Abstract
Fabry disease is characterised by neuropathic pain and accelerated vascular disease. This study evaluates the utility of corneal confocal microscopy (CCM) to non-invasively quantify corneal nerve and endothelial cell morphology and dendritic cell (DC) density in relation to disease severity in subjects with Fabry disease. Seventeen consecutive participants with Fabry disease and 17 healthy control subjects were included in this cross-sectional study. Fabry disease severity was measured using the Mainz Severity Score Index (MSSI). Central corneal sensitivity was assessed with a contact corneal esthesiometer. There was a significant reduction in the corneal sensitivity (5.75 [5.25–6.00] vs. 6.00 [6.00-6.00] cm, P = 0.014), nerve fiber density (NFD) (26.4 ± 10.1 vs. 33.7 ± 7.9 fibers/mm2, P = 0.025) and nerve fiber length (NFL) (15.9 ± 3.4 vs. 19.5 ± 4.4 mm/mm2, P = 0.012) and an increase in DC density (38.3 [17.5–97.3] vs. 13.5 [0–29.4] cells/mm2, P = 0.004) in subjects with Fabry disease compared to the healthy control subjects. The total MSSI score correlated with NFD (ρ = −0.686; P = 0.006), NFL (ρ = −0.692; P = 0.006), endothelial cell density (ρ = −0.511; P = 0.036), endothelial cell area (ρ = 0.514; P = 0.036) and α-galactosidase A enzyme activity (ρ = −0.723; P = 0.008). This study demonstrates reduced corneal sensitivity, corneal nerve fiber damage and increased DCs in subjects with Fabry disease.
Similar content being viewed by others
Introduction
Fabry disease is a rare X-linked disorder due to a deficiency or absence of the lysosomal enzyme α-galactosidase A, which results in the accumulation of the sphingolipid degradation product globotriaosylceramide1. The prevalence of Fabry disease has been reported as 1/476,000 in normal population and 0.95% in chronic kidney disease patients2,3. A common presenting symptom of Fabry disease is neuropathic pain, which usually develops during childhood4,5. Histopathological studies have revealed that glycolipid accumulation in the dorsal root ganglion with axonal degeneration of the small nerve fibers may cause neuropathic pain6,7. Electrophysiological studies, quantitative sensory testing, sural nerve and skin biopsy studies have confirmed neuropathy in subjects with Fabry disease8,9,10.
We have previously demonstrated the clinical utility of corneal confocal microscopy (CCM) as an imaging biomarker that quantifies small nerve fiber damage in diabetic neuropathy, idiopathic small fiber neuropathy, Charcot-Marie-Tooth disease type 1 A, chemotherapy induced peripheral neuropathy, sarcoid neuropathy and HIV neuropathy11,12,13,14,15,16. More recently CCM has also shown corneal nerve degeneration in Parkinsons disease, multiple sclerosis and stroke17,18,19,20. CCM has been used to detect increased Langerhans cell and dendritic cell (DC) density in keratoconus, early diabetic neuropathy, chronic inflammatory demyelinating polyneuropathy and multiple sclerosis18,21,22,23,24.
With regard to Fabry disease, we have previously shown a reduction in corneal sensation and a loss of corneal nerve fibers, which was related to the severity of clinical neuropathy in hemizygous males25. However this study was undertaken using a Tomey corneal confocal microscope which is a first generation device with limited resolution and our study only assessed corneal nerve morphology.
Accelerated vascular disease with progressive renal failure, cardiomyopathy and occlusive cerebro-vascular events are associated with early mortality in subjects with Fabry disease26. Whilst some clinical and experimental studies have shown that accumulation of globotriaosylceramide in endothelial cells may contribute to vascular dysfunction27,28, others have not29. Additionally, activation of innate immunity via dendritic cells has been associated with renal and cardiac inflammation in Fabry disease30,31,32.
In the current study we have undertaken CCM to quantify corneal nerve and endothelial cell morphology and DC density to explore underlying mechanisms and to establish whether CCM could contribute to the development of imaging biomarkers for tissue damage in subjects with Fabry disease.
Results
Seventeen subjects with Fabry disease and 17 age- and sex-matched healthy control participants were enrolled in the study. The baseline characteristics of the subjects with Fabry disease and healthy controls are summarized in Table 1. The mean ages of the subjects with Fabry disease and healthy control participants did not differ (34.6 ± 13.5 vs. 34.5 ± 13.2 years, P = 0.980). Eleven of the 17 subjects (64.7%) were receiving enzyme replacement therapy (ERT), biweekly infusions of agalsidase alpha (Replagal®; Shire Human Genetic Therapies AB, Lund, Sweden). The mean period of ERT use was 12.4 ± 5.7 months (range 5–18 months). Study subjects with Fabry disease were the members of 3 different families. Thirteen participants had c.100 A > C (p.N34H) mutation, 3 participants had c.160 C > T (p.Leu54Phe) mutation and one participant had c.1072_1074delGAG (p.358delE) mutation. The average values of α-galactosidase A enzyme activity were 1.05 ± 0.51 µmol/L/h in males and 2.03 ± 0.63 µmol/L/h in females.
Corneal sensitivity and nerve morphology
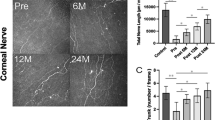
Representative CCM images of the central cornea in a healthy subject and a participant with Fabry disease, demonstrating reduced nerve fibers and increased DCs are shown in Fig. 1. There were significant reductions in the central corneal mechanical thresholds (5.75 [5.25–6.00] vs. 6.00 [6.00 – 6.00] cm, P = 0.014), NFD (26.4 ± 10.1 vs. 33.7 ± 7.9 fibers/mm2, P = 0.025) and NFL (15.9 ± 3.4 vs. 19.5 ± 4.4 mm/mm2, P = 0.012) in participants with Fabry disease compared to control subjects (Table 2, Fig. 2). There were no significant differences in corneal sensitivity and confocal microscopic measures between participants with the p.N34H mutation and p.Leu54Phe mutation (Supplementary Table S1).
Corneal endothelial cells
Corneal endothelial cell density (3220.1 ± 196.5 vs. 3091.1 ± 189.7 cells/mm2, P = 0.060), cell area (250.6 ± 17.2 vs. 261.9 ± 17.7 µm2, P = 0.067), cell perimeter (57.3 ± 1.9 vs. 58.7 ± 2.2 µm, P = 0.054), pleomorphism (36.8 ± 4.0 vs. 35.3 ± 5.4%, P = 0.376) and polymegathism (46.3 ± 3.4 vs. 48.7 ± 4.5%, P = 0.086) did not differ signficantly between subjects with Fabry disease and control subjects (Table 3).
Dendritic cells
There was a significant increase in the central corneal DC density (38.3 [17.5–97.3] vs. 13.5 [0–29.4] cells/mm2, P = 0.004) in subjects with Fabry disease compared to healthy control subjects (Table 2, Fig. 2).
Male vs. Female participants
Male subjects with Fabry disease had a significantly lower NFD (19.7 ± 6.2 vs. 35.9 ± 5.7 fibers/mm2, P < 0.001) and NFL (13.7 ± 2.2 vs. 19.2 ± 1.8 mm/mm2, P < 0.001) compared with female subjects, whereas corneal sensitivity (P = 0.070), NBD (P = 0.193) and DC density (P = 0.887) did not differ. There were no significant differences in any of the study parameters among male and female participants in the control group (data not shown, P > 0.05 for all).
Effect of enzyme replacement therapy
NFD was significantly lower (22.3 ± 8.8 vs. 33.9 ± 7.9 fibers/mm2, P = 0.018) in subjects receiving ERT (9 male, 2 female) compared to subjects not receiving ERT (1 male, 5 female), while corneal sensitivity (P = 0.301), NFL (P = 0.065), NBD (P = 0.884) and DC density (P = 0.591) did not show a significant difference (data not shown, P > 0.05 for all).
Cornea verticillata
Thirteen subjects with Fabry disease (8 male, 5 female; 76.5%) showed cornea verticillata on slit-lamp examination and hyperreflective intracellular inclusions in basal epithelial cells (Fig. 3). There were no significant differences in the Mainz Severity Score Index (MSSI), corneal sensitivity and corneal confocal microscopic measures between subjects with and without cornea verticillata (data not shown, P > 0.05 for all).
Slit-lamp biomicroscopic image showing cornea verticillata in a subject with Fabry disease (a). Corneal confocal microscopic images of the central cornea; showing hyperreflective inclusions in the basal epithelial cell layer of a subject with Fabry disease (b), and basal epithelial cells of a healthy control subject (c) with bright cell borders and dark cytoplasm.
Correlations
The total MSSI score correlated with NFD (ρ = −0.686; P = 0.006), NFL (ρ = −0.692; P = 0.006), endothelial cell density (ρ = −0.511; P = 0.036) and endothelial cell area (ρ = 0.514; P = 0.036) (Fig. 4). The neurological component of the MSSI correlated with central corneal sensitivity (ρ = −0.489; P = 0.046), NFD (r = −0.598; P = 0.022) and NFL (r = −0.626; P = 0.021) (Fig. 5). Central corneal sensitivity correlated with NFD (ρ = 0.613; P = 0.018), NBD (ρ = 0.717; P = 0.003) and NFL (ρ = 0.500; P = 0.041) (Fig. 6). There were significant correlations of the α-galactosidase A enzyme activity with the total MSSI score (ρ = −0.723; P = 0.008), the neurological component of the MSSI (r = −0.515; P = 0.049), NFD (r = 0.498; P = 0.049) and NFL (r = 0.525; P = 0.049) (Fig. 7).
Discussion
Neuropathic pain has been reported to be the most common presenting symptom of Fabry disease4,5, and has been related to loss of small fibers33. Quantification of nerve damage in subjects with Fabry disease is important for the initial diagnosis and assessment of neuropathy progression. Quantitative sensory testing has revealed small fiber dysfunction with a predominant abnormality for cold sensation in Fabry disease8,33. Marked reduction of small myelinated and unmyelinated nerve fibers has been demonstrated in histopathological studies of sural nerve biopsy specimens7,34. Additionally, reduced intraepidermal nerve fiber densities were found in up to 95% of subjects with Fabry disease35,36. In a detailed phenotyping study, warm and cold perception thresholds were elevated and the amplitude of pain related evoked potentials and intraepidermal nerve fiber density were significantly reduced in male but not female subjects with Fabry disease37. Whilst, sural nerve and skin biopsies allow objective quantification of small nerve fiber damage, they are invasive procedures which cannot be used for routine diagnosis and follow-up.
We and others have utilised corneal confocal microscopy to detect corneal nerve loss in a range of peripheral neuropathies11,12,13,14,15. Using a first generation CCM we previously reported significant corneal nerve loss in subjects with Fabry disease25. In the present study, we have found a significant reduction in corneal sensitivity and corneal nerve parameters, which were associated with each other and with disease severity assessed by MSSI as well as α-galactosidase A enzyme activity. Previous studies have reported more severe symptoms, neurological deficits and CCM abnormalities in hemizygous males compared to heterozygous females4,25. We have also found a significantly lower NFD and NFL in males compared to females, which could be explained by the heterogeneity in disease severity among females due to X-chromosome inactivation4. Additionally, we demonstrate an inverse correlation between the MSSI score and endothelial cell density which suggests that corneal endothelial cell abnormality is associated with the disease severity in subjects with Fabry disease.
In the present study whilst there was a trend for a reduction in NBD, this was not significant, unlike our previous study in Fabry disease25. We would attribute this to improved detection of corneal nerve branches due to the better resolution of the HRT III CCM used in this study.
In relation to underlying mechanisms of nerve damage, this is the first study to report increased DCs in the central cornea of subjects with Fabry disease. DCs have been shown to migrate and accumulate in the central cornea in various neuropathies and during inflammation22,23,38,39. Biancini et al.31 have reported that the inflammatory cytokines, IL-6 and TNF-α, are significantly increased in Fabry disease, indicating a pro-inflammatory state in these subjects. There are also reports proposing that Fabry disease has an autoimmune component in its pathophysiology40,41,42. A flow cytometry study has shown a reduction in circulating DCs in peripheral blood samples, suggestive of increased extravasation and migration to peripheral tissues30. The increased DC density in the central cornea of subjects with Fabry disease may reflect this process and therefore CCM may provide further insight into the role of immune mechanisms in Fabry disease.
The reported prevalence of cornea verticillata was 76.9% in heterozygous females and 73.1% in hemizygous males according to the Fabry Outcome Survey43. Hyperreflective intracellular inclusions in the basal epithelium have been found using CCM in subjects with Fabry disease44,45. In this study, whilst cornea verticillata was observed in 71.4% of females and 80.0% of males, there was no difference in the MSSI score, corneal sensitivity or corneal confocal microscopic measures between subjects with and without cornea verticillata. This is in agreement with previous reports suggesting that the presence of cornea verticillata is not associated with disease severity, or the systemic, renal and cardiac manifestations of the disease43,46.
In relation to the effects of treatment with ERT, Schiffmann et al. reported an improvement in the neuropathic pain severity score47 and small nerve fiber function48. Whilst one study showed no change in intraepidermal nerve fiber density after 12–18 months of treatment with ERT10, another study did show an improvement in proximal intraepidermal nerve fiber density after 4 years of ERT33. In our study, despite treatment with ERT, the NFD was lower compared to subjects not on ERT. However, this may be attributed to the fact that in clinical practice only the more severely affected subjects are commenced on ERT.
A limitation of this study is the acquisition and selection of the CCM images by an unmasked observer, which may increase the risk of bias49. However, corneal nerve quantification was undertaken using automated software, and hence the analysis was not operator-dependent. The use of a standardized image selection protocol, as used in this study, has also been shown to have excellent intra- and inter-observer repeatability50. Other limitations are the small sample size which has not allowed us to undertake further subgroup analysis. The cross-sectional nature of the study design also precludes the ability to draw causal inferences. However, we believe that our data show that CCM could have considerable utility in the non-invasive quantification of neural and immune pathology in Fabry disease. Longitudinal studies are required to evaluate the utility of CCM for monitoring changes in corneal nerve morphology and DC density, especially in relation to the effects of ERT or other therapeutic strategies.
Methods
This cross-sectional comparative study was undertaken at a tertiary referral university hospital between February 2017 and November 2017. The study design fulfilled the tenets of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the Necmettin Erbakan University (2017/805). Written informed consent was obtained from all participants after a detailed explanation of the nature and possible consequences of the study.
The diagnosis of Fabry disease had been previously established on the basis of clinical features, biochemical evidence of reduced α-galactosidase A enzyme activity and molecular analysis of the GLA gene. α-Galactosidase A enzyme activity was assessed in dried blood spots and GLA gene mutation analysis was performed by PCR amplification and direct automatic sequencing, as we have previously described in detail3. All consecutive subjects with Fabry disease who were eligible for CCM analysis were enrolled in the study. Control participants were individuals without any systemic or ocular disease. Exclusion criteria were previous ocular trauma or surgery, any corneal pathology, contact lens use, and any other systemic disease that might affect the cornea. None of the participants were receiving anti-inflammatory or immuno-modulatory medications. All subjects underwent clinical and ophthalmological examination, and disease severity was assessed using the MSSI score ranging from 0–76 which includes scoring for general (0–18), neurological (0–20), cardiovascular (0–20), and renal (0–18) symptoms and signs of Fabry disease51.
Corneal Sensitivity Assessment
Central corneal sensitivity was measured using a contact corneal esthesiometer (Cochet-Bonnet; Luneau, France). The esthesiometer is based upon the principle of pressure transmitted axially by a 0.12 mm-diameter nylon monofilament, which was applied with a low pressure perpendicular to the center of the cornea. The filament length was progressively reduced from 6 cm in 5-mm steps until the first response occurred. The longest filament length (cm) resulting in a positive response was verified twice and recorded as a measure of central corneal sensitivity. Both eyes of the study participants were evaluated but only the data obtained form the right eye were included in statistical analyses.
Corneal Confocal Microscopy
All participants underwent examination with a laser scanning CCM using the Rostock Corneal Module/Heidelberg Retina Tomograph lll (Heidelberg Engineering, Germany). The full thickness of the central cornea was scanned using the “section” mode. A standardized image selection protocol was used50. Three high-quality nerve plexus images containing the highest, intermediate and least number of nerve fibers were selected from each subject and the average of these results was considered. Automated CCMetrics software, ver. 2.0 (University of Manchester, UK) was used to quantify the nerve fibers52. Three parameters were quantified: corneal nerve fiber density (NFD), the total number of major nerves/mm2; nerve branch density (NBD), the number of branches emanating from major nerve trunks/mm2; nerve fiber length (NFL), the total length of all nerve fibers and branches (mm/mm2). The same image frames were used to quantify DC density. The number of highly reflective cells with dendriform structures was counted manually and the density was derived as the number of cells in the area of frame assessed in square millimeters (number/mm2) using the proprietary software within the corneal confocal microscope. Corneal endothelial cell morphology was analysed using the Corneal Endothelium Analysis System (CEAS) software (University of Bradford, UK)53 and five parameters were quantified: endothelial cell density (cells/mm2), endothelial cell area (µm2), endothelial cell perimeter (µm), pleomorphism (percentage of hexagonality coefficient, calculated as the number of hexagonal-shaped endothelial cells divided by the total number of cells) and polymegathism (percentage of coefficient of variation in cell size, calculated as the standard deviation of cell area divided by the mean endothelial cell area). For all subjects, only the right eye was included in analyses. All image acquisition and analysis were performed by a single unmasked observer (GB).
Statistical Analysis
Statistical analysis was performed using SPSS ver. 21.0 (Chicago, IL, USA) software. Basic descriptive statistics were calculated on all the data and are reported as the mean ± SD or median (interquartile range [IQR]), as appropriate. The Pearson χ2-test was used to compare categorical variables. Normal distribution of continuous variables was confirmed with the Kolmogorov-Smirnov test. Independent samples t-test for normally distributed data and Mann-Whitney U-test for non-normally distributed data were used to compare the parameters between the subjects with Fabry disease and healthy control participants. The associations between disease severity scores, corneal sensitivity and confocal microscopic parameters were measured using Pearson’s correlation coefficient for normally distributed data and Spearman’s correlation coefficient for non-normally distributed data. A Benjamini-Hochberg correction was applied to adjust the P values for multiple correlations. For all evaluations, a P value of less than 0.05 was considered statistically significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Clarke, J. T. Narrative review: Fabry disease. Ann Intern Med. 146, 425–433 (2007).
Poorthuis, B. J. et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Gen. 105, 151–156 (1999).
Turkmen, K. et al. The prevalence of fabry disease in patients with chronic kidney disease in turkey: The TURKFAB Study. Kidney Blood Press Res. 41, 1016–1024 (2016).
Ries, M. et al. The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr. 162, 767–772 (2003).
Mehta, A. et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 34, 236–242 (2004).
Kahn, P. Anderson-Fabry disease: a histopathological study of three cases with observations on the mechanism of production of pain. J Neurol Neurosurg Psychiatry. 36, 1053–1062 (1973).
Gemignani, F., Marbini, A., Bragaglia, M. M. & Govoni, E. Pathological study of the sural nerve in Fabry’s disease. Eur Neurol. 23, 173–181 (1984).
Luciano, C. A. et al. Physiological characterization of neuropathy in Fabry’s disease. Muscle Nerve. 26, 622–629 (2002).
Toyooka, K. & Said, G. Nerve biopsy findings in hemizygous and heterozygous patients with Fabry’s disease. J Neurol. 244, 464–468 (1997).
Schiffmann, R. et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve. 34, 53–56 (2006).
Malik, R. A. et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 46, 683–688 (2003).
Tavakoli, M. et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 223, 245–250 (2010).
Tavakoli, M. et al. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve. 46, 698–704 (2012).
Ferdousi, M. et al. Corneal confocal microscopy detects small fibre neuropathy in patients with upper gastrointestinal cancer and nerve regeneration in chemotherapy induced peripheral neuropathy. PLoS One. 10, e0139394 (2015).
Culver, D. A. et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci. 58, BIO52–BIO60 (2017).
Kemp, H. I. et al. Use of corneal confocal microscopy to evaluate small nerve fibers in patients with Human Immunodeficiency Virus. JAMA Ophthalmol. 135, 795–800 (2017).
Kass-Iliyya, L. et al. Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord. 21, 1454–1460 (2015).
Bitirgen, G., Akpinar, Z., Malik, R. A. & Ozkagnici, A. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 135, 777–782 (2017).
Petropoulos, I. N. et al. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Vis Sci. 58, 3677–3681 (2017).
Khan, A. et al. Corneal confocal microscopy detects corneal nerve damage in patients admitted with acute ischemic stroke. Stroke. 48, 3012–3018 (2017).
Mandathara, P. S., Stapleton, F. J., Kokkinakis, J. & Willcox, M. D. P. A pilot study on corneal Langerhans cells in keratoconus. Cont Lens Anterior Eye. https://doi.org/10.1016/j.clae.2017.10.005 (2017).
Tavakoli, M., Boulton, A. J., Efron, N. & Malik, R. A. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 34, 7–11 (2011).
Stettner, M. et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 3, 88–100 (2015).
Rajabally, Y. A., Stettner, M., Kieseier, B. C., Hartung, H. P. & Malik, R. A. CIDP and other inflammatory neuropathies in diabetes - diagnosis and management. Nat Rev Neurol. 13, 599–611 (2017).
Tavakoli, M. et al. Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve. 40, 976–984 (2009).
MacDermot, K. D., Holmes, A. & Miners, A. H. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 38, 750–760 (2001).
Yogasundaram, H. et al. Clinical features, diagnosis, and management of patients with Anderson-Fabry cardiomyopathy. Can J Cardiol. 33, 883–897 (2017).
Satoh, K. Globotriaosylceramide induces endothelial dysfunction in fabry disease. Arterioscler Thromb Vasc Biol. 34, 2–4 (2014).
Segura, T. et al. Cerebral hemodynamics and endothelial function in patients with Fabry disease. BMC Neurol. 13, 170 (2013).
Rozenfeld, P., Agriello, E., De Francesco, N., Martinez, P. & Fossati, C. Leukocyte perturbation associated with Fabry disease. J Inherit Metab Dis. 32, S67–77 (2009).
Biancini, G. B. et al. Globotriaosylceramide is correlated with oxidative stress and inflammation in Fabry patients treated with enzyme replacement therapy. Biochim Biophys Acta. 1822, 226–232 (2012).
Rozenfeld, P. & Feriozzi, S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. 122, 19–27 (2017).
Üçeyler, N. et al. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst. 16, 304–314 (2011).
Onishi, A. & Dyck, P. J. Loss of small peripheral sensory neurons in Fabry disease. Histologic and morphometric evaluation of cutaneous nerves, spinal ganglia, and posterior columns. Arch Neurol. 31, 120–127 (1974).
Scott, L. J. C. et al. Quantitative analysis of epidermal innervation in Fabry disease. Neurology. 52, 1249–1254 (1999).
Liguori, R. et al. Small fiber neuropathy in female patients with Fabry disease. Muscle Nerve. 41, 409–412 (2010).
Üçeyler, N. et al. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case-control study. BMC Neurol. 13, 47 (2013).
Mayer, W. J. et al. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 37, 1012–1018 (2012).
Cruzat, A. et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 52, 5136–5143 (2011).
Rahman, P., Gladman, D. D., Wither, J. & Silver, M. D. Coexistence of Fabry’s disease and systemic lupus erythematosus. Clin Exp Rheumatol. 16, 475–478 (1998).
Whybra, C. et al. IgA nephropathy in two adolescent sisters heterozygous for Fabry disease. Pediatr Nephrol. 21, 1251–1256 (2006).
Martinez, P., Aggio, M. & Rozenfeld, P. High incidence of autoantibodies in Fabry disease patients. J Inherit Metab Dis. 30, 365–369 (2007).
Sodi, A. et al. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 91, 210–214 (2007).
Wasielica-Poslednik, J., Pfeiffer, N., Reinke, J. & Pitz, S. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 249, 1689–1696 (2011).
Degirmenci, C. et al. A novel mutation and in vivo confocal microscopic findings in Fabry disease. Saudi J Ophthalmol. 31, 45–47 (2017).
Fumex-Boizard, L., Cochat, P., Fouilhoux, A., Guffon, N. & Denis, P. Relation between ocular manifestations and organ involvement in ten patients with Fabry disease. J Fr Ophtalmol. 28, 45–50 (2005).
Schiffmann, R. et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 285, 2743–2749 (2001).
Schiffmann, R. et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 28, 703–710 (2003).
De Silva, M. E. H., Zhang, A. C., Karahailos, A., Chinnery, H. R. & Downie, L. E. Laser scanning in vivo confocal microscopy (IVCM) for evaluating human corneal sub-basal nerve plexus parameters: protocol for a systematic review. BMJ Open. 7, e018646 (2017).
Kalteniece, A. et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One. 12, e0183040 (2017).
Beck, M. The Mainz Severity Score Index (MSSI): development and validation of a system for scoring the signs and symptoms of Fabry disease. Acta Paediatr Suppl. 95, 43–46 (2006).
Dabbah, M. A., Graham, J., Petropoulos, I. N., Tavakoli, M. & Malik, R. A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 15, 738–747 (2011).
Al-Fahdawi, S. et al. A fully automated cell segmentation and morphometric parameter system for quantifying corneal endothelial cell morphology. Comput Methods Programs Biomed. https://doi.org/10.1016/j.cmpb.2018.03.015 (2018).
Acknowledgements
The preliminary findings of this study were presented at the 30th Summer Symposium of the Turkish Ophthalmological Association, September 23, 2017, İzmir, Turkey.
Author information
Authors and Affiliations
Contributions
G.B. was involved in the design of the work, acquisition, analysis and interpretation of the data and drafted the manuscript. K.T. contributed to design of the work and helped to draft the manuscript. R.A.M. was involved in the design of the work, analysis and interpretation of the data and revised the manuscript. A.O. and N.Z. contributed to analysis of the data and revising the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Kultigin Turkmen has received honorarium fees for lectures on Fabry disease from Shire HGT and Sanofi Genzyme. Other authors declare that they have no financial and non-financial competing interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bitirgen, G., Turkmen, K., Malik, R.A. et al. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep 8, 12244 (2018). https://doi.org/10.1038/s41598-018-30688-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30688-z
This article is cited by
-
Corneal nerve loss and increased Langerhans cells are associated with disease severity in patients with rheumatoid arthritis
Eye (2023)
-
Autophagy-lysosome pathway alteration in ocular surface manifestations in Fabry disease patients
Orphanet Journal of Rare Diseases (2022)
-
Charcot–Marie–Tooth Disease and Implications on Corneal Refractive Surgery
Ophthalmology and Therapy (2022)
-
In vivo confocal microscopy of corneal nerve fiber damage in early course of multiple sclerosis
International Ophthalmology (2022)
-
Implications of Corneal Refractive Surgery in Patients with Fabry Disease
Ophthalmology and Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.