Abstract
Crop improvement programs focus on characteristics that are important for plant productivity. Typically genes underlying these traits are identified and stacked to create improved cultivars. Hence, identification of valuable traits for plant productivity is critical for plant improvement. Here we describe an important characteristic for maize productivity. Despite the fact mature maize ears are typically covered with kernels, we find that only a fraction of ovaries give rise to mature kernels. Non-developed ovaries degenerate while neighboring fertilized ovaries produce kernels that fill the ear. Abortion occurs throughout the ear, not just at the tip. We show that the fraction of aborted ovaries/kernels is genetically controlled and varies widely among maize lines, and low abortion genotypes are rare. Reducing or eliminating ovary abortion could substantially increase yield, making this characteristic a new target for selection in maize improvement programs.
Similar content being viewed by others

Introduction
A grand challenge to our society is feeding the growing human population while facing unfavorable climate change. Plant improvement programs are tasked with increasing yield on less land, in harsher environments, and with reduced inputs for plant growth. A myriad of plant characteristics ranging from disease, pest, and abiotic stress resistance, to photosynthesis, respiration, and nitrogen fixation, are targets for plant improvement. The kernel or grain, the harvested unit for many crops, has also received particular attention. Maize, or corn, is the largest yielding crop in the US with over 380 million metric tons produced in 20161. The maize kernel is a staple food in the diet of much of the world’s population, is a major feed source for livestock production, and provides raw materials for many products that we use in our daily lives.
Grain yield in maize is a product of individual kernel weight and kernel number; hence, factors affecting them have received substantial attention2,3,4,5,6. The potential number of kernels is determined by the number of mature florets on the ear inflorescence. Each floret contains an ovary with a single megametophyte that can develop into a kernel7. Failure of kernels to develop from ovaries, here termed ovary abortion reduces grain number on the ear. Kernel number, rather than kernel weight, has the larger impact on yield8. Therefore, strategies that maximize kernel number could translate into increased yield.
The typical maize ear is covered with kernels, and their removal reveals no evidence of undeveloped ovaries or kernels. Hence, it is surprising, as documented here, that only 60 to 65% of the maize ovaries commonly give rise to a functional, developed kernel under typical field conditions. We show that genetic variation exists for ovary abortion, hence making this trait potentially amendable to genetic selection for improved yield in maize breeding programs.
Results
Ovary abortion is common in maize inbred lines
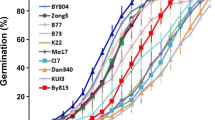
Prompted from observations of poor kernel set in experimental maize lines9, we counted the number of kernels that failed to develop on ears of the 26 parental inbred lines of the maize Nested Association Mapping (NAM) population10. These lines contain tropical, temperate, sweet corn and popcorn types and represent a majority of the common genetic variation found in maize11. The number of kernels that failed to develop was determined by comparing the number of silks 4–5 days after their emergence to the number of developed kernels at maturity. Plotted in Fig. 1a is the percentage of silks that do not give rise to a developed kernel in five inbred lines spanning the phenotypic spectrum. Abortion among the diverse inbred lines ranged from less than 10% to over 80% (Supplementary Table S1). Many ears visually identified as having full kernel set actually had relatively large rates of abortion (Fig. 1b). For example, the imaged B73 ear lost more than 180 kernels (31%), yet appears to have similar kernel set as the CML333 ear which lost less than 10% of its kernels.
Ovary abortion varies among diverse inbred lines. Selected inbred abortion rates (a) and selected ears (b) from 2015 fall nursery with low, average, or high abortion phenotypes. The numbers in parentheses are the percentages of missing kernels on the imaged ears. Error bars represent standard error of the mean.
Importantly, abortion was significantly correlated (r = 0.52, p < 0.001) and heritable (H2 = 0.62) across multiple seasons (Fig. 2). Plants grown in the Florida spring nursery (April-July) are typically exposed to increasing day length and temperature during the growing season, in contrast to the fall season (August-December) when cooler temperatures promote slower plant and kernel development. Despite these different growing conditions, there was no difference in mean abortion between the two seasons (p = 0.42, Welch two-sample t-test). Average abortion based on silk and mature kernel counts in the spring crop was 33% ± 14% standard deviation and 30% ± 15% standard deviation in the fall crop. While the materials analyzed here were inbred maize lines, it is interesting to note that commercial hybrids have also shown substantial kernel loss. A Mycogen hybrid exhibited 43% kernel loss under non-stressed growing conditions9, two Nidera hybrids grown under optimal field conditions lost more than 30% of kernels12, and a Dekalb hybrid varied between 32 and 38% kernel loss in unstressed treatments13. These observations suggest that sensitivity to kernel loss in some hybrids is on par with the mean kernel loss in the inbred lines used in this study.
Ovary abortion among diverse maize inbred lines. The scatterplot shows mean values from 26 NAM parental inbreds grown in 2015 spring and fall nurseries. The plot shows a linear regression trend line (solid line) and one-to-one correspondence line (dashed line). Error bars represent standard error of the mean calculated from 6 ears per data point on average. Correlation coefficient (r) and broad sense heritability (H2) are shown.
Ovary abortion occurs around the time of pollination and is distributed throughout the ear
Failure to form a kernel could occur for a variety of reasons, such as a defective ovary, pollination failure, or abortion of the fertilized ovary. In this study, the terms innate ovary abortion and kernel loss are used for all these scenarios, although failure of a fertilized ovary to develop into a fully mature kernel would be kernel rather than ovary abortion. To define the timing of kernel failure, we compared the number of ovaries on non-pollinated ears, enlarging ovaries during development, and final number of developed kernels in two maize inbred lines, B73 and Mo17. These lines were chosen because of their historic importance in maize improvement programs and because they differ in final kernel number. While B73 ears contained 731 ± 53 ovaries before pollination, mature ears exhibited only 362 ± 61 kernels at maturity (Fig. 3). The loss of 50.5% of potential kernels in B73 occurred early in development since only 400 ± 143 ovaries exhibited any enlargement seven days post-pollination. Analogous data were obtained from the inbred Mo17. Initial ovary number averaged 532 ± 28 while mature ears contained only 134 ± 65 (25.2%) kernels. Like B73, a substantial number of the initial ovaries exhibited no enlargement, since only 240 ± 23 (45.1%) increased in size seven days after pollination. In contrast to B73, not all enlarged ovaries at seven days post-pollination developed into kernels. Despite the apparent late stage loss in Mo17, the majority of potential kernels in both inbred lines were lost before or soon after pollination.
Enlarging ovaries or kernels as a function of development in inbreds B73 and Mo17. Shown at 0 days post-pollination (DPP) is the average number of ovaries on an unpollinated ear. Shown at 40 DPP is the average number of developed kernels on a mature ear at harvest. Error bars represent standard error of the mean calculated from 5 ears per data point on average.
In the maize female inflorescence, florets at the base of the ear mature earlier than those at the tip and are first to draw upon the photoassimlate supply14. These developmental and proximity gradients have implications for which ovaries will set seed when resources are limiting14. For example, drought stress at pollination induces kernel loss primarily at the tip of the ear15,16. To test whether innate abortion is asymmetrically distributed, ears of B73 and Mo17 were divided into five equal sections and the numbers of ovaries, enlarging ovaries, and mature kernels were determined during ear development. The percentages of ovaries giving rise to a developed kernel in the five regions of the ear are shown in Fig. 4. The highest frequency of kernel development occurred in the midsection of B73 ears, with reduced kernel set at the extremities of the ear. Mo17 exhibited an entirely different pattern. The highest percentage of kernel development occurred at the tip of the ear, while the lowest probability for kernel development occurred at the base. Unlike drought stress, the probability of kernel development exhibited a gradient on the ear and was genotype specific. Importantly, cob length following silk emergence of B73 and Mo17 changed only slightly (Fig. 5), showing that ovary position remained stationary during development. This ensured that the frequency of kernel development at various locations on the ear could be determined.
Because kernel loss occurs at the time of pollination or soon after, the number of silks was counted to determine whether inadequate silk number caused kernel losses. The number of silks equaled 84% and 91% of the number of ovaries in B73 and Mo17, respectively (Table 1). A sweet corn inbred containing the shrunken-2 mutation was also evaluated to expand this data set. Ovary number equaled 517 ± 109, whereas 487 ± 93 silks were observed. Silk number in all three lines was comparable to ovary number; hence the number of silks was not the primary factor limiting kernel set12,17.
Temperatures at the Florida nursery are typically hot during kernel set in the summer season. The average daily high and low temperatures during the first 12 to 15 days following fertilization of the B73 and Mo17 genotypes were 33.6 °C and 21.9 °C, respectively (http://fawn.ifas.ufl.edu/). Accordingly, the experiment was repeated in the more moderate climate of Madison, Wisconsin during the unusually mild 2014 growing season where the average high and low daily temperature during pollination were 26 °C and 15 °C, respectively. In Wisconsin, kernel set for Mo17 and B73 equaled 46% and 45% of ovaries, respectively (Table 2). While kernel set for Mo17 was higher in Wisconsin than in Florida, the lower temperatures of Wisconsin did not produce kernel set ratios approaching 100%.
The silk and ovary data show that the silk counts used to measure kernel loss in Fig. 1 and Supplementary Table S1 most likely underestimate the number of ovaries and therefore underestimate kernel loss. Incorporation of this adjustment leads to the conclusion that only 61.4% of the ovaries give rise to a developed kernel, on average, in the NAM inbred lines.
Kernel development accompanies disintegration of neighboring ovaries
Examination of typical maize ears revealed no ovaries remained on the cob following kernel removal. To gain further insight into the disappearance of ovaries during development, we measured the number of ovaries on non-pollinated ears at two days post-silking and at the time of normal ear maturity (40 days post-pollination in the Florida spring environment). Non-pollinated Mo17 ears contained 460.5 ovaries at two days post-silking and 411.5 ovaries (89.4%) at maturity. Corresponding ovary counts for non-pollinated B73 were 730.5 and 680.3 (93.1%). Hence in the absence of fertilization and kernel development, ovaries remain intact and do not disintegrate during the maturation of the plant. However, upon fertilization the non-developing ovaries and the space they occupy are consumed by expansion of neighboring kernels. Time-lapse images of a W22 inbred ear illustrate how space of undeveloped ovaries is filled during development (Fig. 6). Ovaries not enlarging can be distinguished on the ear eight days post-pollination (DPP). The space occupied by the non-developing ovaries at eight DPP is covered by developing kernels during the next 17 days of development. The lack of visual evidence of ovary abortion on the mature ear shows that the degree of kernel loss cannot be determined by examining the ear at harvest (Fig. 1b).
Taken together, these data show that only 60 to 65% of maize ovaries in diverse inbred lines commonly develop into mature kernels. Ovaries not fertilized, or those that are fertilized but do not develop, are consumed as nearby kernels develop. How the “develop/do not develop” decision is made is not understood. It is interesting to note that mutant maize kernels lacking the gene expression necessary for early kernel development, termed empty pericarp, are not selected against in this process since the pericarp develops and is present in the mature ear. Many of the defective kernel mutants exhibit typical Mendelian 3 to 1 ratios in F2 generations18,19,20,21,22, suggesting that post-fertilization seed developmental defects are not a factor in the abortion decision.
Discussion
We found that ovary abortion is significant in maize grown in conventional field conditions, and genetic variation exists for this trait. Hence, maize breeders now have an additional trait to exploit in maize breeding programs. The extent to which reduced ovary abortion and how it is impacted by stress conditions will affect yield is presently unknown. However, it is noteworthy that expression of trehalose phosphate phosphatase (TPP) in developing maize florets increased kernel number and increased yield in hybrids under non-stressed conditions23. Furthermore, maize transgenes encoding an enzyme in the starch biosynthetic pathway reduced ovary abortion, and thereby increased kernel number without decreasing individual kernel weight9,24. These data suggest that mitigating ovary abortion, even in a hybrid context9, can positively impact yield under optimal cultivation conditions.
The cellular mechanisms underlying ovary abortion are not understood. Many previous studies of ovary abortion in maize have involved the imposition of an environmental stress at the time of kernel set, when maize is most susceptible to kernel loss. Drought stress imposed three days prior to silking is sufficient to substantially reduce kernel set25,26. Failure to set kernels is not due to lack of fertilization, but rather abortion of the floral ovary near the time of pollination. Two explanations for this abortion have been put forward.
One hypothesis focuses on altered carbohydrate metabolism2,27,28. Starch reserves in ovaries are depleted after several days of drought stress when senescence-related transcripts begin to accumulate marking the onset of abortion4,5. Addition of exogenous sucrose to water-stressed plants partially restores kernel set2, suggesting that carbohydrate status of the ovary is critical for determining whether an ovary will set seed during environmentally stressful conditions.
Consistent with the hypothesis that inadequate carbohydrate supply reduces kernel set, the sugar trehalose-6-phosphate (T6P) has been implicated in ovary abortion in maize. T6P is a metabolic intermediate of the trehalose biosynthetic pathway and is an important signaling molecule that integrates sugar status with growth and development in plants29,30,31,32. T6P is produced by trehalose phosphate synthase (TPS) from UDP-glucose and glucose-6-phosphate, and T6P is converted to trehalose via trehalose-6-phosphate phosphatase (TPP)33. The T6P concentration closely follows that of sucrose in most tissues. However, in floral tissues T6P and sucrose levels were found to be inversely related around the time of pollination, with T6P levels increasing as sucrose levels decrease34. Decoupling of the T6P/sucrose relationship in floral tissues may be important for seed set. Nuccio et al.23 found that reducing T6P by transgenically expressing TPP in maize florets increased sucrose content and significantly increased kernel number. The increase in kernel number was most likely due to reduced ovary abortion, although the ovary number was not counted. The slight increase in sucrose in floral organs was proposed to mitigate ovary abortion and increased kernel number in drought stressed plants.
In an alternative hypothesis, Oury et al.17,35, invoked drought-induced reductions in cell expansion and silk growth as the major cause of ovary abortion. These investigators noted that low light intensities caused the same reduction in the amount of photosynthate entering the ovary as caused by water stress, yet severe abortion did not occur. They found that water stress delayed silk emergence and the time period of total silk growth. Under extreme drought, some silks did not emerge from the husks and hence could not be used for fertilization. Furthermore, expression analysis showed that genes involved in cell wall properties were among the first to be altered, rather than genes involved in carbohydrate metabolism. Hence, these observations point to lack of silk growth as the cause of reduced kernel set. Oury et al.17,35, do note that their data do not exclude the possibility that carbohydrate status may also play a role, so the two models are not mutually exclusive. Genetic variants, as noted here, should aid in identifying the actual mechanism of drought induced seed abortion.
While studies of stress-induced ovary abortion invariably find that most abortion occurs at the tip of maize ears14,17, it is surprising that the abortion described here under typical field conditions does not follow this pattern. In this case, abortion occurs throughout the ear. While abortion in B73 is greatest at the tip, a substantial number of kernels are also lost at the middle and base of the ear. The pattern in Mo17 is striking. The highest seed set is actually at the tip of the ear, with a gradient of reduced seed set toward the base of the ear. Hence, abortion can occur at any location along the ear, and does not always proceed basipetally. This observation has bearing on the mechanism of abortion. It is unclear why one floret would fail, while neighboring florets proceed to kernel development. Currently proposed mechanisms, based upon basipetal patterns of abortion, predict that neighboring ovaries have different ovary/silk growth rates17,35,36 or carbohydrate levels28,32,34,36. However in multiple hybrid lines, neighboring ovaries were found to have very similar silk growth rates and carbohydrate contents17,35. It is possible that florets can suppress the development of their immediate neighbors through well-established dominance signals37. But it should be noted that pollinations of the NAM inbred lines were conducted synchronously, such that all available silks received pollen at once. This should have ensured synchronous fertilization of the ovaries and avoided the confounding effects of early fertilized kernels preventing the development of kernels on later silking florets as found in natural pollinations36.
In summary, our observations reveal a little reported, but potentially important phenotype, innate ovary abortion, which could have important implications for maize breeding. A substantial number of ovaries do not develop under typical field conditions, and there is genetic variation for this trait. Hence, ovary abortion is a viable target phenotype for breeding maize hybrids with higher seed set. This study reveals patterns of abortion not noted in previous studies and identifies genetic variation can be used to decipher the mechanisms of kernel abortion.
Materials and Methods
Plants were grown at the University of Florida Plant Science Research and Education Center in Citra, Florida, or at the University of Wisconsin farm facility near Madison, Wisconsin. Plots in Florida were sown with a low planting density of approximately 30,000 plants ha−1 using 36 inch row spacing. Fertilizer was applied four times during the growth period, totaling 240 lbs N, 54 lbs P and 225 lbs K per acre. Water was provided as needed by overhead irrigation. Plots were kept well-watered. Each inbred was grown in a single block surrounded by other corn lines to maximize pollination. Pollinations within each block of B73 and Mo17 inbred lines were synchronized6 such that pollination initiation of experimental ears within each inbred occurred on the same day (June 13, 2013 for B73 and June 17, 2013 for Mo17). Only plants having silks visible under the coverings for one to two days were chosen for pollination. Coverings were removed and ears were hand pollinated with pollen from sibling plants. Ears remained uncovered to allow subsequent pollinations from neighboring plants. Ears were harvested at 0, 7, 14, 21 and 28 days post-pollination (DPP). Ears were also harvested at maturity (around 40 days in the Florida spring environment). Each developmental time point was represented by at least five ears. Ear length was measured and each ear was divided into five equally-sized sections. The number of enlarging and non-enlarging ovaries, as well as the number of developed kernels on mature ears, was determined in each section. Ovary number on non-pollinated ears harvested at the normal time of maturity was also determined. Silk number on ears harvested at 0 DPP was also determined. Non-pollinated ears harvested at approximately two days post-silking as well as pollinated ears at maturity were harvested in Wisconsin and shipped to Florida for counting. All data were obtained by manual counting.
The 26 NAM inbred lines have a wide range of flowering times. To minimize the influence of pollination date on measured traits, the lines were planted at staggered dates according to their flowering times and all pollinations were conducted within a 17 day and 13 day window in the 2015 spring and fall nurseries, respectively. Pollinations for the NAM parental lines were conducted as described for B73 and Mo17 above except that ears were cut back to the ear tip one day prior to pollination and the ears were covered after sibling pollinations preventing pollination from neighboring plants. Ovary number was estimated from the number of silks counted at the time of pollination (Table 1) and therefore slightly underestimate the total number of ovaries on the ear (Tables 1 and 2).
Statistical Analysis
Broad-sense heritability (H2) was defined as: H2 = MSgen (MSgen + MSyear + MSer + MSgenMSer)−1, where MSgen is the genotype mean square, MSyear is the season mean square, and MSer is the error mean square from a two-way ANOVA.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Crop Production. (National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA) (2017).
Zinselmeier, C., Jeong, B. R. & Boyer, J. S. Starch and the control of kernel number in maize at low water potentials. Plant Physiol 121, 25–36 (1999).
Campos, H., Cooper, A., Habben, J., Edmeades, G. & Schussler, J. Improving drought tolerance in maize: a view from industry. Field Crops Research 90, 19–34, https://doi.org/10.1016/j.fcr.2004.07.003 (2004).
McLaughlin, J. E. & Boyer, J. S. Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann Bot 94, 675–689, https://doi.org/10.1093/aob/mch193 (2004).
McLaughlin, J. E. & Boyer, J. S. Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Ann Bot 94, 75–86, https://doi.org/10.1093/aob/mch123 (2004).
Uribelarrea, M., Carcova, J., Borras, L. & Otegui, M. Enhanced kernel set promoted by synchronous pollination determines a tradeoff between kernel number and kernel weight in temperate maize hybrids. Field Crops Research 105, 172–181, https://doi.org/10.1016/j.fcr.2007.09.002 (2008).
Willemse, M. & Went, J. The Female Gametophyte. (Springer-Verlag, 1984).
Borras, L. & Gambin, B. Trait dissection of maize kernel weight: Towards integrating hierarchical scales using a plant growth approach. Field Crops Research 118, 1–12, https://doi.org/10.1016/j.fcr.2010.04.010 (2010).
Hannah, L. C. et al. A shrunken-2 transgene increases maize yield by acting in maternal tissues to increase the frequency of seed development. Plant Cell 24, 2352–2363, https://doi.org/10.1105/tpc.112.100602 (2012).
McMullen, M. D. et al. Genetic properties of the maize nested association mapping population. Science 325, 737–740, https://doi.org/10.1126/science.1174320 (2009).
Liu, K. et al. Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 165, 2117–2128 (2003).
Rossini, M., Maddonni, G. & Otegui, M. Inter-plant variability in maize crops grown under contrasting N x stand density combinations: Links between development, growth and kernel set. Field Crops Research 133, 90–100, https://doi.org/10.1016/j.fcr.2012.03.010 (2012).
Uhart, S. A. & Andrade, F. H. Nitrogen Deficiency in Maize: II. Carbon-Nitrogen Interaction Effects on Kernel Number and Grain Yield. Crop Science 35, 1384–1389 (1995).
Carcova, J. & Otegui, M. Ovary growth and maize kernel set. Crop Science 47, 1104–1110, https://doi.org/10.2135/cropsci2006.09.0590 (2007).
Setter, T., Flannigan, B. & Melkonian, J. Loss of kernel set due to water deficit and shade in maize: Carbohydrate supplies, abscisic acid, and cytokinins. Crop Science 41, 1530–1540, https://doi.org/10.2135/cropsci2001.4151530x (2001).
Setter, T. L. & Parra, R. Relationship of Carbohydrate and Abscisic Acid Levels to Kernel Set in Maize under Postpollination Water Deficit. Crop Science 50, 980–988, https://doi.org/10.2135/cropsci2009.07.0391 (2010).
Oury, V., Tardieu, F. & Turc, O. Ovary Apical Abortion under Water Deficit Is Caused by Changes in Sequential Development of Ovaries and in Silk Growth Rate in Maize. Plant Physiol 171, 986–996, https://doi.org/10.1104/pp.15.00268 (2016).
Neuffer, M. G. & Sheridan, W. F. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95, 929–944 (1980).
Sheridan, W. F. & Neuffer, M. G. Defective Kernel Mutants of Maize II. Morphological and Embryo Culture Studies. Genetics 95, 945–960 (1980).
McCarty, D. R. et al. Steady-state transposon mutagenesis in inbred maize. Plant J 44, 52–61, https://doi.org/10.1111/j.1365-313X.2005.02509.x (2005).
Settles, A. M. et al. Sequence-indexed mutations in maize using the UniformMu transposon-tagging population. BMC Genomics 8, 116, https://doi.org/10.1186/1471-2164-8-116 (2007).
Hasnain, G. et al. Identification and characterization of the missing pyrimidine reductase in the plant riboflavin biosynthesis pathway. Plant Physiol 161, 48–56, https://doi.org/10.1104/pp.112.208488 (2013).
Nuccio, M. L. et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33, 862–869 (2015).
Hannah, L. C., Shaw, J. R., Clancy, M. A., Georgelis, N. & Boehlein, S. K. A brittle‐2 transgene increases maize yield by acting in maternal tissues to increase seed number. Plant Direct 1, e00029, https://doi.org/10.1002/pld3.29 (2017).
Schussler, J. & Westgate, M. Maize kernel set at low water potential. 1. Sensitivity to reduced assimilates during early kernel growth. Crop Science 31, 1189–1195, https://doi.org/10.2135/cropsci1991.0011183X003100050023x (1991).
Schussler, J. & Westgate, M. Maize kernel set at low water potential. 2. Sensitivity to reduced assimilates at pollination. Crop Science 31, 1196–1203, https://doi.org/10.2135/cropsci1991.0011183X003100050024x (1991).
Makela, P., McLaughlin, J. & Boyer, J. Imaging and quantifying carbohydrate transport to the developing ovaries of maize. Annals of Botany 96, 939–949, https://doi.org/10.1093/aob/mci246 (2005).
Westgate, M. & Boyer, J. Reproduction at low silk and pollen water potentials in maize. Crop Science 26, 951–956, https://doi.org/10.2135/cropsci1986.0011183X002600050023x (1986).
Nunes, C. et al. The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol 162, 1720–1732, https://doi.org/10.1104/pp.113.220657 (2013).
Schluepmann, H., Pellny, T., van Dijken, A., Smeekens, S. & Paul, M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100, 6849–6854, https://doi.org/10.1073/pnas.1132018100 (2003).
Lunn, J. E. et al. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397, 139–148, https://doi.org/10.1042/BJ20060083 (2006).
Ramon, M. & Rolland, F. Plant development: introducing trehalose metabolism. Trends Plant Sci 12, 185–188, https://doi.org/10.1016/j.tplants.2007.03.007 (2007).
Londesborough, J. & Vuorio, O. Purification of trehalose synthase from bakers-yeast - Its temperature-dependent activation by fructose 6-phosphate and inhibition by phosphate. European Journal of Biochemistry 216, 841–848, https://doi.org/10.1111/j.1432-1033.1993.tb18206.x (1993).
Henry, C. et al. Differential Role for Trehalose Metabolism in Salt-Stressed Maize. Plant Physiol 169, 1072–1089, https://doi.org/10.1104/pp.15.00729 (2015).
Oury, V. et al. Is Change in Ovary Carbon Status a Cause or a Consequence of Maize Ovary Abortion in Water Deficit during Flowering? Plant Physiology 171, 997–1008, https://doi.org/10.1104/pp.15.01130 (2016).
Carcova, J., Uribelarrea, M., Borras, L., Otegui, M. & Westgate, M. Synchronous pollination within and between ears improves kernel set in maize. Crop Science 40, 1056–1061, https://doi.org/10.2135/cropsci2000.4041056x (2000).
Bangerth, F. Dominance among fruits sinks and the search for a correlative signal. Physiologia Plantarum 76, 608–614, https://doi.org/10.1111/j.1399-3054.1989.tb05487.x (1989).
Acknowledgements
We thank Timothy Boehlein, Brandon Futch, Emily Boehlein, John Baier and Chi-Wah Tseung for technical assistance. This work was supported by National Institute of Food and Agriculture 2010-04228 (LCH, AMS, SKB and WT) and National Science Foundation grant IOS-1444456 (AMS and JLG) and the Vasil-Monsanto Endowment (AMS).
Author information
Authors and Affiliations
Contributions
L.C.H. and J.L.G. prepared the manuscript and designed the experiments. J.L.G., S.K.B., J.R.S., A.W. and W.J. executed the experiments. A.M.S. and W.T. provided experimental design support and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gustin, J.L., Boehlein, S.K., Shaw, J.R. et al. Ovary abortion is prevalent in diverse maize inbred lines and is under genetic control. Sci Rep 8, 13032 (2018). https://doi.org/10.1038/s41598-018-31216-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31216-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.