and Stamatis Zogaris for help with host fish specimen collection. We kindly thank Matthew Nicholls for English revision of the final draft and Timo K. Pajunen from the Finnish Museum of Natural History for his curatorial services. This study was funded by the Czech Science Foundation (project number 15-19382S).
Author information
Authors and Affiliations
Contributions
A.Š. designed and supervised the study. A.Š., J.V. and R.Š. organized the field trip and fish and parasite collection. J.V. and R.Š. collected and identified fish in the field and provided the background on the host phylogeny and distribution. A.Š. and M.B. processed fish and collected parasites during the field trip. M.B. performed microscopical observations, determination of Dactylogyrus species and all laboratorty procedures. M.B. and Y.D. performed phylogenetic and statistical analyses. M.B. wrote the draft of the manuscript. A.Š., Y.D., J.V. and R.Š. revised the manuscript. All authors read and approved final version of manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Benovics, M., Desdevises, Y., Vukić, J. et al. The phylogenetic relationships and species richness of host-specific Dactylogyrus parasites shaped by the biogeography of Balkan cyprinids. Sci Rep 8, 13006 (2018). https://doi.org/10.1038/s41598-018-31382-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31382-w
This article is cited by
-
First report of Dactylogyrus formosus from India: a case of co-existence with Paradactylogyrus catlaius causing mass mortality in Indian major carp, Catla catla
Parasitology Research (2024)
-
A host choosy gill parasite (Dactylogyrus spp.) in fish: an insight into host-parasite interaction for developing control strategies
Aquaculture International (2024)
-
Morphological and Genetic Divergence in a Gill Monogenean Parasitizing Distant Cichlid Lineages of Lake Tanganyika: Cichlidogyrus nshomboi (Monogenea: Dactylogyridae) from Representatives of Boulengerochromini and Perissodini
Evolutionary Biology (2022)
-
Molecular footprint of parasite co-introduction with Nile tilapia in the Congo Basin
Organisms Diversity & Evolution (2022)
-
Molecular and morphological phylogeny of host-specific Dactylogyrus parasites (Monogenea) sheds new light on the puzzling Middle Eastern origin of European and African lineages
Parasites & Vectors (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.