Abstract
While biophysical stimuli from polymeric matrices are known to significantly affect the fates of human mesenchymal stem cells (hMSCs), the stimulatory effects of nano-sized silicon-based matrices on hMSCs have not been thoroughly investigated. We previously demonstrated that vertically aligned, single-crystalline silicon nanowires (SiNWs) can control the osteogenicity of hMSCs via controllable spring constants from SiNWs matrix. However, other possible differentiation fates of hMSCs on SiNWs have not been explored. We hypothesize that tunable spring constant from artificial SiNWs matrices can direct different types of hMSC differentiations. The spring constants of tunable SiNW matrices can be consistently controlled by tuning the SiNW length. The results of gene expression and cell stiffness suggest that hMSCs differentiations are sensitive to our distinguishable spring constants from the SiNWs groups, and simultaneously conduct osteogenicity and adipogenicity. These findings suggest that SiNW matrices can regulate the fates of hMSCs when the SiNW characteristics are carefully tuned.
Similar content being viewed by others
Introduction
Regenerative medicine is a multidisciplinary field that combines biology, materials science and engineering, and mechanical design to ameliorate complex diseases, physical imperfections, and disorders in humans. Self-renewing and multipotent stem cells are ideal for treating such complicated conditions. Multilineage stem cells that are typically collected from bone marrow, umbilical cord tissue, and placenta, are indispensable to artificial tissue engineering1,2,3,4 and neuroregeneration5,6,7. Before the full potential of stem cell therapy in artificial tissue engineering can be attained, it is necessary to develop precise approaches to manipulate stem cell fates8.
To cure physiological problems such as organ failure8,9 and type I diabetes10 using hematopoietic stem cells8, stem cell fates must be precisely controlled. However, the desired therapeutic effects can be achieved only by using stem cells that undergo specific transitions resulting from complex induction factors and stimuli from microenvironments. Biophysical and biochemical stimuli are two common means to direct the stem cell fate transitions. Biophysical stimulation involves elasticity of polymeric substrates11,12,13, electric-field induction14, and photostimulation15, whereas biochemical stimulation is primarily achieved via growth factors16,17, protein mediation18, and drug carriers19.
Regulation pathways and types of stimuli strongly affect stem cell fates. Elasticity of a flat polymeric matrix11,12,13 is one of the most straightforward methods of biophysical stimulation for manipulating stem-cell fate. Several studies have demonstrated that mesenchymal stem cell (MSC) fates are affected by the elasticity13,20 and topography of the extracellular matrix21. Moreover, osteogenesis and adipogenesis are favored by stiff and flexible matrices, respectively12,22. While the relationship between stem cell fate transition and the elasticity of flat culture plates has been evaluated, little is known about the effects of silicon nanowires (SiNWs) on stem-cell differentiation and variations in cell stiffness. We evaluated the effects of SiNW stiffness (spring constant, K, determined theoretically and by in situ measurements)23 on the differentiation of human MSCs (hMSCs) stimulated by SiNW matrices and the distributions of hMSC stiffness after differentiation. The SiNW matrix is an excellent platform for evaluating how extracellular stimulation from matrices of various stiffnesses, mechanotransduction, and microenvironment affect stem-cell fate. The ultimate goal is to profile a map of hMSC differentiation with regard to SiNWs stimulations for use in clinical applications.
First, based on theoretical calculations of K using beam theory24 and in situ nano-indentation measurements25,26, we evaluated the consistency between the theoretical and experimental values of K and investigated the effects of SiNW dimensions on the mechanical properties of SiNWs groups. Subsequently, hMSCs were cultured on the SiNWs groups to evaluate cell fate after differentiation. Finally, we mapped elasticity distributions of the fixed and living hMSCs that adhered to the SiNWs. Based on the above evaluations, we analyzed the correlations among SiNW dimensions, hMSC fate regulation, and mechanical properties.
Stiffness of SiNWs groups
In our previous study, we designed six SiNWs groups, according to SiNWs preparation time, to generate tunable spring constants. SiNWs Group I, the shortest SiNWs group, regulated osteogenic differentiation in hMSCs23. An idea that can other SiNWs groups direct the fates of hMSCs appeared. Therefore, in this study, we attempted to identify stem cell fates that can be controlled using different SiNWs groups. We fabricated vertically aligned, dense, and length-controllable SiNW arrays23,27 as cell-culture matrices on single-crystalline Si (100) chips using electroless metal deposition (EMD). In the EMD process, silver nanoparticles (AgNPs) in an aqueous silver nitrate solution [AgNO3(aq)] served as the oxidizing agent to form SiOx nanospots. Upon etching with fluorine ions, these SiOx nanospots generated vertical pits because of the anisotropic etching behavior of orientated Si chips. EMD was performed under a constant concentration of electrolyte solution [0.03 M AgNO3(aq) + 4.6 M HF(aq)] and fixed temperature at 50 °C ± 1 °C, and different etching periods (5–60 min) were applied to prepare six groups of dense SiNW arrays with various dimensions (Table S1). These fabrication conditions produced a series of SiNWs with different K, which served as the dominant source of biophysical stimulation in this work. The etching process that was used is stable and achieves a constant etching rate of 1.06 μm/min23,27. For each SiNWs group, the average diameter (D1) and length (L1) were determined on the basis of 300 individual SiNWs, and the theoretical value of K (KTheo,SiNW) was calculated using beam theory24 [Equation (1); Table 1 and Fig. 1]:
where E is the Young’s modulus of (100) Si (E = 170 GPa), L is SiNW length, and D is the SiNW diameter. During the EMD process23,27, etching period was proportional to L1 because of anisotropic etching behavior. D1 remained within a small range of 160–190 nm. (100)-orientated single-crystalline Si and anisotropic etchants, AgNPs and fluorine ions, both settled the uniform and directional etching reaction23,27.
Spring constants of individual SiNWs and SiNW bundles obtained by beam theory and in situ TEM indentation. The theoretical spring constants of individual SiNWs and SiNW bundles in the six SiNWs groups were calculated using beam theory [Equation (1)] from the SiNW dimensions (L and D). At least 300 individual SiNWs were analyzed for each group. The spring constants of SiNW bundles were also determined on the basis of force–displacement curves measured by in situ TEM picoindentation (PI 95 TEM Picoindenter, Hysitron, USA). The TEM image in the inset (JEM 2010, Jeol, Japan) shows that the SiNWs easily formed bundles because of capillarity (stiction).
Interestingly, the SiNWs uniformly synthesized on (100) Si chips easily formed the micro-bundles because of capillary action or stiction28. While stiction is a drawback of microelectromechanical systems (MEMS), the assembled SiNW micro-bundles are important for delivering mechanical stimuli to hMSCs. Each individual SiNW and SiNW bundle behaves like a mechanical spring (beam), effectively supporting hMSCs adhesion and stimulating hMSCs growth and differentiation. The inset of Fig. 1 shows a SEM image of the SiNW bundles. Notably, the longer, more flexible SiNWs aggregated with surrounding nanowires to a greater extent than did the shorter SiNWs, to form bundles with larger diameters (D2). As shown in Table 1, KTheo,SiNW was inversely proportional to SiNW length. The same trend was observed in the spring constants of SiNW bundles (KTheo,bundles); however, the different diameter scales of the SiNWs and bundles (nm vs. μm, reflectively) affected the magnitudes of KTheo,SiNW (mN/m) and KTheo,bundles (N/m).
The spring constants of the SiNW bundles (KReal,bundles) were also experimentally measured using in situ transmission electron microscopy (TEM) picoindentation. First, a focused ion beam microscope was used to prepare SiNW samples on Cu TEM grids. In an in situ TEM picoindentation instrument, the SiNW bundles were indented to a depth of 400 nm using a cone-shaped flat-end tip with a 5-μm punching diameter. The force–displacement curves were recorded during indentation. Based on these force–displacement curves, the following values of KReal,bundles were calculated for the six SiNWs groups (Table 1): 1.20 ± 0.28 (Group I), 0.96 ± 0.11 (Group II), 0.81 ± 0.16 (Group III), 0.52 ± 0.11 (Group IV), 0.38 ± 0.07 (Group V), and 0.086 ± 0.004 kN/m (Group VI). The theoretical and real spring constants of the SiNWs decreased significantly with increasing SiNW length (Fig. 1). The shortest SiNWs (Group I) were the stiffest with the highest spring constants, whereas the longest SiNWs (Group VI) were the softest with the lowest spring constants. The effect of the minor differences in diameter among the six SiNWs groups on the spring constants was weaker than that of SiNW length. However, K was higher for the SiNW bundles than the single SiNWs, indicating that the large diameters of the bundles (D2) enhanced the spring constants. The spring constant measurements made by nanoindentation were in agreement with those calculated by beam theory (Table 1).
Osteogenicity and adipogenicity of hMSCs stimulated by SiNWs
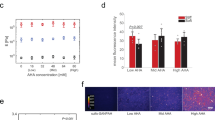
We hypothesize that SiNWs matrices with different spring constants initiate a series of reactions through integrin heterodimers, focal adhesion kinase, and vinculin send stimulating messages to the nucleus that eventually regulate the fate transitions of hMSCs. Substrate stiffness has been shown to affect stem cell fates11,12,13,22. We found the stimulations from Group I SiNWs conducted to the cultivating hMSCs under maintaining medium greatly boost the osteogenicity of hMSCs23. In addition to osteogenicity, we investigated other fate transitions that were promoted by SiNWs groups in this study. To decode the fate regulations of SiNWs groups, we used osteogenic markers (COL1α1 and RUNX-2) and adipogenic markers [PPARγ and fatty acid-binding protein 4 (FABP4)] to determine the fates of hMSCs after SiNW stimulation.
For osteogenicity of hMSCs, gene expressions showed the obvious trend on SiNWs groups between COL1α1 and RUNX-2 [Fig. 2(a,b)] under adipogenic medium. These gene expressions, after a series of RNA extraction and qRT-PCR amplification, quantitatively indicated that SiNWs in Group I with the highest spring constant (KReal,bundles = 1.2 kN/m) obviously promote the COL1α1 and RUNX-2 expression in adipogenic medium. COL1α1 and RUNX-2 expressions were enhanced 10.1- and 2.4-fold, respectively, in Group I than in Group VI. Intriguingly, Group I SiNWs, under maintain medium exhibited osteogenicity that was similar to that which was observed in our previous study5. The statistical significance of osteogenic gene markers indicate that Group I SiNWs were strongly favorable to hMSC osteogenicity under both the maintain and adipogenic media, compared to the other SiNWs groups5.
The adipogenicity of the hMSCs [Fig. 2(c,d)] exhibited completely different trend on gene expression, compared to osteogenicity. Adipogenic gene expression reached a plateau on Group IV SiNWs on PPARγ and FABP4. Expression of PPARγ and FABP4 was enhanced by 3.3- and 2.9-fold, respectively, in Group IV compared to those of Group I SiNWs. However, there is no statistical significance of PPARγ expression among the six SiNWs groups. In contrast, Group IV SiNWs obtained the statistical significance on FABP4. The Group IV SiNWs with moderate spring constant (KReal,bundles = 0.5 kN/m) is favorable to adipogenicity of hMSCs. The results indicate that the SiNWs with different spring constants selectively triggered osteogenicity and adipogenicity in hMSCs. We then applied Group I, IV, and VI SiNWs to investigate the effects of spring constant on cell morphology, cell stiffness, and fate regulation.
Cell morphology and stiffness of fixed and living hMSCs on SiNWs
The above results confirm that the SiNWs with different spring constants affected the fates of hMSCs in this study. Next, we evaluated the cell morphology and stiffness for fixed and living cells on SiNWs after four days of induced differentiation. The fixed hMSCs grown on SiNWs were dehydrated, stained, and sputtered with Pd–Au thin films prior to morphological assessment using scanning electron microscopy (SEM). The SiNWs arrays formed bundles as a result of capillarity [Fig. 3(a)], and the physical properties of the bundles determined the cell adhesion/growth behaviors23 [Fig. 3(a,b)]. The fixed hMSCs were observed on the tips of the SiNW micro-bundles, and sensed that SiNWs are non-cytotoxic23,29. The produced protrusion [red arrow in Fig. 3(a)] from hMSCs covered these surrounding SiNWs micro-bundles and received the simulations from their stiffness. Group I SiNWs, which were the shortest and produced the stiffest bundles, supported cell adhesion similar to that of flat substrate that cells grown along X–Y plane more than along Z direction and cell wrapped the tips of these nanowire bundles30. In contrast, the SiNWs in Groups IV, V, and VI formed more flexible and distinct bundles because of their greater lengths. This forced the cells to grow primarily along the Z direction by expanding protrusions to congregate the surrounding bundles toward the cell body23. The red arrows in Fig. 3(a) highlight the bent tips of SiNW bundles resulting from the contraction force generated by the hMSCs. The side-view SEM images indicate that the Group IV and VI SiNW bundles clearly penetrated the cell membrane23.
Schematic, SEM images, and cell stiffness of fixed and living hMSCs on SiNWs. Schematic showing (a) fixed and (b) living hMSCs differentiated on SiNWs. Cell stiffness of (c) fixed and (d) living hMSCs differentiated on SiNWs in PBS-containing petri dishes; the force–displacement curves were evaluated using Dimension Icon (Bruker, USA) and Bioscope Catalyst (Bruker, USA) instruments, respectively. (b: P < 0.05; c: P < 0.01).
Second, living hMSCs grown on SiNWs in adipogenic medium after four days of cultivation were mapped by in situ atomic force microscope (AFM) indentation in petri dishes containing PBS [Fig. 3(b)]. In contrast to the fixed hMSCs on SiNWs, the living cells were embedded and flattened on the SiNW matrix, and SiNW bundles in Groups I, IV, and VI penetrated the cells [green arrows in Fig. 3(b)]30. Less cell penetration was observed for Group I SiNWs because the surfaces of these SiNWs were relatively flat, and most of the penetrating peaks occurred at the outer region of cell body. Group IV and VI SiNWs exhibited a greater degree of cell penetration and contributed around the cell body due to the distinct SiNW bundles.
Finally, we measured the stiffnesses of fixed [Fig. 3(c)] and living [Fig. 3(d)] hMSCs on SiNWs. These differentiated hMSCs on SiNWs groups should present their distinguishable mechanical properties between osteogenesis and adipogenesis31. The highest cell stiffness was observed for cells on Group I SiNWs, which stimulated osteogenicity in the hMSCs. In contrast, the Group IV SiNWs, which stimulated adipogenicity, resulted in the lowest hMSC stiffness. As indicated by the adipogenic gene markers in Fig. 2(c), no clear adipogenicity was observed in the hMSCs on Group V–VI SiNWs. Figure 3(c,d) indicate that cells on Group VI SiNWs had the lowest cell stiffness but did not regulate hMSCs into adipogenicity. This implies that mechanical simulation by Group VI SiNWs may have resulted in other cell fates of hMSCs. Collectively, the different SiNWs fabricated in the present study were able to direct hMSCs into osteogenicity or adipogenicity.
Discussion
SiNWs have been shown to initiate unique mechanical stimulations and perform signal transductions through integrin heterodimers, pFAK, and vinculin to the, resulting in the successful induction of osteogenicity in hMSCs23. In this study, we sought other possible fates of hMSCs regulated by chemically equivalent SiNWs with different physical properties (dimensions, spring constants, and stiffness). We adopted beam theory24 to compute the theoretical spring constants of individual SiNWs and SiNW bundles (Table 1 and Fig. 1). When cells were cultured on SiNWs, they produced protrusions that grabbed (bent) the surrounding SiNW bundles, pulling them toward the cell body [Fig. 3(a)]. These bending SiNW bundles strongly supported cell adherence, growth, and stimulation, in accordance with beam theory.
In beam theory, each SiNW or SiNW bundles are considered to be perfect cylindrical objects without the imperfections bent with the lateral loading; however, in reality, the SiNW bundles were formed from tens of nanowires with weak capillarity (stiction force) and imperfections among the SiNWs. These geometric differences explain the observed variations between KTheo,bundles and KReal,bundles. However, for all the samples, SiNW length was inversely related to spring constant; thus, the shortest SiNWs (Group I) exhibited the stiffest (largest) spring constant, whereas the longest (Group VI) had the softest (lowest) spring constant.
The SiNWs were employed as sources of biomechanical stimulation to induce hMSC growth and differentiation. González-Cruz et al. demonstrated that adipogenesis was positively correlated with cell height and negatively correlated with cell stiffness, whereas osteogenesis was positively correlated with cell stiffness31. A quintessential transition of osteogenicity and adipogenicity was observed in Fig. 2; the optimal levels of hMSC osteogenicity and adipogenicity were observed for the Group I and Group IV SiNWs, respectively. Thus, based on gene expression, the SiNWs were able to regulate the hMSCs into specific fates under adipogenic induction medium. Interestingly, Group I SiNWs strongly regulated the osteogenic differentiation of hMSCs under either maintain medium or adipogenic induction medium. Moreover, the Group I SiNWs resulted in the greatest cell stiffness, whereas cells adhered on Group IV SiNWs had the lowest stiffness (Fig. 3). While hMSCs grown on SiNWs groups with different stiffness, hMSCs received stress and stimulations and subsequently adapted to remodel and re-assemble their cytoskeleton, cell morphology, and gene expression31,32,33. After this adapting process, the stiffness of such differentiated cells is also affected by substrate stiffness11,12,13,20,22,23,31,32,33. The above results indicate that SiNWs with controllable spring constants are capable of regulating the osteogenicity and adipogenicity of hMSCs in vitro.
A fate-regulation map of the hMSCs is shown in Fig. 4. The Group I SiNWs (length = 9 μm; spring constant of SiNW bundles = 323 N/m) induced osteogenicity in the hMSCs, whereas the Group IV SiNWs (length = 26 μm; spring constant of SiNW bundles = 65 N/m) favored adipogenicity. The regions labeled A, B, and C in Fig. 4 indicate other possible fate regulations. According to the SiNW fabrication process from flat Si substrate, we divided this map into five regions. Group I and IV SiNWs represent osteogenicity and adipogenicity region, respectively. Region A means test samples did not perform EMD process (0 min, present as clean flat Si surface) or perform EMD process at very short period of time (<3 min, present as porous Si surface). In Region A, the mechanical property of the porous Si surface is similar to that of flat surfaces. However, unlike polymer based culture matrices, the porous and flat Si have very limited variations in their Young’s modulus11,12. This culture matrix in Region A has very low degree of freedom in substrate elasticity and may provide limited contributions to hMSCs differentiation. The spring constant of Group I SiNWs was nearly five times higher than that of Group IV SiNWs, and these SiNWs produced opposite differentiation results. Region B is the transition area between Group I and IV SiNWs. Region C is beyond Group V SiNWs. Unlike Group I and IV SiNWs, rest of the groups did not regulate hMSCs into obvious osteogenicity or adipogenicity. However, there might be some other possible differentiations of hMSCs in Region B and C. Like Group II and Group VI SiNWs, their spring constants were three times higher and seven times lower than that of the Group IV SiNWs, respectively, and might be potential candidates for directed other differentiations of hMSCs.
In summary, we successfully manipulated the spring constants of SiNWs, and the resulting SiNW matrices strongly supported hMSC adhesion, growth, and differentiation. The high-spring-constant SiNWs (Group I) induced osteogenicity in the hMSCs, whereas the low-spring-constant SiNWs (Group IV) favored adipogenicity. The gene expressions of osteogenic and adipogenic markers and cell stiffnesses were consistent with our hypothesis. This in vitro approach for the control of stem cell differentiation by SiNW matrices guarantees the consistent regulation of cell fate.
Methods
SiNW fabrication
A (100)-orientated Si single-crystal wafer was sliced into 1 × 1 cm2 pieces for SiNW fabrication, morphological observation, and hMSC cellular tests. Electroless metal deposition (EMD)23,27 was used to produce vertically aligned SiNW matrices. First, the Si chip was immersed in concentrated silver nitrate (AgNO3) solution for 10 min at 50 °C ± 1 °C to pre-deposit silver nanoparticles (AgNPs) as oxidizing agents onto the polished Si surface. Subsequently, we transferred the chips with AgNPs into the EMD electrolyte solution, which was composed of AgNO3/hydrofluoric acid (HF)/H2O. The EMD process was carried out at 50 °C ± 1 °C for different time periods to obtain uniform SiNW arrays: 5 min (Group I), 10 min (Group II), 15 min (Group III), 20 min (Group IV), 30 min (Group V), and 60 min (Group VI). Table S1, tabulated in the Supplementary information, lists the conditions for SiNW fabrication.
During the etching reaction, the pre-deposited AgNPs first oxidized the contact surface, leaving numerous tiny SiOx areas. These oxides were immediately etched away from the Si substrate by F−, forming many nano-pits on the surface. Much of the Ag+ from AgNO3 in the electrolyte was reduced into AgNPs on the Si surface; thus, the Si surface was continually oxidized, and HF continuously etched away the oxides. In this way, vertically aligned SiNWs were uniformly formed on the (100) Si single-crystal substrates. In addition, the huge amount of the concentrated AgNPs gradually transformed into Ag dendrites, which covered the entire sample. After the desired EMD reaction time, the samples were removed from the electrolyte solution, dipped in distilled water to stop the etching reaction, and transferred into HNO3 solution to dissolve the Ag dendrites.
These SiNWs samples were gently washed twice with distilled water to remove any residual EMD electrolyte and then dried on a hotplate at 120 °C for 10 min. Prior to cell culturing, the SiNW samples and flat Si chips were sterilized in a steam autoclave at 121 °C for 15 min and washed twice with PBS for 10 min.
Evaluation of SiNW dimensions
The lengths and diameters of the SiNWs in all six groups were determined by SEM (JSM-6309, JEOL, Japan). The measurements were made for 300 individual SiNWs (n = 300), and their averages and standard deviations were reported. Based on the measured lengths and diameters, we used Hook’s law (Equation S1) and beam theory (Equation S2) to calculate the theoretical spring constants of the SiNWs (Equation 1):
and
where F is the applied force, K is the spring constant, x is the displacement, E is the Young’s modulus of flat (100) Si, l is the length of the object, w is the width of the object, t is the thickness of the object, and E is the Young’s modulus of (100) Si ( = 170 GPa), which is independent of dimensional factors. Beam theory is usually employed to calculate the spring constant of a cantilever; thus, l, w, and t are regarded as the dimensional factors of the cantilever. The samples in this study were cylindrical nanowires; thus, we replaced w and t with diameter D. Thus, to evaluate the theoretical spring constants of the SiNWs in this study, we transformed Equation S2 into Equation 1.
SiNW characteristics
Determination of SiNW physical properties by in situ TEM picoindentation
We measured the actual SiNW spring constants by in situ TEM picoindentation using three pieces of equipment: a dual-beam focused ion beam (FIB) microscope (Nova-200 DBFIB NanoLab, FEI, US), a high-resolution TEM instrument (JEM-2010, JEOL, JAPAN), and a TEM picoindenter (PI-95 TEM Picoindenter, HYSITRON, US). Briefly, FIB was used to fabricate SiNW testing chips with dimensions of 10–30 μm (length) × 10–15 μm (width) × 1.5–2 μm (thickness). These chips were glued tightly on TEM Cu grids using an in situ Omniprobe for in situ TEM picoindentation.
Picoindentation was conducted using a flat-end cylindrical punch (contact area = 5 μm2) in front of top surface of the prepared SiNW sample fixed on the TEM holder. The indentation process was observed at an acceleration voltage of 200 KV. Indentation was performed to a depth of 400 nm, and the load, time, and displacement were recorded.
Total RNA isolation, reverse transcription, and quantitative real-time polymerase chain reaction (RT-PCR)
A total of 1 × 105 hMSCs were seeded onto SiNWs in 24-well plates in MesenPRO RS medium (GIBCO®, Invitrogen, Grand Island, NY, USA) incubated for 24 h. The cells were then transferred to adipogenic induction medium for 72 h. Total RNA was extracted using TRIzol reagent (Invitrogen). The messenger RNA in 2 μg total RNA was reverse transcribed to complementary DNA using MMLV High-Performance Reverse Transcriptase (EPICENTRE® Biotechnologies) followed by PCR amplification.
The gene expressions of hMSCs on the various SiNWs were detected by quantitative real-time polymerase chain reaction (qRT-PCR) using a StepOne PlusTM Real-time PCR System (Applied Biosystems). Intron spanning primers specific for each gene were designed using the Universal ProbeLibrary Assay Design Center and detected using the corresponding probes from the Universal ProbeLibrary (Roche Applied Sciences, Mannheum, Germany). The following primer and probe sequences were applied for qPCR: peroxisome proliferator-activated receptors gamma (PPARγ): forward primer 5′-GACAGGAAAGACAACAGACAAATC-3′, reverse primer 5′-GGGGTGATGTGTTTGAACTTG-3′, probe number 7; fatty acid binding protein 4 (FABP4): RUNX-2 forward primer 5′-CTACCACCCCGCTGTCTTC-3′, reverse primer 5′-CAGAGGTGGCAGTGTCATCA-3′, probe number 29 and COL1α1: forward primer 5′ATGTTCAGCTTTGTGGACCTC3′, reverse primer 5′CTGTACGCAGGTGATTGGTG3′, probe number 15. The average threshold cycle (Ct) for each gene was normalized to that of GAPDH. The details of semi-qRT-PCR analysis are given in Table S2 in the Supplementary information.
Elasticity measurements of hMSCs adhered on SiNWs
Immuno-staining of living hMSCs on SiNWs
For fixed cells, cells were fixated, dehydrated, and coated with Au–Pd for adherence to SiNWs. Cell morphology was fixed, and the cells could be observed by optical microscopy (OM) because of the strong reflection of the Au–Pd coatings. Consequently, we could determine the locations of the fixed hMSCs by OM-equipped AFM.
For living cells adhered to SiNWs in a liquid environment, the SiNW matrix is a promising anti-reflecting surface that absorbs more than 90% of light, and the liquid environment also inhibits observation. Thus, OM-equipped AFM is not suitable for determining the locations of living cells. Thus, we used an immune-staining approach to locate the living cells. The living cells and their locations were confirmed by staining with calcein acetoxymethyl ester (Calcein AM, Sigma-Aldrich, St. Louis, MO, USA), which appears green under fluorescence microscope. The adherent cells were stained with 2 μM Calcein AM for 30 min before measurement.
Elasticity measurements of fixed and living hMSCs on SiNWs
The elasticity of fixed hMSCs adhered on SiNWs was determined by AFM (Dimension Icon®, Bruker, USA) in contact mode in air phase with an SNL-10 tip (silicon tip on a nitride lever coated with 45-nm Ti–Au on the back side). The spring constant of the cantilever was 0.35 Nm−1, and its frequency was 50–80 kHz. Before indentation, polydimethylsiloxane was employed as a standard substrate to calibrate subsequent measurements. For fixed cells on SiNWs (dead cells), the SNL-10 tip was applied directly without sterilization. Randomly, 30–40 cells were indented in the central region to obtain their force curves for all SiNW groups.
In contrast, the elasticities of living cells adhered on SiNWs were measured using a Bioscope Catalyst instrument (Bioscope Catalyst, Bruker, USA) in peak force quantitative nanomechanical mapping mode was Advanced TEC- SPM-Sensor with tip at the end of the cantilever (ATEC-FM-10, without coating). The spring constant of the cantilever was 2.8932 Nm−1, its frequency was 85 kHz, its tip radius was 10 ± 2 nm, and its half angle was 12°. Prior to the indentation experiments, the AFM probes were cleaned and sterilized in ethanol to remove contaminants on the probe surface. The relationships between displacement and indentation of the cantilever in contact with the cells were obtained in force curves. In this study, the contact point was defined as the point at which the slope of the force curve was approximately zero. To determine the elasticity of living cells, 20 cells were measured for each SiNW group. The Young’s moduli were calculated using Sneddon fits34.
Statistical analysis
One-way analysis of variance with Tukey’s post hoc tests was conducted using IBM SPSS 12.0 software. Differences considered to be statistically significant at P < 0.05 and <0.01 are demoted as b and c, respectively (b: P < 0.05; c: P < 0.01).
References
Zhao, C., Tan, A., Pastorin, G. & Ho, H. K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 31, 654–668 (2013).
Song, M. J., Dean, D. & Tate, M. L. K. Mechanical modulation of nascent stem cell lineage commitment in tissue engineering scaffolds. Biomaterials 34, 5766–5775 (2013).
Correia, C. et al. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomaterilia 8, 2483–2493 (2012).
Wang, L., Johnson, J. A., Zhang, Q. & Beahm, E. K. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomaterilia 9, 8921–8931 (2013).
Portmann-Lanz, C. et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 194, 664–673 (2006).
Serakinci, N., Fahrioglu, U. & Christensen, C. Mesenchymal stem cells, cancer challenges and new Directions. Eur. J. Cancer 50, 1522–1530 (2014).
Zhao, L. X. et al. Modification of the brain-derived neurotrophic factor gene: a portal to transform mesenchymal stem cells into advantageous engineering cells for neuroregeneration and neuroprotection. Exp. Neurol. 190, 396–406 (2004).
Hadar, A., Philip, L. & Arnon, N. Tissue regeneration potential in human umbilical cord blood. Best Pract. Res. Clin. Haematol. 23, 291–303 (2010).
Marmotti, A. et al. Minced umbilical cord blood fragments as a source of cells for orthopaedic tissue engineering: an in vitro. Stem Cells Int. 2012, 1–13 (2012).
Mabed, M. The Potential Utility of Bone Marrow or Umbilical Cord Blood Transplantation For the Treatment of Type I Diabetes Mellitus. Biol. Blood Marrow Transplant 17, 455–464 (2011).
ven-Ram, S., Artym, V. & Yamada, K. M. Matrix control of stem cell fate. Cell 126, 645–647 (2006).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Hronik-Tupaj, M., Rice, W. L., Crinin-Golomb, M., Kaplan, D. L. & Georgakoudi, I. Osteoblast differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed. Eng. Online 10, 1–22 (2011).
Ong, W. K. et al. The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials 34, 1911–1920 (2013).
Choi, J. S. & Harley, B. A. C. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials 33, 4460–4468 (2012).
Qutachi, O., Shakesheff, K. M. & Buttery, L. D. K. Delivery of definable number of drug or growth factor loaded poly (DL-lactic acid-co-glycolic acid) microparticles within human embryonic stem cell derived aggregates. J. Control Release 168, 18–27 (2013).
Mathews, S., Bhonde, R., Gupta, P. K. & Totey, S. Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation 84, 185–192 (2012).
Yu, D. et al. Blockade of Peroxynitrite-Induced Neural Stem Cell Death in the Acutely Injured Spinal Cord by Drug-Releasing Polymer. Stem Cells 27, 3121–3121 (2009).
Mammoto, A. et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 (2009).
Curtis, A. & Wilkinson, C. Topographical control of cells. Biomaterials 18, 1573–1583 (1997).
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Kuo, S. W. et al. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. Biomaterials 33, 5013–5022 (2012).
Lam, D. C. C., Yang, F., Chong, A. C. M., Wang, J. & Tong, P. Experiments and theory in strain gradient elasticity. J. Mech. Phys. Solids 51, 1477–1508 (2003).
Zhang, X., Godfrey, A., Winther, G., Hansen, N. & Huang, X. Plastic deformation of submicron-sized crystals studied by in-situ Kikuchi diffraction and dislocation imaging. Mater. Charact. 70, 21–27 (2012).
Dubach, A., Raghavan, R., Löffler, J. F., Michler, J. & Ramanurtry, U. Micropillar compression studies on a bulk metallic glass in different structure states. Scripta Materialia 60, 567–570 (2009).
Chen, C. Y., Wu, C. S., Chou, C. J. & Yen, T. J. Morphological control of single-crystalline silicon nanowire arrays near room temperature. Adv. Mater. 20, 3811–3815 (2008).
Ali, S. M., Jennings, J. M. & Phinney, L. M. Temperature dependence for in-use stiction of polycrystalline silicon MEMS cantilevers. Sensors and Actuators A:. Physical 113, 60–70 (2004).
Qi, S., Yi, C., Ji, S., Fong, C. C. & Yang, M. Cell adhesion and spreading behavior on vertically aligned silicon nanowire arrays. ACS Appl. Mater. Interfaces 1, 30–34 (2009).
Xie, X. Mechanical model of vertical nanowire cell penetration. Nanoletters 13, 6002–6008 (2013).
González-Cruz, R. D., Fonseca, V. C. & Darling, E. M. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. PNAS 109, E1523–E1529 (2012).
Solon, J., Levental, I., Sengupta, K., Georges, P. C. & Janmey, P. A. Fibroblast adaption and stiffness matching to soft elastic substrates. Biophys J. 93, 4453–4461 (2007).
Thomas, G., Burnham, N. A., Camesano, T. A. & Wen, Q. Measuring the mechanical properties of living cells using atomic force microscopy. J. Vis. Exp. 76, e50497-1–8 (2013).
Sneddon, I. N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile, Int. J. Engng. Sci. 3, 47–57 (1965).
Acknowledgements
The authors would like to gratefully acknowledge the financial support from the Ministry of Science and Technology, Taiwan (MOST 104-2221-E-007-040-MY3, MOST 106-2321-B-010-008, MOST 106-2911-I-010-502, MOST 106-3114-B-010-002, and MOST 106-2221-E-007-038-MY3) and from the Ministry of Education (“Aim for the Top University Plan” for National Tsing Hua University & Nation Yang Ming University under project number 102N2015E1 and 102N2043E1). Authors appreciate Mr. Jimmy Juan and Mr. Ryan Stromberg from Hysitron for their great helps in in-situ picoindenter and technical assistances; F.S. Kao and Y.H. Liao from Materials and Chemical research laboratory in The Industrial Technology Research Institute (ITRI) for their supports of measurements and analysis of cell elasticity in AFM.
Author information
Authors and Affiliations
Contributions
H.I. Lin, S.W. Kuo, T.J. Yen and Oscar K. Lee all performed this study; H.I. Lin and T.J. Yen design this research, took all tests in physical characteristics of SiNWs, growth profile and mechanical properties of hMSCs and their analysis; S.W. Kuo and Oscar K. Lee performed all the cellular tests and their analysis; H.I. Lin and S.W. Kuo contributed equally in this work and entitled as co-first author; T.J. Yen and Oscar K. Lee entitled as co-corresponding author; H.I. Lin and S.W. Kuo wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, HI., Kuo, SW., Yen, TJ. et al. SiNWs Biophysically Regulate the Fates of Human Mesenchymal Stem Cells. Sci Rep 8, 12913 (2018). https://doi.org/10.1038/s41598-018-30854-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30854-3
This article is cited by
-
Biointerface design for vertical nanoprobes
Nature Reviews Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.