Abstract
This study aimed to assess the predictors of acute kidney injury (AKI) during colistin therapy in a cohort of patients with bloodstream infections (BSI) due to colistin-susceptible Gram-negative bacteria, focusing on the role of serum albumin levels. The study consisted of two parts: (1) a multicentre retrospective clinical study to assess the predictors of AKI during colistin therapy, defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria; and (2) bioinformatic and biochemical characterization of the possible interaction between human serum albumin and colistin. Among the 170 patients included in the study, 71 (42%), 35 (21%), and 11 (6%) developed KDIGO stage 1 (K1-AKI), KDIGO stage 2 (K2-AKI), and KDIGO stage 3 (K3-AKI), respectively. In multivariable analyses, serum albumin <2.5 g/dL was independently associated with K1-AKI (subdistribution hazard ratio [sHR] 1.85, 95% confidence interval [CI] 1.17–2.93, p = 0.009) and K2-AKI (sHR 2.37, 95% CI 1.15–4.87, p = 0.019). Bioinformatic and biochemical analyses provided additional information nurturing the discussion on how hypoalbuminemia favors development of AKI during colistin therapy. In conclusion, severe hypoalbuminemia independently predicted AKI during colistin therapy in a large cohort of patients with BSI due to colistin-susceptible Gram-negative bacteria. Further study is needed to clarify the underlying causal pathways.
Similar content being viewed by others
Introduction
Colistin is a polymyxin antibiotic with in vitro activity against most aerobic Gram-negative rods1,2. The use of colistin for the treatment of infections in humans was largely abandoned in the last quarter of the past century, owing to concerns of nephrotoxicity and neurotoxicity1,3,4. In the last two decades, the lack of dependable alternatives for treating infections due to multidrug resistant (MDR) Gram-negative bacteria has led to an increasing use of colistin, as well as to a renewed interest in its effectiveness and tolerability in humans1,5,6.
In recent years, several clinical studies have investigated risk factors for nephrotoxicity, or more appropriately acute kidney injury (AKI), during colistin treatment, with heterogeneous results. Indeed, possible increases in the risk of AKI due to increasing age, comorbid conditions, prolonged course of treatment, high colistin dosage, concomitant nephrotoxic agents, pre-existing renal failure, hypertension, and obesity have been variously reported6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. In addition, hypoalbuminemia has also been suggested to possibly predispose to AKI during colistin therapy15,18,24,27. However, the true impact of hypoalbuminemia remains unclear because of conflicting results in different studies, possibly relying on the wide heterogeneity in patient population, type of infections, and study design.
In the present study, we assessed the predictors of AKI during colistin therapy in a cohort of patients with bloodstream infections (BSI) due to colistin-susceptible Gram-negative bacteria, focusing on the role of serum albumin levels. In addition, we performed docking simulations and biochemical characterization of colistin binding to human serum albumin (HSA), in order to verify whether an interaction between colistin and albumin exist that might add to the discussion on any possible causal relationship between hypoalbuminemia and AKI during colistin therapy.
Materials and Methods
The present study consists of two parts: (1) a multicentre retrospective clinical study; and (2) bioinformatic and biochemical characterization of the possible interaction between human serum albumin (HSA) and colistin.
Part 1 – Multicentre retrospective clinical study
Study design
The multicentre retrospective clinical study was conducted in the following Italian hospitals: (i) Ospedale Policlinico San Martino – IRCCS per l’Oncologia, 1200 beds, in Genoa; (ii) Azienda Sanitaria Universitaria Integrata di Trieste, 600 beds, in Trieste; (iii) ASST Monza – Ospedale San Gerardo, 1200 beds, in Monza; and (iv) Città di Lecce Hospital – gruppo GVM Care and Research, 150 beds, in Lecce. All patients with BSI caused by colistin-susceptible Gram-negative bacteria and treated with intravenous colistin from January 2011 to June 2016 were identified through the computerized databases of the four hospitals. Exclusion criteria were: (i) missing key data; (ii) less than 48 h of intravenous colistin; and (iii) hemodialysis. The primary study outcome measure was AKI during colistin treatment, defined as a time-to-event endpoint. Patients were followed until the 14th day of colistin therapy or death, whichever came first.
Definitions
BSI was defined as the presence of at least one blood culture positive for Gram-negative bacteria in presence of signs and symptoms of infection30. AKI was defined on the basis of serum creatinine levels according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (Table 1)31.
Data collection
The following demographic and clinical variables were collected from clinical charts as baseline data at the time of colistin initiation: age; gender; presence of diabetes mellitus, presence of solid neoplasms, presence of hematological malignancies, presence of chronic renal failure, presence of severe hepatic failure (defined as liver cirrhosis according to histology or in presence of a clinical diagnosis supported by laboratory, endoscopy, and radiologic findings32), presence of septic shock (defined as hypotension not responding to fluid therapy and requiring vasoactive agents), presence of polymicrobial BSI, ward of stay (intensive care unit [ICU] vs. non-ICU), presence of central venous catheter, neutropenia (defined as absolute neutrophil count of <500/mm3), serum total bilirubin, serum hemoglobin, serum creatinine, serum albumin. The following data were also collected with regard to the management and course of BSI: dosage and length of colistin therapy, whether colistin was given as monotherapy or in combination, time to adequate therapy (defined as the number of days elapsing from the first positive blood culture to initiation of at least one antibiotic with in vitro activity against the causative agent of BSI), serum creatinine during colistin therapy.
Microbiology
The Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France) was routinely used for identifying the causative agent of BSI and for susceptibility testing. Colistin susceptibility testing was performed using the Vitek 2 system or the Etest (bioMérieux, Marcy l’Etoile, France). Susceptibility results were interpreted according to the latest EUCAST criteria (EUCAST breakpoint tables for interpretation of MICs and zone diameters, version 6.0, 2016; http://www.eucast.org).
Statistical analysis
Baseline demographic and clinical data of patients were described with numbers and percentages for categorical variables, and with median and interquartile range (IQR) for continuous variables.
The main analysis was the identification of predictors of AKI during colistin therapy. To this aim, the possible association of demographic and clinical factors with AKI was assessed in univariable Fine-Gray models, with AKI as the outcome of interest and death as a competing event33. The Day 0 was the day of colistin initiation. Patients who discontinued colistin before 14 days of therapy were right-censored at the time of discontinuation. In different univariable models, serum albumin was either dichotomized according to an arbitrary cut-off based on its median concentration in the study population (<2.5 g/dl vs. ≥2.5 g/dl), or considered as a continuous variable. All the variables were tested for their possible association with three different dependent variables, reflecting the three different stages of AKI according to KDIGO classification: (i) KDIGO stage 1 (K1-AKI); (ii) KDIGO stage 2 (K2-AKI); (iii) KDIGO stage 3 (K3-AKI).
To assess the independent role of variables, factors showing a potential association with AKI in univariable analyses (p < 0.10) were included in an initial multivariable Fine-Gray model, and further selected through a stepwise backward procedure based on the Akaike information criterion. Different multivariable analyses were conducted according to all the possible combinations of independent (dichotomous or continuous albumin) and dependent (K1-AKI, K2-AKI, K3-AKI) variables. The proportionality assumption was checked by plotting Schoenfeld residuals and by verifying the absence of statistically significant interactions between time and covariates in Fine-Gray models.
The cumulative incidence of K1-AKI, K2-AKI, and K3-AKI, in patients with or without severe hypoalbuminemia (<2.5 and ≥2.5 g/dL, respectively) was calculated by means of the Aalen-Johansen method, considering death as a competing event and applying right-censoring at the time of colistin discontinuation34. Statistical analyses were performed using the R Statistical Software (version 3.3.0, R Foundation for Statistical Computing, Vienna, Austria).
Part 2 - Bioinformatic and biochemical analyses
Bioinformatic analysis
Docking simulations of colistin binding to HSA were performed using the crystal structure of ligand-free HSA (PDB ID: 1AO6)35. The colistin three-dimensional structure was obtained from the Drug Bank Database (https://www.drugbank.ca/drugs/DB00803). Simulations were carried out using DockingApp36, a user friendly interface for the docking program AutoDock Vina37. In all the simulations, the search space (docking grid) included the whole HSA structure in order to carry out “blind” predictions of the colistin binding sites. The simulations were carried out both by keeping all protein residues rigid and by allowing flexibility only of the residues building up the walls of the FA sites (FA1 to FA9) (see38). Residues for which flexibility was allowed are reported in the supplementary material (Table S1). Rotatable bonds of colistin structure were kept flexible in all the simulations.
Biochemical analysis
As spectrofluorimetric and spectrophotometric binding studies were not informative of colistin binding to HSA due to the lack of optical variations following the interaction, we performed an indirect assay. In particular, colistin binding to HSA was evaluated by measuring the MG1655 E. coli strain (ATCC® 47046; Manassas, VA, USA) growth. Briefly, the activity of colistin (Sigma-Aldrich, St. Louis, MO, USA) and HSA (Sigma-Aldrich) on the MG1655 E. coli strain was tested in 96-well microtiter plates. In order to obtain high cell densities, bacterial cells were grown overnight in Mueller Hinton Broth 2 (MH II) (Sigma-Aldrich) and then diluted to an OD600 of 0.001 in MH II broth containing increasing concentrations of colistin (1, 1.25, and 1.5 μg/mL) and HSA (25, 50, 100, and 200 μg/mL). Microtiter plates were incubated for 20 h at 37 °C. Bacteria growth was measured at a wavelength of 600 nm using a microplate reader (Spark, Tecan, Switzerland). Biochemical results are shown as the means ± standard deviation (SD) derived minimally from three independent experiments. Differences between means, assessed by the Student’s t-test (GraphPad InStat 3.1 Software Inc., San Diego, CA, USA), were considered significant when p values were ≤0.05.
Ethical approval and informed consent
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the regional ethics committee of the coordinating center (Regional Ethics Committee of Liguria Region, registry number 473REG2016) and subsequently by the local ethics committees/institutional review boards of the other participating centers. No specific informed consent was required because of the retrospective nature of the analyses.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Part 1 – Multicentre retrospective clinical study
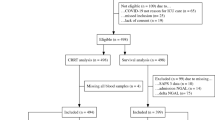
During the study period, 214 patients with BSI due to colistin-susceptible Gram-negative bacteria were treated with intravenous colistin, and 170 of them (79%) were included in the study (Fig. 1). Their median age was 68 years (IQR 58–76), and 111 were males (65%). Their complete demographic and clinical characteristics are reported in Table 2. Overall, 71 (42%), 35 (21%), and 11 (6%) patients developed K1-AKI, K2-AKI, and K3-AKI during colistin therapy, respectively. Crude mortality within 14 days from the first positive blood culture was 17% (29/170).
Table 3 shows univariable and multivariable analysis of predictors of K1-AKI during colistin therapy, considering baseline serum albumin as a dichotomous variable (<2.5 g/dL vs. ≥2.5 g/dL). In univariable comparisons, increasing age and serum albumin <2.5 g/dL were associated with K1-AKI. In multivariable analysis, only serum albumin <2.5 g/dL remained independently associated with K1-AKI (subdistribution hazard ratio [sHR] 1.85, 95% confidence interval [CI] 1.17–2.93, p = 0.009). As a continuous variable, serum albumin showed an association with K1-AKI in univariable analysis (sHR 0.63 for each increase of 1 g/dL in baseline serum albumin, 95% IC 0.42–0.93, p = 0.021), whereas statistical significance was not retained in the final multivariable model (sHR 0.68 for each increase of 1 g/dL in baseline serum albumin, 95% IC 0.45–1.03, p = 0.066; for details see Supplementary Table S2).
As shown in Table 4, serum albumin <2.5 g/dL resulted also the only independent predictor of K2-AKI (sHR 2.37, 95% CI 1.15–4.87, p = 0.019). As a continuous variable, serum albumin was associated with K2-AKI both in univariable (sHR 0.52 for each increase of 1 g/dL in baseline serum albumin, 95% IC 0.31–0.88, p = 0.014) and in multivariable (sHR 0.57 for each increase of 1 g/dL in baseline serum albumin, 95% IC 0.34–0.95, p = 0.03) models, as detailed in Supplementary Table S3.
With regard to the potential association of serum albumin with K3-AKI, despite a trend towards increased risk of K3-AKI in patients with serum albumin <2.5 g/dL, no statistically significant association was detected in univariable analysis (sHR 3.16, 95% CI 0.85–11.8, p = 0.086). A similar result was observed when serum albumin was considered as a continuous variable (sHR 0.46 for each increase of 1 g/dL in baseline serum albumin, 95% CI 0.20–1.05, p = 0.064). The details of univariable analyses for K3-AKI are available in Supplementary Table S4. No multivariable analysis was conducted because of the low number of K3-AKI events.
The cumulative incidence of K1-AKI, K2-AKI, and K3-AKI in patients with serum albumin <2.5 g/dL and ≥2.5 g/dL is shown in Fig. 2.
Cumulative incidence of acute kidney injury in the study population. KDIGO, Kidney Disease: Improving Global Outcomes31; AKI, acute kidney injury.
Part 2 - Bioinformatic and biochemical analyses
Docking simulations of colistin binding to ligand-free HSA, with the search space extended to the whole protein, indicated the preferential binding of this drug to the FA8 site, with a number of five complexes observed in a maximum of nine poses (Fig. 3A and Table 5)39. In particular, colistin recognition to the FA8 site of ligand-free HSA is based on hydrogen bonds with Asp187, Arg218, Arg222, Glu292, and Lys436 (Fig. 3B). Of note, this binding mechanism is reminiscent of that observed for thyroxine recognition40. In other poses of docking simulation, colistin has been found to be placed in the FA9 site, which is located in the upper region of the FA8 site (Fig. 3A and Table 5)41,42.
Colistin recognition by ligand-free HSA. (A) Overall view of the best apparent free energy poses of ligand-free HSA-colistin complexes. (B) Overall view of the atomic details of colistin recognition at the FA8 site of HSA. Colistin is rendered in stick (orange). The picture was drawn with the UCSF-Chimera package39. For details, see text.
As shown in Fig. S1, E. coli growth was completely inhibited in the presence of colistin, at all the concentrations tested. The addition of HSA to the broth significantly reduced the anti-microbial effect of colistin as a consequence of the reduced bioavailability due to its binding to albumin. Of note, the addition of HSA to the bacteria broth did not affect E. coli growth (Fig. S1).
Discussion
In a retrospective cohort of 170 patients with BSI due colistin-susceptible Gram-negative bacteria treated with colistin, as many as 42% developed at least mild AKI during colistin therapy, and 6% developed severe AKI. Severe hypoalbuminemia at colistin initiation (<2.5 g/dL) was associated with the development of AKI during colistin treatment.
Although with the necessary premise that direct comparisons are somewhat hampered by the use of different definitions of AKI in different studies, it is of note that the rates of AKI during colistin therapy detected in our cohort seem coherent with current literature43,44. For example, in a very recent study in 149 critically-ill patients receiving intravenous colistin, decreases of ≥25%, ≥50% and ≥75% in creatinine clearance during colistin treatment were observed in 49%, 39%, and 8% of cases, respectively44. These results likely reflect the low therapeutic index of colistin45, and in turn the need for proper use and prompt identification of factors favoring the development of AKI during the course of treatment.
Other authors have already investigated hypoalbuminemia as a possible predictor of AKI during colistin therapy. In two previous studies, serum albumin concentrations <3.2 g/dL and <2 g/dL were associated with development of AKI during treatment in 47 and 71 patients with various types of infections, respectively18,27. Low serum albumin concentrations also predicted AKI during colistin therapy in other 42 patients with heterogeneous infections15. In the study of Lee and colleagues, lower serum albumin concentrations were associated with the development in AKI in 285 colistin-treated patients with estimated glomerular filtration rate (GFR) ≥ 60 mL/min/1.73 m2, but not in 44 patients with estimated GFR < 60 mL/min/1.73 m2 (although possibly because of the low sample size of this latter subgroup)24. Finally, Sorlì and colleagues observed that in 102 colistin-treated patients with various types of infections, hypoalbuminemia was associated with the development of AKI in univariable but not in multivariable analyses17. These heterogeneous results might rely, at least in part, on the different patient populations analyzed in the different studies, including various combinations of critically- and non-critically-ill patients, as well as various types of infections and causative agents. In the present study, we observed an independent association between severe hypoalbuminemia (<2.5 g/dL) and development of AKI during colistin therapy in an cohort of 170 patients with BSI due to colistin-susceptible Gram-negative bacteria. Notably, these results suggest that severe hypoalbuminemia is a true risk factor for AKI in those patients in whom colistin is probably used the most nowadays, and in whom it may remain the only active therapeutic option46,47,48.
The binding of colistin to albumin has also been evaluated in the present work, thereby providing some insights into their interaction in the serum of colistin-treated patients. Our bioinformatics and biochemical analyses demonstrated that colistin binds to albumin with a very high affinity, as also supported by the in vitro E. coli growth assay. However, as far as a causal relationship is concerned, it is important to note that this does not automatically imply an increased colistin nephrotoxicity because of reduced albumin binding in the serum of hypoalbuminemic patients. Indeed, as already observed by Nation and colleagues, hypoalbuminemia might promote reductions in the total concentration of colistin in serum (bound plus unbound) but perhaps not in that of the unbound drug, which is ultimately the toxic entity49. In this light, other possible explanations for the increased risk of AKI in hypoalbuminemic patients receiving colistin therapy should be necessarily considered. For example, severe hypoalbuminemia is responsible for altered regulation of fluids (and drugs) distribution, which might jeopardize renal perfusion and colistin ability to clear the infection and prevent/reduce any infection-related kidney injury50. In addition, hypoalbuminemia might also contribute to AKI through reduced anti-oxidant activities, less efficacious scavenging of reactive oxygen species, and impaired preservation of renal tubular cells51,52. Finally, hypoalbuminemia might simply be a proxy for poor clinical conditions, thus potentially influencing the development of AKI independent of any binding with colistin. With so many different but reasonable explanations at stake, it is therefore plausible that the development of AKI in hypoalbuminemic patients during colistin therapy is a complex and multifactorial process, in which not only the magnitude but also the existence of any possible contribution of reduced albumin binding still remains unclear. However, we think our findings nonetheless add to the literature, nurturing the discussion on the possible mechanisms trough which hypoalbuminemia leads to AKI in colistin-treated patients, and providing baseline biochemical information that might help in designing further dedicated studies to enrich our knowledge.
This study has some important limitations. The major one is that we did not have retrospective data on serum colistin levels, a well-known factor that may independently influence the development of AKI45. This also prevented us from assessing whether or not severe hypoalbuminemia influenced colistin concentrations in a clinically significant way49,53. Another potential confounding factor we could not adequately explore retrospectively is intravenous albumin therapy, which might have altered serum albumin levels (and their impact on AKI) in a time-dependent manner during colistin treatment. However, it is worth noting that intravenous albumin was likely administered the most in the case of severe hypoalbuminemia, which remained associated with AKI despite any possible interfering effect of subsequent corrections of serum albumin levels. This indirectly testifies to the absence of important biases related to albumin administration. It should also be noted that broth microdilution is the EUCAST reference method for colistin susceptibility testing, and not the Etest or the Vitek 2 automated system. However, although some patients with resistant strains might have been included because of the use of the Etest54, it should be noted that the majority of patients were selected according to Vitek 2 results. Reasonably, this did not hinder their correct selection, since colistin MICs are usually overestimated rather than underestimated by the Vitek 2 system55. Finally, we decided to use Fine-Gray regression with death as a competing event since our study was focused on the impact of hypoalbuminemia on the development of AKI. Indeed, hypoalbuminemia had been previously associated with increased mortality28,56,57, and we were thus interested not only in whether hypoalbuminemia predisposes to AKI, but also in whether it predisposes first to AKI than to mortality. However, the interpretation of the possible impact on AKI of other variables, which were not the primary focus of our study, might be less immediate than usual when using competing risk methods58. Therefore, any related conclusion should be drawn cautiously from this study.
Conclusions
Severe hypoalbuminemia was an independent predictor of AKI during colistin therapy in a large cohort of patients with BSI due to colistin-susceptible Gram-negative bacteria. Further study is needed to clarify the underlying causal pathways.
References
Yahav, D., Farbman, L., Leibovici, L. & Paul, M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 18, 18–29 (2012).
Falagas, M. E. & Kasiakou, S. K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40, 1333–1341 (2005).
Koch-Weser, J. et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 72, 857–868 (1970).
Nation, R. L. & Li, J. Colistin in the 21st century. Curr Opin Infect Dis 22, 535–543 (2009).
Velkov, T., Roberts, K. D., Nation, R. L., Thompson, P. E. & Li, J. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8, 711–724 (2013).
Couet, W., Grégoire, N., Marchand, S. & Mimoz, O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect 18, 30–39 (2012).
Florescu, D. F. et al. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis 54, 670–680 (2012).
Temocin, F. et al. Incidence and Risk Factors for Colistin-Associated Nephrotoxicity. Jpn J Infect Dis 68, 318–320 (2015).
Gauthier, T. P. et al. Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob Agents Chemother 56, 2392–2396 (2012).
Doshi, N. M., Mount, K. L. & Murphy, C. V. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy 31, 1257–1264 (2011).
Pogue, J. M. et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 53, 879–884 (2011).
Ko, H. J. et al. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin Pract 117, c284–288 (2011).
Deryke, C. A., Crawford, A. J., Uddin, N. & Wallace, M. R. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother 54, 4503–1505 (2010).
Phe, K., Johnson, M. L., Palmer, H. R. & Tam, V. H. Validation of a model to predict the risk of nephrotoxicity in patients receiving colistin. Antimicrob Agents Chemother 58, 6946–6948 (2014).
Omrani, A. S. et al. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann Clin Microbiol Antimicrob 14, 3 (2015).
Hartzell, J. D. et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48, 1724–1728 (2009).
Sorlí, L. et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 13, 380 (2013).
Kim, J., Lee, K. H., Yoo, S. & Pai, H. Clinical characteristics and risk factors of colistin induced nephrotoxicity. Int J Antimicrob Agents 34, 434–438 (2009).
Pike, M. & Saltiel, E. Colistin- and polymyxin-induced nephrotoxicity: focus on literature utilizing the RIFLE classification scheme of acute kidney injury. J Pharm Pract 27, 554–561 (2014).
Durante-Mangoni, E. et al. Acute kidney injury during colistin therapy: a prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clin Microbiol Infect 22, 984–989 (2016).
Rattanaumpawan, P., Ungprasert, P. & Thamlikitkul, V. Risk factors for colistin associated nephrotoxicity. J Infect 62, 187–190 (2011).
Falagas, M. E., Fragoulis, K. N., Kasiakou, S. K., Sermaidis, G. J. & Michalopoulos, A. Nephrotoxicity of intravenous colistin: a prospective evaluation. Int J Antimicrob Agents 26, 504–507 (2005).
Kwon, K. H. et al. Colistin treatment in carbapenem-resistant Acinetobacter baumannii pneumonia patients: Incidence of nephrotoxicity and outcomes. Int J Antimicrob Agents 45, 605–609 (2015).
Lee, Y. J. et al. Association between colistin dose and development of nephrotoxicity. Crit Care Med 43, 1187–1193 (2015).
Balkan, I. I. et al. Colistin nephrotoxicity increases with age. Scand J Infect Dis 46, 678–685 (2014).
Tuon, F. F. et al. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents 43, 349–352 (2014).
Kwon, J. A. et al. Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents 35, 473–477 (2010).
Benattar, Y. D. et al. The Effectiveness and Safety of High-Dose Colistin: Prospective Cohort Study. Clin Infect Dis 63, 1605–1612 (2016).
Grignolo, S. et al. Good tolerability of high dose colistin-based therapy in patients with haematological malignancies. Infection 45, 505–511 (2017).
Russell, J. A. Management of sepsis. N Engl J Med 355, 1699–1713 (2006).
Kellum, J. A. & Lameire, N. Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care 17, 204 (2013).
Bartoletti, M. et al. A Prospective Multicentre Study of the Epidemiology and Outcomes of Bloodstream Infection in Cirrhotic Patients. Clin Microbiol Infect [Epub ahead of print]; https://doi.org/10.1016/j.cmi.2017.08.001 (2017).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94, 496–509 (1999).
Aalen, O. O. & Johansen, S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scandinavian Journal of Statistics 5, 141–150 (1978).
Sugio, S., Kashima, A., Mochizuki, S., Noda, M. & Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng 12, 439–446 (1999).
Di Muzio, E., Toti, D. & Polticelli, F. DockingApp: a user friendly interface for facilitated docking simulations with AutoDock Vina. Journal of Computer-Aided Molecular Design 31, 213–218 (2017).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem 31, 455–461 (2010).
Polticelli, F. et al. Cantharidin inhibits competitively heme-Fe(III) binding to the FA1 site of human serum albumin. J Mol Recognit 30 (2017).
Pettersen, E. F. et al. UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004).
Petitpas, I. et al. Structural basis of albumin-thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. Proc Natl Acad Sci USA 100, 6440–6445 (2003).
Bhattacharya, A. A., Grüne, T. & Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303, 721–732 (2000).
Fanali, G. et al. Human serum albumin: from bench to bedside. Mol Aspects Med 33, 209–290 (2012).
Kelesidis, T. & Falagas, M. E. The safety of polymyxin antibiotics. Expert Opin Drug Saf 14, 1687–1701 (2015).
Zavascki, A. P. & Nation, R. L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob Agents Chemother 61, e02319–16 (2017).
Forrest, A. et al. Pharmacokinetic/Toxicodynamic Analysis of Colistin-Associated Acute Kidney Injury in Critically Ill Patients. Antimicrob Agents Chemother 61, e01367–17 (2017).
Giacobbe, D. R. et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21, 1106.e1–8 (2015).
Del Bono, V. et al. Meropenem for treating KPC-producing Klebsiella pneumoniae bloodstream infections: should we get to the PK/PD root of the paradox? Virulence 8, 66–73 (2017).
Bassetti, M. et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 24, 133–144 (2018).
Nation, R. L. et al. Reply to Corona and Cattaneo. Clin Infect Dis 65, 870–871 (2017).
Nicholson, J. P., Wolmarans, M. R. & Park, G. R. The role of albumin in critical illness. Br J Anaesth 85, 599–610 (2000).
Wiedermann, C. J., Wiedermann, W. & Joannidis, M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med 36, 1657–1665 (2010).
Iglesias, J. et al. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol 277, F711–F722 (1999).
Corona, A. & Cattaneo, D. Dosing colistin properly: let’s save “our last resort old drug”! Clin Infect Dis 65, 870 (2017).
Matuschek, E., Åhman, J., Webster, C. & Kahlmeter, G. Antimicrobial susceptibility testing of colistin - evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter spp. Clin Microbiol Infect [Epub ahead of print]; https://doi.org/10.1016/j.cmi.2017.11.020 (2017).
Chew, K. L., La, M. V., Lin, R. T. P. & Teo, J. W. P. Colistin and Polymyxin B Susceptibility Testing for Carbapenem-Resistant and mcr-Positive Enterobacteriaceae: Comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth Microdilution. J Clin Microbiol 55, 2609–2616 (2017).
Goldwasser, P. & Feldman, J. Association of serum albumin and mortality risk. J Clin Epidemiol 50, 693–703 (1997).
Jellinge, M. E., Henriksen, D. P., Hallas, P. & Brabrand, M. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: a prospective, observational, cohort study. PLoS One 9, e105983 (2014).
Andersen, P. K., Geskus, R. B., de Witte, T. & Putter, H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 41, 861–870 (2012).
Acknowledgements
The authors wish thank Rita Francesca Tobaldi and Anil Minnicelli for their help with data collection.
Author information
Authors and Affiliations
Contributions
D.R.G., A.d.M. and S.D.B. made substantial contributions to study concept and design; acquisition of data; analysis and interpretation of data; first drafting of the manuscript; critical revision of the manuscript for important intellectual content. L.L., V.D.B., M.R., D.C. and C.V. made substantial contributions to study concept and design and critical revision of the manuscript for important intellectual content. A.N., A.G., A.Ma. A.S., P.A., R.L. and M.M. made substantial contributions to study concept and design; analysis and interpretation of data. E.C., A.C., K.P., L.P., A.B., L.E.V., P.O. and A.Mi. made substantial contributions to acquisition of data and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giacobbe, D.R., di Masi, A., Leboffe, L. et al. Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment. Sci Rep 8, 11968 (2018). https://doi.org/10.1038/s41598-018-30361-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30361-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.