Abstract
We carried out systematic review and meta-analysis to evaluate whether peripheral levels of pro-inflammatory markers including Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF- α) and C-Reactive Protein (CRP) are significantly higher in elderly with depression and Alzheimer’s disease. We searched Pubmed, PsycINFO and Embase, and thirty-four relevant studies (2609 with Depression, 1645 with Alzheimer’s disease and 14363 Controls) were included. Compared with controls, IL-1β (pooled standardized mean difference [SMD]: 0.642; 95% confidence interval [CI]: 0.078–1.206; significant heterogeneity: I2 = 86.28%) and IL-6 (pooled SMD: 0.377; 95% CI: 0.156–0.598; significant heterogeneity: I2 = 88.75%) were significantly elevated in depression. There was no difference in TNF-α (p = 0.351) and CRP (p = 0.05) between those with depression and controls. Compared with controls, IL-1β (pooled SMD: 1.37, 95% CI: 0.06–2.68, significant heterogeneity: I2 = 96.01%) was significantly elevated in Alzheimer’s disease. There were no differences in IL-6 (p = 0.138), TNF-α (p = 0.451) and CRP (p = 0.07) between elderly with Alzheimer’s disease and controls. After Bonferroni adjustment, only IL-6 remained significantly higher in depression. Elderly with depression have higher IL-6 than controls, while those with Alzheimer’s disease did not have higher peripheral inflammatory markers.
Similar content being viewed by others
Introduction
Depression and Alzheimer’s disease are the most common psychiatric disorders amongst people aged 60 years and above. In recent years, depression resulted in 5.7% of Years Lived with Disability (YLD) in people above 60 years old and the annual global cost of dementia was US$604 billion1. In the future, the health burden due to depression and dementia will continue to increase because the number of people aged 60 years and above is expected to increase from 901 million in 2015 to 1.4 billion in 20302. Furthermore, elderly with depression and dementia are associated with higher risk of suicide3, chronic obstructive lung disease4, stroke5,6 and pneumonia7, resulting in premature death. Dementia does not only affect elderly themselves but also affects the mental health of their caregivers8, leading to loss of productivity in the society9. It is thus prudent to understand the pathophysiology behind depression as well as Alzheimer’s disease which is one of the most common subtypes of dementia in elderly.
Inflammation has long been thought to play a vital role in the pathophysiology of psychiatric disorders in general adults10. In depression, the inflammatory response system (IRS) activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to production of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), as well as increase in turnover of serotonin and catecholamines11. The IRS is driven by pro-inflammatory cytokines that are produced by macrophages, T cells and Natural Killer cells in response to immune activation12. The psycho-neuro-inflammatory theory was supported by previous studies which showed that stimulation of the HPA-axis resulted in the release of CRH by pro-inflammatory cytokines such as interleukin (IL)-113, IL-6 and tumor necrosis factor (TNF-α)14. Since then, multiple studies demonstrated increased levels of acute phase proteins and cytokines such as C-reactive protein (CRP), IL-1β and IL6 in depression15,16,17,18,19,20. Elevation in peripheral inflammatory cytokines were also seen in other psychiatric disorders such as: IL-6, TNF-α and TGF-β in acute psychosis;21 IL-2R22 and IL-623,24 in schizophrenia; IL-1β25,26, IL-6 and TNF-α26 in panic disorders; IL-1β and TNF-α in obsessive compulsive disorders27, and IL-1β, IL-6 and TNF-α in post-traumatic stress disorder (PTSD)28,29. The elevation of peripheral IL-1β, IL-6 and TNF-α could be a potential biomarker of vulnerability for psychiatric disorders in adults but their roles remain unknown for elderly.
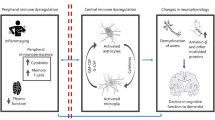
Inflammation may play a pathological role in psychiatric disorders in elderly because a pro-inflammatory state is associated with ageing. Aging facilitates the pro-inflammatory state by disrupting peripheral immune system leading to excessive innate immune activity with release of pro-inflammatory cytokines and decrease in anti-inflammatory molecules30. The disruption in the periphery-central nervous system (CNS) immune communication via the blood-brain barrier (BBB) partly attributed by the peripheral injury further contributes to the pro-inflammatory shift of CNS. There is also altered discordant CNS response to inflammation with the upregulation of genes controlling inflammatory processes, as demonstrated by an increase in pro-inflammatory gene expression in late life of mice31 and humans32. Microglia and astrocytes are the two main cell components of the CNS that play a pivotal role in the central neuro-inflammatory process responsible for neuro-biological hemostasis. With the pro-inflammatory shift of the aging CNS, there is proliferation of both activated microglia and astrocytes resulting in continuous production of pro-inflammatory cytokines33. This process is further amplified in depression and Alzheimer’s disease, whereby chronic stress34 and amyloid beta (Aβ)35 respectively disrupts the microglia activity, leading to further release of the pro-inflammatory cytokines, interleukins and chemokines. In addition, depression and Alzheimer’s disease cause alteration in the BBB, allowing permeability of the peripheral inflammatory cells into the CNS36, further enhancing a state of neuro-inflammation with consequent tissue damage. For depression, Martinez-Cengotitabengoa et al.37 performed a systematic review and found that elevation of peripheral IL-8, IL-6, and TNF-α could indicate vulnerability to late-life depression. This review was based on qualitative review of 6 studies and the findings require further verification by a meta-analysis. For all-cause dementia, a meta-analysis found that elevation of peripheral IL-6 and CRP levels were associated with increased risk of developing dementia38. Nevertheless, another meta-analysis found that there was no significant difference in mean CRP levels between elderly with and without Alzheimer’s disease39. The role of TNF-α as causative and or as contributor to pathogenesis of dementia remains inconclusive40,41.
Hitherto, no meta-analysis has specifically compared the peripheral levels of proinflammatory markers including IL-1β, IL-6, TNF-α and CRP between elderly with depression or Alzheimer’s disease and controls without any psychiatric disorder. Convergent evidence of higher peripheral IL-1β, IL-6, TNF-α and CRP levels would support the psychoneuroimmunology theory as contributory to depressive syndromes and Alzheimer’s disease in elderly. Therefore, we conducted a systematic review, meta-analysis and meta-regression. Our hypothesis was that elderly with depression or Alzheimer’s disease had higher levels of IL-1β, IL-6, TNF-α and CRP than controls without psychiatric disorders. We aimed to identify factors that could influence the peripheral levels of these inflammatory markers.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guideline for conducting and reporting meta-analysis and systematic reviews42.
Search Strategy
A comprehensive literature search was conducted through the major databases of PubMed, PsycINFO and Embase from inception to March 16, 2017. Searches including the following broad terms: cytokines, interleukins (interleukin, IL*, IL-1β, IL-6), tumor necrosis factor (TNF, tumor necrosis factor), c-reactive protein (CRP, c-reactive protein), elderly (late onset, late life, geriatric, elderly), mood disorders (depress*, depression, depressive, depressed, major depressive disorder, bipolar, manic-depressive) and Alzheimer’s disease (Alzheimer’s disease, Alzheimer, dementia, Alzheimer’s dementia, AD) where * indicates truncation43. A total of 2478 titles and abstracts were shortlisted, and references of the retrieved trials and review articles were also searched.
Data extraction
The following information was extracted from each article: family name of the first author, country, year of publication, sample size (number of controls and number of elderly with depression or dementia), levels of pro-inflammatory cytokines or CRP, measure of depression or dementia, mean age, proportion of female gender, duration of education, severity of depression or dementia, proportion of diabetes, cerebrovascular disorder and cardiovascular disease. All information was recorded on a standardized data collection form and was checked by the first and last author. The quality of the 18 studies was appraised using adaptations of the Newcastle–Ottawa cohort scale for cross-sectional and case–control studies, which assessed the selection of study participants, comparability of outcome groups and assessment of outcome measures.
Selection criteria
The two authors (AN and RCH) independently reviewed all abstracts and articles to evaluate whether the study would meet criteria for inclusion in the meta-analysis. If there was any disagreement between the authors about the inclusion of a study, a third author (MWZ) reviewed the study and made a decision. Studies were included if they fulfilled the following criteria: (1) Compared the blood levels of IL-1β, IL-6, TNF-α and CRP between elderly patients with depression or Alzheimer’s disease versus controls; (2) Patients with depression, Alzheimer’s disease and controls were at least 60 years old; (3) Study had sufficient information to calculate effect size. Studies were excluded if they: (1) studied patients with depression or Alzheimer’s disease who were younger than 60 years; (2) were animal studies; (3) did not measure levels of IL-1β, IL-6, TNF-α and CRP in the blood; (4) did not include a control group; (5) had a sample size of less than 30. If two studies were conducted in the same city within 5 years of publication, we would remove one of the studies that had smaller sample size to avoid overlapping of participants44,45,46. A total of 2444 articles were rejected because they did not meet the inclusion criteria. Thirty-four relevant articles were extracted and included for this meta-analysis.
Statistical analyses
All statistical analysis was performed using the “metafor” function in R based on DerSimonian-Laird random-effects model47 and based on methods established by previous study comparing levels of biological markers between a disease group and controls without the disease48. The random-effects model was used because it assumes varying effect sizes between studies, because of differing study design and study population49. Differences in mean levels of IL-1β, IL-6, TNF-α and CRP between elderly who had Alzheimer’s disease or depression and controls were calculated using a standardized mean difference based on random-effect model. Random-effect model attempted to generalize findings beyond the included studies by assuming that the selected studies are random samples from a larger population50. Test of heterogeneity were conducted with the Q statistic that is distributed as a X2 variate under the assumption of homogeneity of effect sizes43. Between- study heterogeneity was assessed with the I2 statistic. As a guide, I2 values of 25% may be considered low, 50% as moderate, and 75% high3,6. Regression test was performed to examine publication bias if there are 10 or more studies for the outcome. Bonferroni adjustment for multiple testing in meta-analysis produced a rejection p-value of 0.05 divided by the total number of outcomes51. In this meta-analysis, there were 4 outcomes (IL-1β, IL-6, TNF-α and CRP). As a result, the rejection p value was 5/4 = 0.0125.
For results with considerable heterogeneity, meta-regression was performed to identify demographic and disease-related variables which might contribute to the heterogeneity if there were 5 or more studies with the variables. The regression coefficients and the associated z values and p values were reported in the meta-regression analysis48. Patient-related factors included mean age, gender, years of education, severity of depression or Alzheimer’s disease, history of diabetes, hyperlipidemia, coronary heart disease52.
Results
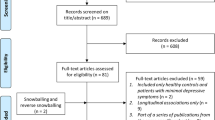
From an initial 2,478 potentially relevant articles, we assessed 176 full-text articles for eligibility. Finally, we included 34 articles in our analysis (Fig. 1). These studies comprising 2609 elderly with depression, 1645 elderly with Alzheimer’s disease and 14363 controls.
Majority of the studies were from western countries and characteristics of the population studied are shown in Table 1 and Table 2. Of these, five articles compared levels of IL-1β between depression and controls;53,54,55,56,57 seven articles compared IL-1β for Alzheimer’s disease and controls;41,44,57,58,59,60,61 nine articles compared IL-6 levels between depression and controls;53,55,57,62,63,64,65,66,67 eight articles compared IL-6 levels between Alzheimer’s disease and controls;57,59,60,68,69,70,71,72 five articles compared levels of TNF- α between depression and controls;53,55,64,66,73 ten articles compared the levels of TNF- α between Alzheimer’s disease and controls;41,59,60,69,70,71,72,74,75,76 nine articles compared the levels of CRP between depression and controls;55,56,63,64,65,66,67,77,78 eleven articles compared levels of CRP between Alzheimer’s disease and controls60,69,79,80,81,82,83,84,85,86,87. All the cytokine levels were converted to pg/ml.
Meta-Analysis for Elderly with and without Depression
Figure 2 shows the results of the 5 studies that compared the peripheral IL-1β levels between elderly suffering from depression and controls53,54,55,56,57. Elderly with depression were significantly higher in their peripheral IL-1β levels than control participants (pooled SMD with random-effects model: 0.642, 95% CI: 0.078–1.206, z = 2.232, p = 0.026). A significant level of between-study heterogeneity was found (τ2 = 0.337, Q = 29.16, df = 4, p < 0.001, I2 = 86.28%). When we undertook meta-regression to explore the impact of a priori sources of heterogeneity, no significant association was observed for age (β = −0.023 p = 0.767) and proportion of women (β = 1.070, p = 0.677).
Figure 3 shows the results of the 9 studies that compared the peripheral IL-6 levels between elderly suffering from depression and controls53,55,57,62,63,64,65,66,67. Elderly with depression were significantly higher in their peripheral IL-6 levels than control participants (pooled SMD with random-effects model: 0.377, 95% CI: 0.156–0.598, z = 3.348, p < 0.001). A significant level of between-study heterogeneity was found (τ2 = 0.085, Q = 75.75, df = 8, p < 0.001, I2 = 89.44%). Meta-regression shows that mean age of participants is a significant moderator (β = −0.093, p = 0.0363) but proportion of women is not (β = 1.108, p = 0.560). After adjustment of age, the adjusted pooled SMD became 0.2895. Publication bias was not detected (p = 0.1909).
Figure 4 shows the results of the 5 studies that compared the peripheral TNF- α levels between elderly suffering from depression and controls53,55,64,66,73. There was no significant difference in peripheral TNF- α levels between elderly with and without depression (pooled SMD with random-effects model: 0.112, 95% CI: −0.123–0.348, z = 0.968, p = 0.351). A significant level of between-study heterogeneity was found (τ2 = 0.044, Q = 15.80, df = 4, p = 0.003, I2 = 74.68%). Meta-regression was not conducted as there were too few studies.
Figure 5 shows the results of the 9 studies that compared the peripheral CRP levels between elderly suffering from depression and controls55,56,63,64,65,66,67,77,78. Elderly with depression were nominal higher in their peripheral CRP levels than control participants but not statistically significant (pooled SMD: 0.499, 95% CI: 0.001–0.999, z = 1.961, p = 0.050). A significant level of between-study heterogeneity was found (τ2 = 0.562, Q = 698.5, df = 8, p < 0.001, I2 = 98.9%). No significant association was revealed for mean age of participants (β = 0.049, p = 0.499) or proportion of elderly women (β = −0.012, p = 0.650) from meta-regression. Publication bias was not detected (p = 0.734).
Bonferroni adjustment for multiple testing produced a rejection p-value of 0.0125. This would mean that the outcome involving peripheral levels of IL-1β was no longer statistically significant. However, the outcome involving peripheral levels of IL-6 remained statistically significant.
Meta-Analysis for Elderly with and without Alzheimer’s disease
Figure 6 shows the results of the 6 studies that compared the peripheral IL-1β levels between elderly suffering from Alzheimer’s disease and controls41,57,58,59,60,61. Elderly with Alzheimer’s disease were significantly higher in their peripheral IL-1β levels than control participants (pooled SMD: 1.37, 95% CI: 0.06–2.68, z = 1.98, p = 0.041). A significant level of between-study heterogeneity was found (τ2 = 2.31, Q = 175.54, df = 7, p < 0.001, I2 = 96.01%). Meta-regression showed that mean age (β = −0.72, p = 0.247) and proportion of elderly women (β = 0.031, p = 0.626) were not statistical significant. Regression for MMSE was not done as there were less than 5 studies with MMSE score.
Figure 7 shows the results of the 8 studies that compared the peripheral IL-6 levels between elderly suffering from Alzheimer’s disease and controls57,59,60,68,69,70,71,72. There was no significant difference in peripheral IL-6 levels between elderly with and without Alzheimer’s disease (pooled SMD with random-effects model: 0.228, 95% CI: −0.074–0.528, z = 1.482, p = 0.138). A significant level of between-study heterogeneity was found (τ2 = 0.117, Q = 19.36, df = 7, p = 0.007, I2 = 63.84%). Meta-regression showed that MMSE score was statistically significant (β = −0.1048, p = 0.010) while no significant association was observed for mean age (β = −0.098, p = 0.819) and proportion of women (β = 0.002, p = 0.853).
Figure 8 shows the results of the 10 studies that compared the peripheral TNF-α levels between elderly suffering from Alzheimer’s disease and controls41,59,60,69,70,71,72,74,75,76. There was no significant difference in peripheral TNF-α levels between elderly with and without Alzheimer’s disease (pooled SMD with random-effects model: 0.18, 95% CI: -0.29–0.65, z = 0.338, p = 0.451). A significant level of between-study heterogeneity was found (τ2 = 0.542, Q = 95.14, df = 11 p < 0.001, I2 = 88.44%). Meta-regression revealed that mean age (β = 0.0466, p = 0.654), proportion of women (β = −0.031, p = 0.594), and MMSE (β = −0.2466, p = 0.2908) were not statistically significant. No publication bias was detected (p = 0.0802).
Figure 9 shows the results of the 11 studies that compared the peripheral CRP levels between elderly suffering from Alzheimer’s disease and controls60,69,79,80,81,82,83,84,85,86,87. There was no significant difference in peripheral CRP levels between elderly with and without Alzheimer’s disease (pooled SMD with random-effects model: 0.638, 95% CI: −0.055–1.331, z = 1.804, p = 0.0712). A significant level of between-study heterogeneity was found (τ2 = 1.293, Q = 492.4, df = 10, p < 0.001, I2 = 97.97). Meta-regression revealed that mean age (β = 0.0796, p = 0.527) and proportion of women (β = −0.035, p = 0.274), were not statistically significant. Publication bias was detected (p < 0.001). As Mancinella’s study reported an extremely large effect, a sensitivity analysis was conducted by excluding it in the analysis. Without this study, the pooled SMD became −0.142 (95% CI: −0.654–0.370) (See Fig. 10) and publication bias was no longer detected (p = 0.368).
Bonferroni adjustment for multiple testing produced a rejection p-value of 0.0125. This would mean that the outcome involving peripheral levels of IL-1β was no longer statistically significant.
Discussion
In this meta-analysis, we found that elderly with depression had significantly higher peripheral levels of IL-1β (p = 0.026), IL-6 (p < 0.001) but not TNF-α (p = 0.351) and CRP (p = 0.05). After Bonferroni adjustment, only peripheral levels of IL-1β remained significantly higher in elderly with depression than controls. However, elderly with Alzheimer’s disease only had significantly higher peripheral levels of IL-1β (p = 0.047) but not IL-6 (p = 0.138), TNF-α (p = 0.735) or CRP (p = 0.0712). After Bonferroni adjustment, none of the above inflammatory markers remained significant. Heterogeneity was noted in the studies for depression and Alzheimer’s disease. Factors such as age range of the elderly, proportion of gender in subjects, severity of the condition (depression or Alzheimer’s disease), presence and extent of medical comorbidities, study design and methodical variance which includes the types of assay kits used to measure cytokines, may contribute to the observed heterogeneity. Nevertheless, it is not feasible to match for all these factors in the meta-analysis. In this meta-analysis, there was no publication bias. Our results with Bonferroni adjustment are different from a previous meta-analysis which was based on 7 studies and found that higher levels of IL-6 and CRP were associated with increased risk of all-cause dementia38. Furthermore, this previous meta-analysis did not study IL-1β and TNF-α38. Ravaglia et al.88 reported that peripheral inflammatory markers are associated with increased risk of vascular dementia but not Alzheimer’s disease. This postulation might explain why this meta-analysis found that all peripheral inflammatory markers were not increased in Alzheimer’s disease. Many previous studies examining blood cytokine levels and cognitive impairment were also inconclusive in results89 and this could possibly be attributed by lack of longitudinal data on cytokine expression, inadequate methodological standardization and standardization of patient characteristics. Further prospective studies are required to study the role of each proinflammatory cytokine in different types of dementia and correlate with clinical features, neuroimaging and presence of beta amyloid and neurofibrillary tangles. Our results suggest that peripheral pro-inflammatory marker IL-6 is more likely to be increased in depression as compared to Alzheimer’s disease in elderly. The increased peripheral IL-6 levels in elderly with depression indicate vascular risk factors and atherosclerosis59 which can contribute to depression. Primary prevention strategies targeting metabolically mediated comorbidity (e.g., cardiovascular disease) may prevent depression in elderly90. Previous study revealed the potential therapeutic effects of antidepressant fluoxetine by reducing central and peripheral levels of IL-6 in the alleviation of depressive symptoms91.
In this meta-analysis, elderly with depression or Alzheimer’s disease were found to have significantly higher peripheral IL-1β levels before Bonferroni adjustment. IL-1β is produced by macrophages, endothelial cells, and astrocytes59. IL-1β was found to be a significant risk factor for the development of depressive symptoms92. IL-1β crosses the blood brain barrier93 and subsequently alters the HPA-axis11. IL-1β reduces the re-uptake of serotonin from the synaptic cleft resulting in depressive symptoms94. For Alzheimer’s disease, Griffin et al.95 found that IL-1β plays an important role in causing neurodegenerative changes. IL-1β drives the synthesis of β-amyloid precursor protein, resulting in the production and deposition of β-amyloid plaques in the brain of patients with Alzheimer’s disease95,96. It is also involved in tau phosphorylation97, a key pathogenic process in Alzheimer’s disease7.
IL-6 is produced by macrophages, T-lymphocytes, endothelial cells, smooth muscle cells and adipocytes59. IL-6 modulates the HPA-axis which stimulates CRH, ACTH and cortisol98. IL-1β and IL-6 could also work together and synergistically induce systemic immune response, leading to psycho-neuro-immunological changes in patients suffering from depression98. IL-6 also induces production of indoleamine 2,3-dioxygenase (IDO), leading to a decrease in tryptophan and the production of tryptophan catabolites (TRYCATs), and this is associated with depressive symptoms99. In contrast, peripheral IL-6 level was not significantly higher in elderly with Alzheimer’s disease as compared to controls. This finding could be supported by previous research which found that elevations observed in peripheral IL-6 levels preceded the onset of Alzheimer’s disease but not during the course of Alzheimer’s disease70.
CRP is an acute-phase protein of hepatic origin that increases following IL-6 secretion by macrophages and T cells. In this meta-analysis, there was no significant difference in peripheral CRP levels in elderly with depression and Alzheimer’s disease as compared to controls before and after Bonferroni’s correction. Eriksson et al.100 proposed that increased peripheral levels of CRP in mid-life played a more detrimental role leading to Alzheimer’s disease but not in older people. Our findings revealed that peripheral CRP level was not increased but IL-1β was significantly raised in elderly with Alzheimer’s disease as compared to controls before Bonferroni adjustment. Licastro et al.86 postulated that the IL-1β increment in elderly with Alzheimer’s disease might be due to central immune response.
TNF-α is produced by macrophages, adipocytes and astrocytes70. In this meta-analysis, peripheral levels of TNF-α were not significantly increased in elderly with depression as well as Alzheimer’s disease as compared to controls before and after Bonferroni adjustment. Firstly, our findings might suggest that peripheral TNF-α is a less sensitive marker as compared to IL-1β and IL-6. Secondly, our findings support previous postulation that the levels of peripheral TNF- α was found to be significantly lower in mild to moderate Alzheimer’s disease as compared to severe Alzheimer’s disease101. When we included participants with different severity of Alzheimer’s disease, the peripheral TNF – α levels were not significantly higher than controls.
Limitations
This meta-analysis has several limitations. Firstly, one major limitation is lack of repeated measurements for inflammatory markers in elderly with depression and Alzheimer’s disease in this meta-analysis. We cannot ignore the limitations of performing a meta-analysis on cross-sectional studies. The causality of high levels of peripheral inflammatory markers in elderly with depression cannot be established102. Based on the current study, it is not possible to delineate whether inflammation plays a causal role in depression or dementia, or if inflammatory processes in cardiovascular diseases that are common in the elderly lead to increased depressive symptoms in elderly or vice versa. Secondly, this meta-analysis focused on peripheral inflammatory markers which might not reflect inflammatory process in the central nervous system.
Conclusion
In conclusion, our meta-analysis found that elderly with depression had elevated peripheral levels of IL-1β, IL-6 but not CRP and TNF-α, and elderly with Alzheimer’s disease only had increased peripheral levels of blood IL-1β. After correction for multiple testing, only peripheral levels of IL-6 remained significantly higher in elderly with depression. The involvement of peripheral IL-6 in elderly with depression reflects a high proportion of metabolic comorbidities which are modifiable. Further studies are necessary to evaluate the association between high peripheral IL-6 levels and the subsequent risk of developing depression in old age.
References
World Health Organisation & Alzheimer’s Disease International. Dementia: A Public Health Priority. World Health Organization. http://www.who.int/mental_health/publications/dementia_report_2012/en/ (2012).
UN: Population Division. World population projected to reach 9.7 billion by 2050. Department of Economic and Social Affairs. http://www.un.org/en/development/desa/news/population/2015-report.html (2015).
Ho, R. C., Ho, E. C., Tai, B. C., Ng, W. Y. & Chia, B. H. Elderly suicide with and without a history of suicidal behavior: implications for suicide prevention and management. Archives of suicide research 18, 363–375 (2014).
Zhang, M. W., Ho, R. C., Cheung, M. W., Fu, E. & Mak, A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. General hospital psychiatry 33, 217–223 (2011).
Loh, A. Z., Tan, J. S., Zhang, M. W. & Ho, R. C. The global prevalence of anxiety and depressive symptoms among caregivers of stroke survivors. Journal of the American Medical Directors Association 18, 111–116 (2016).
Mak, K. K., Kong, W. Y., Mak, A., Sharma, V. K. & Ho, R. C. Polymorphisms of the serotonin transporter gene and post-stroke depression: a meta-analysis. J Neurol Neurosurg Psychiatry 84, 322–328 (2012).
Puri, B., Hall, A. & Ho, R. Revision Notes in Psychiatry, Third Edition, Taylor & Francis (2013).
Sallim, A. B., Sayampanathan, A. A., Cuttilan, A. & Ho, R. C. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. Journal of the American Medical Directors Association 16, 1034–1041 (2015).
Ho, R. C., Mak, K. K., Chua, A. N., Ho, C. S. & Mak, A. The effect of severity of depressive disorder on economic burden in a university hospital in Singapore. Expert review of pharmacoeconomics & outcomes research 13, 549–559 (2013).
Liu, Y., Ho, R. C. & Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Journal of affective disorders 139, 230–239 (2012).
Maes, M. Major depression and activation of the inflammatory response system, in Cytokines, stress, and depression. Springer (pp. 25–46 (1999).
Lu, Y. et al. Prevalence of anxiety and depressive symptoms in adolescents with asthma: A meta‐analysis and meta‐regression. Pediatric allergy and immunology 23, 707–715 (2012).
Sapolsky, R., Rivier, C., Yamamoto, G., Plotsky, P. & Vale, W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238, 522–524 (1987).
Besedovsky, H. et al. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. The Journal of steroid biochemistry and molecular biology 40, 613–618 (1991).
Maes, M. et al. Depression‐related disturbances in mitogen‐induced lymphocyte responses and interleukin‐1β and soluble interleukin‐2 receptor production. Acta Psychiatrica Scandinavica 84, 379–386 (1991).
Maes, M. et al. Higher α 1-antitrypsin, haptoglobin, ceruloplasmin and lower retinol binding protein plasma levels during depression: further evidence for the existence of an inflammatory response during that illness. Journal of affective disorders 24, 183–192 (1992).
Maes, M., Bosmans, E., Meltzer, H. Y., Scharpé, S. & Suy, E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? The American journal of psychiatry 150, 1189–1193 (1993).
Maes, M. et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry research 54, 143–160 (1994).
Maes, M. et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of affective disorders 34, 301–309 (1995).
Maes, M. et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 853–858 (1997).
Mahadevan, J. et al. An exploratory study of immune markers in acute and transient psychosis. Asian Journal of Psychiatry 25, 219–223 (2017).
Rapaport, M. H. et al. Increased serum soluble interleukin-2 receptors in schizophrenic monozygotic twins. European archives of psychiatry and clinical neuroscience 243, 7–10 (1993).
Ganguli, R. et al. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry research 51, 1–10 (1994).
Neelamekam, S., Nurjono, M. & Lee, J. Regulation of interleukin-6 and leptin in schizophrenia patients: a preliminary analysis. Clinical Psychopharmacology and Neuroscience 12, 209–214 (2014).
Brambilla, F. et al. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry research 54, 135–142 (1994).
Hoge, E. et al. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depression and anxiety 26, 447–455 (2009).
Brambilla, F. et al. Plasma Interleukin-1β and Tumor Necrosis Factor Concentrations in Obsessive–Compulsive Disorders. Biological psychiatry 42, 976–981 (1997).
Lindqvist, D. et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain, behavior, and immunity 42, 81–88 (2014).
Oganesyan, L., Mkrtchyan, G., Sukiasyan, S. & Boyajyan, A. S. Classic and alternative complement cascades in post-traumatic stress disorder. Bulletin of experimental biology and medicine 148, 859–861 (2009).
Gruver, A. L., Hudson, L. L. & Sempowski, G. D. Immunosenescence of ageing. J Pathol. 211, 144–156 (2007).
Cheol-Koo, L., Weindruch, R. & Prolla, T. A. Gene-expression profile of the ageing brain in mice. Nature genetics 25, 294–297 (2000).
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004).
Sparkman, N. L. & Johnson, R. W. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 15, 323–330 (2008).
Kreisel, T. et al. The role of microglia in stress-induced depression. Brain Behav. Immun 40, e2–e3 (2014).
Pasqualetti, G., Brooks, D. J. & Edison, P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep 15(4), 17 (2015).
Syvanen, S. & Eriksson, J. Advances in PET imaging of P-glycoprotein function at the blood-brain barrier. ACS Chemical Neuroscience 4(2), 225–37 (2013).
Martínez-Cengotitabengoa, M. et al. Peripheral inflammatory parameters in late-life depression: A systematic review. International journal of molecular sciences 17, 2022 (2016).
Koyama, A. et al. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 68, 433–440 (2012).
Gong, C. et al. A Meta-Analysis of C-Reactive Protein in Patients With Alzheimer’s Disease. American Journal of Alzheimer’s Disease & Other Dementias 31, 194–200 (2016).
Engelhart, M. J. et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology 61, 668–672 (2004).
Yasutake, C., Kuroda, K., Yanagawa, T. & Okamura, T. & Yoneda, H. Serum BDNF, TNF-α and IL-1β levels in dementia patients. European Archives of Psychiatry and Clinical Neuroscience 256, 402–406 (2006).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. & PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 151, 264–269 (2009).
Ho, R. C. et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. The American Journal of Geriatric Psychiatry 19, 607–617 (2011).
Zuliani, G. et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. Journal of the neurological sciences 272, 164–170 (2008).
Teunissen, C., Durieux-Lu, S., Blankenstein, M., Oude Voshaar, R. C. & Comijs, H. C. The inflammatory marker GDF-15 is not independently associated with late-life depression. Journal of psychosomatic research 83, 46–49 (2016).
Toledo, J. B. et al. Cardiovascular risk factors, cortisol, and amyloid-β deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & Dementia 8, 483–489 (2012).
R Core Team. R: A language and environment for statistical computing. http://www.R-project.org/. Vienna, Austria (2013).
Ho, R. C. et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmunity reviews 15, 124–138 (2016).
Mak, A., Cheung, M. W. L., Chiew, H. J., Liu, Y. & Ho, R. C. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 41, 830–839 (2012).
Cheung, M. W. L., Ho, R. C., Lim, Y. & Mak, A. Conducting a meta‐analysis: basics and good practices. International journal of rheumatic diseases 15, 129–135 (2012).
Cleophas, T.J. & Zwinderman, A.H. Modern Meta-Analysis: Review and Update of Methodologies. Springer International Publishing (2017).
Quek, Y. H., Tam, W. W., Zhang, M. W. & Ho, R. C. M. Exploring the association between childhood and adolescent obesity and depression: a meta‐analysis. Obesity Reviews 18, 742–754 (2017).
Charlton, R. A. et al. Associations between pro‐inflammatory cytokines, learning, and memory in late‐life depression and healthy aging. International Journal of Geriatric Psychiatry 33, 104–112 (2018).
Diniz, B. S., Teixeira, A. L., Talib, L., Gattaz, W. F. & Forlenza, O. V. Interleukin-1β serum levels is increased in antidepressant-free elderly depressed patients. The American Journal of Geriatric Psychiatry 18, 172–176 (2010).
Milaneschi, Y. et al. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biological psychiatry 65, 973–978 (2009).
Thomas, A. J. et al. Increase in interleukin-1β in late-life depression. American Journal of Psychiatry 162, 175–177 (2005).
Torres, K. C. et al. Increased frequency of cluster of differentiation 14 (CD14+) monocytes expressing interleukin 1 beta (IL‐1β) in Alzheimer’s disease patients and intermediate levels in late‐onset depression patients. International journal of geriatric psychiatry 29, 137–143 (2014).
De Luigi, A. et al. Inflammatory markers in Alzheimer’s disease and multi-infarct dementia. Mechanisms of ageing and development 122, 1985–1995 (2001).
Zuliani, G. et al. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. Journal of psychiatric research 41, 686–693 (2007).
Villarreal, A. E. et al. Serum-based protein profiles of Alzheimer’s disease and mild cognitive impairment in elderly Hispanics. Neurodegener Dis Manag 6, 203–213 (2016).
Forlenza, O. V. et al. Increased serum IL-1β level in Alzheimer’s disease and mild cognitive impairment. Dementia and geriatric cognitive disorders 28, 507–512 (2009).
Dimopoulos, N., Piperi, C., Psarra, V., Lea, R. W. & Kalofoutis, A. Increased plasma levels of 8-iso-PGF 2α and IL-6 in an elderly population with depression. Psychiatry research 161, 59–66 (2008).
Elderkin-Thompson, V., Irwin, M. R., Hellemann, G. & Kumar, A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. The American Journal of Geriatric Psychiatry 20, 753–763 (2012).
Forti, P. et al. A. Blood inflammatory proteins and risk of incident depression in the elderly. Dementia and geriatric cognitive disorders 29, 11–20 (2010).
Nadrowski, P. et al. Associations between cardiovascular disease risk factors and IL-6 and hsCRP levels in the elderly. Experimental gerontology 85, 112–117 (2016).
Penninx, B. W. et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biological psychiatry 54, 566–572 (2003).
Vogelzangs, N., Comijs, H. C., Oude Voshaar, R. C., Stek, M. L. & Penninx, B. W. Late-life depression symptom profiles are differentially associated with immunometabolic functioning. Brain, behavior, and immunity 41, 109–115 (2014).
Kalman, J. et al. Serum interleukin‐6 levels correlate with the severity of dementia in Down syndrome and in Alzheimer’s disease. Acta Neurologica Scandinavica 96, 236–240 (1997).
Ciabattoni, G. et al. Determinants of platelet activation in Alzheimer’s disease. Neurobiology of aging 28, 336–342 (2007).
Uslu, S. et al. Levels of amyloid beta-42, interleukin-6 and tumor necrosis factor-alpha in Alzheimer’s disease and vascular dementia. Neurochemical research 37, 1554–1559 (2012).
Huang, C. W. et al. Potential blood biomarker for disease severity in the Taiwanese population with Alzheimer’s disease. American Journal of Alzheimer’s Disease & Other Dementias 28, 75–83 (2013).
Bonotis, K. et al. Systemic immune aberrations in Alzheimer’s disease patients. Journal of neuroimmunology 193, 183–187 (2008).
Marinho, P. E., Castro, C. M., Raposo, M. C., Guerra, R. O. & de Andrade, A. D. Depressive symptoms, inflammatory markers and body composition in elderly with and without chronic obstructive pulmonary disease (COPD). Archives of gerontology and geriatrics 54, 453–458 (2012).
Álvarez, A., Cacabelos, R., Sanpedro, C., García-Fantini, M. & Aleixandre, M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiology of aging 28, 533–536 (2007).
Solerte, S., Cravello, L., Ferrari, E. & Fioravanti, M. Overproduction of IFN‐γ and TNF‐α from Natural Killer (NK) Cells Is Associated with Abnormal NK Reactivity and Cognitive Derangement in Alzheimer’s Disease. Annals of the New York Academy of Sciences 917, 331–340 (2000).
Laske, C. et al. Identification of a blood-based biomarker panel for classification of Alzheimer’s disease. International Journal of Neuropsychopharmacology 14, 1147–1155 (2011).
Bremmer, M. et al. Inflammatory markers in late-life depression: results from a population-based study. Journal of affective disorders 106, 249–255 (2008).
Kop, W. J. et al. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. The American journal of cardiology 89, 419–424 (2002).
Mulder, S. D. et al. Evaluation of intrathecal serum amyloid P (SAP) and C-reactive protein (CRP) synthesis in Alzheimer’s disease with the use of index values. Journal of Alzheimer’s Disease 22, 1073–1079 (2010).
O’Bryant, S. E. et al. Decreased C-reactive protein levels in Alzheimer disease. Journal of geriatric psychiatry and neurology 23, 49–53 (2010).
Lawlor, B. A., Swanwick, G. R., Feighery, C., Walsh, J. B. & Coakley, D. Acute phase reactants in Alzheimer’s disease. Biological Psychiatry 39, 1051–1052 (1996).
Lepara, O. et al. Elevated serum homocysteine level is not associated with serum C-reactive protein in patients with probable Alzheimer’s disease. Journal of neural transmission 116, 1651–1656 (2009).
Mancinella, A. et al. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Archives of gerontology and geriatrics 49, 185–194 (2009).
Davis, G., Baboolal, N., Nayak, S. & McRae, A. Sialic acid, homocysteine and CRP: potential markers for dementia. Neuroscience letters 465, 282–284 (2009).
Hsu, P. F. et al. C-Reactive protein predicts incidence of dementia in an elderly Asian community cohort. Journal of the American Medical Directors Association 18(277), e7–277 (2017).
Licastro, F. et al. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? Journal of neuroimmunology 103, 97–102 (2000).
Yarchoan, M. et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. Journal of the neurological sciences 333, 9–12 (2013).
Ravaglia, G. et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiology of aging 28, 1810–1820 (2007).
Brosseronm, F., Krauthausen, M., Kummer, M. & Heneka, M. T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 50, 534–544 (2014).
McIntyre, R. S. et al. Should depressive syndromes be reclassified as “metabolic syndrome type II. Annals of Clinical Psychiatry 19, 257–264 (2007).
Lu, Y. et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PloS one 12, e0186700 (2017).
van den Biggelaar, A. H. et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Experimental gerontology 42, 693–701 (2007).
Banks, W. A., Farr, S. A. & Morley, J. E. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation 10, 319–327 (2002).
Ramamoorthy, S. et al. Regulation of the human serotonin transporter by interleukin-1β. Biochemical and biophysical research communications 216, 560–567 (1995).
Griffin, W. et al. Glial‐neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’in disease progression. Brain pathology 8, 65–72 (1998).
Forloni, G., Demicheli, F., Giorgi, S., Bendotti, C. & Angeretti, N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Molecular brain research 16, 128–134 (1992).
Li, Y., Liu, L., Barger, S. W. & Griffin, W. S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. Journal of Neuroscience 23, 1605–1611 (2003).
Maes, M. et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry research 49, 11–27 (1993).
Maes, M., Anderson, G., Kubera, M. & Berk, M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert opinion on therapeutic targets 18, 495–512 (2014).
Eriksson, U. K. et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer’s disease and dementia. Journal of Alzheimer’s Disease 23, 361–369 (2011).
Paganelli, R. et al. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild–moderate Alzheimer’s disease patients. Experimental gerontology 37, 257–263 (2002).
Ho, R. C., Fu, E. H., Chua, A. N., Cheak, A. A. & Mak, A. Clinical and psychosocial factors associated with depression and anxiety in Singaporean patients with rheumatoid arthritis. International journal of rheumatic diseases 14, 37–47 (2011).
Author information
Authors and Affiliations
Contributions
R.C.H. designed the study; A.N. performed the systematic review; A.N., W.W.T., M.W.Z. critically reviewed the studies; A.N. drafted the manuscript; C.S.H., S.F.H., R.S.M., R.C.H. reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ng, A., Tam, W.W., Zhang, M.W. et al. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci Rep 8, 12050 (2018). https://doi.org/10.1038/s41598-018-30487-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30487-6
This article is cited by
-
Characterization of preclinical Alzheimer’s disease model: spontaneous type 2 diabetic cynomolgus monkeys with systemic pro-inflammation, positive biomarkers and developing AD-like pathology
Alzheimer's Research & Therapy (2024)
-
Impacts of inflammatory cytokines on depression: a cohort study
BMC Psychiatry (2024)
-
Association between symptoms of depression and inflammatory parameters in people aged over 90 years
BMC Geriatrics (2024)
-
Gut microbiota connects the brain and the heart: potential mechanisms and clinical implications
Psychopharmacology (2024)
-
Association between dietary magnesium intake, inflammation, and neurodegeneration
European Journal of Nutrition (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.