Abstract
MPMV precursor polypeptide Pr78Gag orchestrates assembly and packaging of genomic RNA (gRNA) into virus particles. Therefore, we have expressed recombinant full-length Pr78Gag either with or without His6-tag in bacterial as well as eukaryotic cultures and purified the recombinant protein from soluble fractions of the bacterial cultures. The recombinant Pr78Gag protein has the intrinsic ability to assemble in vitro to form virus like particles (VLPs). Consistent with this observation, the recombinant protein could form VLPs in both prokaryotes and eukaryotes. VLPs formed in eukaryotic cells by recombinant Pr78Gag with or without His6-tag can encapsidate MPMV transfer vector RNA, suggesting that the inclusion of the His6-tag to the full-length Pr78Gag did not interfere with its expression or biological function. This study demonstrates the expression and purification of a biologically active, recombinant Pr78Gag, which should pave the way to study RNA-protein interactions involved in the MPMV gRNA packaging process.
Similar content being viewed by others
Introduction
Retroviruses are a group of viruses that require packaging/encapsidation of their “full-length”, unspliced, single-stranded, RNA genome (gRNA) into assembling viral particles for the continuity of their life cycle. During this process, two copies of the gRNA dimerize and are preferentially packaged into the assembling virions compared to the spliced viral RNA and the large pool of cellular RNAs of the infected host cell1,2,3,4,5,6,7. Such specificity towards packaging of gRNA is a result of intricate interaction(s) between the cis-acting sequences on the gRNA and the trans-acting viral Gag protein. Retroviral cis-acting sequences which interact with Gag polyprotein are generally located at the 5′ end of the gRNA and have been designated as the “packaging signal” or “psi” (ψ). For almost all retroviruses, the ψ sequences required for gRNA packaging have been identified as a structurally-conserved region generally present both upstream and downstream of the major splice donor (mSD) within the 5′ untranslated region (5′ UTR) and often extending into the 5′ end of the Gag open reading frame (ORF)1,2,3,4,5,6,8.
Among the proteins implicated in selective gRNA packaging into virus particles, the nucleocapsid (NC) region of the retroviral Gag polyprotein is a primary candidate, as this highly basic protein contains Cys-His boxes that can interact with Zn2+ ions to facilitate protein/RNA interactions4,9. Mutational analysis of the NC domain of several retroviral gag genes has shown that it is one of the most critical proteins involved in gRNA packaging10,11,12,13,14. However, additional lines of evidence indicate that NC may not be the only determinant of specific gRNA packaging, and other Gag domains may also be involved, including matrix15, capsid, the p2 spacer peptide between CA and NC16,17,18, and the terminal p6 late domain19. Furthermore, it is thought that rather than recognizing monomeric RNA substrates, NC probably recognizes dimeric genomes, an interaction that is thought to initiate the multimerization of the Gag polyprotein on the RNA templates, eventually leading to encapsidation of the gRNA into the assembling virus particle20,21,22. Together, these observations suggests that specific selection of gRNA from cellular and spliced RNAs is a complex phenomenon that happens in the context of the whole Gag polyprotein, as has recently been shown for the human immunodeficiency virus type 1 (HIV-1)23,24,25,26.
Based on these observations, a simplistic model shown in Fig. 1(a) suggests that the gRNA is preferentially packaged by virtue of the presence of the cis-acting Gag binding site on the structured RNA packaging determinant. In the case of the spliced RNAs, part of the packaging sequence is spliced out, thereby excluding them from encapsidation into the newly formed viral particles, a process that also disrupts the structure of the packaging determinants (Fig. 1(a)). Such a model offers a possible mechanism for discriminating between spliced and unspliced viral mRNAs1 as has recently been shown in the case of HIV-1 where the stem loop 1 (SL1) is located in the region harboring the packaging determinants of HIV-1 and is capable of binding HIV-1 Pr55Gag with high affinity23,24,25,26,27. Furthermore, the sequences downstream of this region enhance HIV-1 RNA packaging, while the sequences upstream inhibit packaging efficiency23. Such RNA binding studies that challenge the earlier observations where SL3 was thought to contain the primary packaging determinants28 have not been accomplished in most retroviruses owing to the unavailability of respective purified full-length Gag precursor proteins.
(a) Schematic representation of a simplistic model of retroviral genomic RNA packaging. The presence of an intact RNA secondary structure of the packaging signal (psi; ψ) facilitates genomic RNA packaging, whereas loss of its structure in the spliced RNA excludes its packaging. (b) Illustration of the MPMV Gag proteins expressed from the prokaryotic expression vector either with (FN1) or without (FN1A) the His6-tag (6xH). The amino acid residues LE were introduced upstream of the His6-tag into the FN1 vector during the cloning process.
The Mason-Pfizer monkey virus (MPMV) is a non-transforming, prototypic simple, type D retrovirus, which has been shown to be involved in causing immunodeficiency in infected new-born Rhesus monkeys29,30. Among type D retroviruses, MPMV RNA packaging is the most thoroughly investigated31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47, primarily because MPMV-based vectors are considered as potential tools for delivering therapeutic genes in human gene transfer studies. MPMV-based vectors are good candidates for human gene transfer studies because: i) MPMV promoter is transcriptionally active in human cells, thereby obviating the need of replacing MPMV promoters with those of other human viral promoters, and ii) the presence of MPMV constitutive transport element (CTE) should greatly facilitate the nuclear export of the therapeutic genes for their effective expression in the target cells34,48,49,50.
A number of studies have focused on identifying the MPMV sequences (at the sequence as well as secondary RNA structure levels) responsible for gRNA packaging and dimerization31,36,37,38,39,42,44,47. There is now a consensus that sequences that are responsible for MPMV packaging are highly structured, bipartite in nature, and divided into two regions both upstream and downstream of the major splice donor site31,38,39. However, not much is known as to how Pr78Gag selects gRNA. For instance, it remains largely unclear whether discrimination between gRNA and spliced RNA is mediated by the initial binding step to Pr78Gag, or whether other pathways such as the gRNA nuclear export and subcellular localization are also involved, as has been proposed for HIV-151,52,53,54,55,56,57. The void in understanding selective packaging of gRNA among retroviruses is largely due to the unavailability of biologically active full-length Gag polyprotein which has been proposed to interact with the packaging sequences on the full-length, unspliced, and dimerized gRNA23.
The gag gene of MPMV encodes a polypeptide, Pr78Gag that is the precursor of the viral structural proteins responsible for formation of MPMV particles. Pr78Gag is proteolytically cleaved into six proteins (Fig. 1(b)): namely NH2-p10 (MA), pp24 (and its C-terminal cleaved product, referred as pp24/16), p12, p27 (CA), p14 (NC), and p4-COOH46,58,59. Cleavage of the polyprotein is achieved by a protease (PR) encoded for by the virally-encoded pro gene58,59,60. Like most retroviruses, MPMV Pr78Gag assembles to form an immature capsid and expression of the pro gene results in the maturation of the virus particles61.
Since Pr78Gag is a critical component of the packaging process, understanding the biochemical and biophysical properties of MPMV Pr78Gag is of paramount importance to understand MPMV biology. Overexpression and purification of Pr78Gag in bacteria has been reported before; however, the protein was mainly found within inclusion bodies and had to be solubilized and denatured for purification and then refolded for further analysis62. Furthermore, its suitability for RNA binding assays was never established. Therefore, to overcome this caveat, we have expressed large amounts of recombinant Pr78Gag in soluble fractions of Escherichia coli (E. coli) containing a C-terminal hexa-histidine (His6) tag to facilitate protein purification. This was followed by immobilized metal affinity chromatography (IMAC) to purify the protein to homogeneity employing high-pressure liquid chromatography (HPLC)19,63,64. The availability of purified MPMV full-length Gag polyprotein should allow us to investigate how Pr78Gag is involved in selectively packaging gRNA over spliced viral and cellular RNAs which will further enhance our understanding of the molecular intricacies involved in MPMV gRNA packaging, especially in delineating RNA-protein interactions that take place during MPMV replication.
Results and Discussion
Bacterial Expression of Recombinant MPMV Pr78Gag-His6-tag Protein
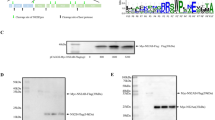
In order to express full-length MPMV Pr78Gag, we created a recombinant bacterial expression plasmid (FN1) in a fashion that this plasmid should produce a fusion protein comprising of full-length MPMV Pr78Gag and a LEHHHHHH tag at the C-terminus (Pr78Gag-His6-tag), with a predicted molecular weight of ~74101 Daltons (Fig. 1(b)). Such a T7 RNA polymerase promoter-based expression plasmid (FN1) facilitated high level expression of His6-tagged MPMV Pr78Gag by BL21(DE3) bacterial cells when induced with IPTG because of the presence of a chromosomal copy of the T7 RNA polymerase gene under the dependency of the lac promoter (Fig. 2).
Schematic representation of the construction of the recombinant Pr78Gag. (a) Full length nucleic acid and amino acid sequence of MPMV Pr78Gag. (b) Design of the modified pET28b(+) vector expressing the full length MPMV Pr78Gag (FN1) cloned into NcoI and XhoI sites and expressed from the bacteriophage T7 promoter. (c) A silent mutation introduced to remove an internal NcoI site for the ease of cloning.
Expression of the recombinant Pr78Gag-His6-tagged protein was confirmed by growing FN1-transformed BL21(DE3) cultures at 37 °C followed by IPTG induction and were further grown at 28 °C. At 0, 2, 4, 6, and 8 hours post induction, total protein lysates were prepared from both induced as well as un-induced cultures and the expression of the recombinant MPMV Pr78Gag-His6-tag was monitored based on size on SDS-PAGE. As shown in Fig. 3, a distinct band of ~78 kDa corresponding to the expected size of the recombinant full-length Pr78Gag-His6-tag was observed in the induced cultures at 2, 4, 6, and 8 hours (lanes 4–7) but not in un-induced cultures (lane 3) or cultures containing only pET28b(+) vector without any MPMV Gag sequences (lane 2). These results reveal successful expression of the recombinant full-length MPMV Pr78Gag. However, we cannot be certain whether this recombinant full-length Gag protein was capable of making VLPs or was present in the soluble bacterial fraction. Klikova and colleagues have reported earlier that culturing bacteria at 37 °C post induction resulted in the confinement of MPMV Gag polyprotein in the inclusion bodies containing aberrantly assembled spiral like structures62. Keeping these observations in mind, the recombinant MPMV Pr78Gag was expressed employing sub-optimal conditions such as low temperature in all subsequent experiments. Furthermore, although the expression of recombinant MPMV full-length Pr78Gag-His6-tag was observed between 2–8 hours post IPTG induction (Fig. 3; lanes 4–7), for subsequent experiments we chose to purify protein from cultures that were induced only for 4 hours sub-optimally at 28 °C following IPTG induction in order to avoid any possible aggregation and/or sequestration of Gag in inclusion bodies as has been reported earlier62.
Expression of recombinant Pr78Gag in Escherichia coli lysates. Coomassie Brilliant Blue-stained SDS-polyacrylamide gel showing expression of recombinant full-length Pr78Gag prepared from total cell lysates from un-induced and IPTG-induced BL21(DE3) bacterial cells which were cultured for 0, 2, 4, 6, and 8-hours at 28 °C.
The Bacterially-expressed MPMV Pr78Gag-His6-tag Protein Forms VLPs
It has previously been shown that not only MPMV Gag62,65,66, but other retroviral Gag proteins67,68 can form immature VLPs. Therefore, we tested whether the recombinant MPMV Pr78Gag either with or without His6-tag at the C-terminus was able to assemble into VLPs within bacterial cells or the presence of His6-tag in any way hindered this process. Towards this end, the full-length MPMV Gag recombinant clone FN1 (with His6-tag) and FN1A (without His6-tag) were expressed in BL21(DE3) cells at 28 °C and tested for their ability to form immature VLPs using transmission electron microscopy (TEM). The ultrathin sections were negatively stained with 1% uranyl acetate and visualized. Electron micrographs of the IPTG-induced bacterial expression plasmids (FN1 and FN1A) revealed a predominant population of intra-cytoplasmic structures appearing as electron dense rings of ~55–65 nanometer (nm) in size resembling immature VLPs (Fig. 4(a–c)). This range of size (55–65 nm) of VLPs is consistent with the earlier published observations of MPMV VLPs assembled in bacteria from full-length Gag62 or mutated Gag66. No such VLP structures were observed when FN1- and FN1A-transformed bacterial cells were not induced with IPTG (Fig. 4(d,e)). Similarly, no VLP-like structures were observed when the cloning vector, pET28b(+), by itself was transformed in BL21(DE3) and induced employing similar conditions (data not shown). These results suggest that clone FN1 containing full-length MPMV Gag with a His6-tag at the C-terminus as well as FN1A without the His6-tag were able to express full-length Gag proteins capable of assembling VLPs in bacteria. Based on these results, it can be further concluded that the presence of His6-tag at the C-terminus does not interfere with the recombinant full-length MPMV Pr78Gag expression as well as VLP formation. This observation is further strengthened by the fact that when sequences for FN1 (with His6-tag) and FN1A (without His6-tag) were subjected to online ExPASy-Compute pI/Mw tool which allows the computation of the theoretical isoelectric point (pI) of proteins, it predicted minimal difference (0.15) in these proteins (FN1-with His6-tag: pI: 7.07 versus FN1A-without His6-tag: pI: 7.22).
Assembly of virus like particles (VLPs) by recombinant Pr78Gag in Escherichia coli. Transmission electron micrographs showing VLPs assembled in E. coli BL21(DE3) cells transformed with (a) the FN1 clone containing His6-tag and (b,c) with a FN1A clone without the His6-tag. (d,e) BL21(DE3) uninduced BL21(DE3) cells transformed with FN1 and FN1A, respectively. (Scale bar = 100 nm; 60,000X magnification).
MPMV Pr78Gag-His6-tag Protein is Expressed in the Soluble Fraction in Bacteria
In order to determine whether the recombinant MPMV Pr78Gag-His6-tag protein was present in the soluble bacterial fraction, expression of the full-length MPMV Gag clone in FN1 was induced with IPTG at 28 °C for 4 hours and lysed as described in Material and Methods. Insoluble material (containing cell debris and inclusion bodies, if any) was removed by centrifugation and the soluble fraction from different cultures was either stored at −80 °C or immediately monitored for the expression of the recombinant MPMV Pr78Gag-His6-tag protein by SDS-PAGE and immunoblotting. Staining with Coomassie Brilliant Blue revealed presence of a distinct band of ~78 kDa corresponding to the expected size of recombinant MPMV Pr78Gag-His6-tag protein (Fig. 5(a); lane 2). The identity and recombinant nature (Pr78Gag-His6-tag fusion protein) of this band was established by immunoblotting using HRP-conjugated α-His6 monoclonal antibody (Fig. 5(b); lane 2) as well as α-MPMV Pr78 polyserum (Fig. 5(c); lane 2). These results show that under the conditions used by us, the recombinant Pr78Gag-His6-tag protein was expressed primarily in the soluble fraction in contrast to the inclusion bodies, as reported earlier62.
Expression of recombinant Pr78Gag in the soluble fraction of E. coli. (a) Coomassie Brilliant Blue-stained SDS-polyacrylamide gel with lysates from the soluble fraction of bacteria transformed with FN1 expressing recombinant full-length MPMV Pr78Gag-His6-tag fusion protein (lane 2), followed by IMAC purification (lane 3). The same lysates were analyzed with (b) a monoclonal anti-His6 monoclonal antibody, and (c) an anti-Pr78Gag polyserum, respectively.
Further Purification of the Soluble Fraction Containing Pr78Gag-His6-tag Fusion Protein by Immobilized Metal Affinity Chromatography (IMAC)
Having established that the expressed recombinant Pr78Gag-His6-tag protein was soluble, we wanted to further increase the purity of our protein since two additional faint bands could be observed underneath the primary protein band when detected by the α-MPMV Pr78 polyserum, but not by α-His6 antibody which could be degraded products (Fig. 5(b) versus Fig. 5(c)). Thus, the purified bacterial lysate (containing soluble fraction of recombinant Pr78Gag-His6-tag protein) was further clarified employing IMAC, as described in Materials and Methods. The buffering conditions used in these protocols (non-denaturing conditions and especially the presence of 1.0 M NaCl), not only allowed the protein to bind to the column, but also avoided protein aggregation and precipitation. The IMAC-purified protein was then monitored for purity of the recombinant MPMV Pr78Gag-His6-tag protein by SDS-PAGE and immunoblotting. Coomassie Brilliant Blue stain of the SDS-PAGE demonstrated that most bacterial proteins that were present in the soluble fraction prior to IMAC purification were eliminated following IMAC purification (compare lane 2 with lane 3 in Fig. 5(a)). Immunoblotting of IMAC-purified protein with HRP-conjugated anti-His6 monoclonal antibody (Fig. 5(b); lane 3) and anti-MPMV Pr78 polyserum (Fig. 5(c); lane 3) further confirmed the purity of the protein, as observed by the disappearance of the additional faint bands seen earlier (compare lane 2 with lane 3 in Fig. 5(c)). These results confirm that MPMV full-length Gag is truly fused with the His6-tag, allowing its binding to the HisTRAPTM column and subsequent elution in the purified form, further authenticating the recombinant nature of MPMV Pr78Gag- His6-tag fusion protein (compare Fig. 5(b); lane 3 with Fig. 5(c); lane 3).
Concentration and further Purification of the IMAC-purified Pr78Gag-His6-tag Protein by Gel Filtration Chromatography
Following IMAC purification, the bacterially-expressed full-length Pr78Gag-His6-tag protein was concentrated and further purified by gel filtration/size exclusion chromatography under non-denaturing conditions. As in the case of IMAC purification, non-denaturing conditions (especially the presence of 1.0 M NaCl) were employed to prevent protein aggregation and precipitation. Based on a sharp absorbance peak at 280 nm, fractions of 500 µl each were collected over several hours (Fig. 6(a)). Additionally, two other smaller peaks were also noticed that represented the degradation products or unidentified proteins of much lower molecular weight compared to Pr78Gag-His6-tag protein, as established by SDS-PAGE analysis (data not shown), and therefore were eliminated for any further downstream applications.
Fractionation of IMAC-purified recombinant Pr78Gag protein by size exclusion chromatography. (a) Absorbance versus elution time chromatogram plotted from the data obtained from a Superdex 200 column showing peak fractions with maximum absorbance containing purified recombinant full-length MPMV Pr78Gag-His6-tag fusion protein expressed from FN1. (b) Coomassie Brilliant Blue-stained SDS-polyacrylamide gel showing the resolution of purified recombinant full-length MPMV Pr78Gag-His6-tag fusion protein expressed from FN1 in fractions 18–26. (c) Western blot analysis of pooled peak fractions (22–24) of purified recombinant full-length MPMV Pr78Gag-His6-tag fusion protein analyzed with anti-Pr78Gag polyserum, and (d) anti-6x-His monoclonal antibody, respectively.
Protein fractions representing the sharp peak (fractions 19–26) were further analyzed by separation on SDS-PAGE. As shown in Fig. 6(b), fractions collected from the MPMV Pr78Gag-His6-tag peak were characteristically pure, with varying amounts of protein. Fractions representing the highest amount of pure protein (peaks 22–24) were pooled and once again concentrated using Ultra 15 (30,000 molecular weight cut-off membrane) concentrators. To establish the purity of recombinant full-length MPMV Pr78Gag-His6-tag Gag fusion protein, we measured the A260/A280 ratio by spectrophotometry. Our spectrophotometric analysis revealed the A260/A280 ratio to be 0.61, indicating that our recombinant full-length MPMV Pr78Gag-His6-tag fusion protein’s purity was greater than 95%. The concentrated protein was further analyzed by immunoblotting using anti-MPMV Pr78 polyserum and HRP-conjugated anti-His6 monoclonal antibody. Figure 6(c,d), in close corroboration with the SDS-PAGE analysis (Fig. 6(b)), clearly demonstrate that the pooled protein fractions contained pure MPMV Pr78Gag-His6-tag fusion protein. The protein yield, following IMAC purification and gel filtration/size exclusion chromatography, was estimated to be 3.8 mg and 0.23 mg per liter, respectively.
The Recombinant Pr78Gag-His6-tag Protein can Assemble in Vitro to Form VLPs
The recombinant full-length Gag proteins from HIV-1 and feline immunodeficiency virus (FIV) have been shown to assemble in vitro to form VLPs19,64,69,70. Therefore, we tested the ability of our purified recombinant full-length MPMV Pr78Gag-His6-tag fusion protein expressed in bacteria to assemble in vitro to form VLPs. Since the presence of nucleic acids along with purified Gag protein has been shown to be a prerequisite for VLP formation19,64,69,70, therefore, purified recombinant Pr78Gag-His6-tag fusion protein was mixed with yeast tRNA, as described in Materials and Methods (in an appropriate buffer to avoid formation of protein aggregates). This mixture was then dialyzed against a buffer that was of low salt concentration compared to the buffer in which the protein-RNA mixture was prepared. As an appropriate negative control, only yeast tRNA was also dialyzed without any protein in the same buffer and manner as the protein-RNA mixture. Following dialysis, the protein-RNA mixture as well as the control tRNA suspension (without any protein) was recovered from the cassette and concentrated after which ~1/25th of the concentrate was spotted onto carbon coated grids, dried, and stained for observation under an electron microscope.
Analysis of various electron micrographs taken from different fields revealed the assembly of VLPs in the form of compact electron-dense rings of approximately 30–35 nm in size resembling immature VLPs (Fig. 7(a–f)). The size of these in vitro assembled VLPs corroborated well with the earlier observations which have reported a similar size (~20–30 nm) obtained following in vitro assembly using purified Gag as opposed to the larger size of full-length Gag particles (~55–65 nm) produced in vivo in eukaryotic cells19,64,69,70. These VLPs were assembled efficiently despite the fact that the purified protein was frozen and thawed which suggests that the purified protein remained biologically active following freeze-thaw cycle. In contrast, yeast tRNA alone, without any purified MPMV full-length Gag, did not show any VLP-like structure (Fig. 7(g,h)). These results suggest that MPMV recombinant full-length Gag His6-tag fusion protein has the inherent property of forming VLPs by virtue of its intrinsic multimerizing ability, as has been reported previously in the case of HIV-119,64,69,70,71.
In vitro assembly by purified recombinant Pr78Gag protein to form virus like particles (VLPs). (a–f) Transmission electron micrographs showing in vitro-assembled virus like particles from purified recombinant full-length MPMV Pr78Gag-His6-tag fusion protein expressed from FN1 in the presence of yeast tRNA. (g,h) Electron micrographs of negative controls comprising of assembly buffer and yeast tRNA only without any protein. Scale bar = 50 nm; 135,000X magnification.
Recombinant Pr78Gag-His6-tag Protein Expressed in Eukaryotic Cells can form VLPs Capable of Packaging Unspliced Transfer Vector RNA
Finally, in order to ensure that MPMV full-length Pr78Gag-His6-tag is able to encapsidate transfer vector RNA in eukaryotic cells, we created two full-length MPMV Gag eukaryotic expression plasmids, with or without the His6-tag (FN7 and FN9, respectively (Fig. 8(a)). To ensure proper export of the MPMV Pr78Gag-His6-tag mRNA out of the nucleus, the MPMV CTE was inserted immediately downstream of the MPMV Gag stop codon (Fig. 8(a)). The ability of these full-length Gag expression plasmids to encapsidate MPMV RNA was tested by employing a two-plasmid genetic complementation assay (Fig. 8(b)). In this assay, either FN7 or FN9 were co-transfected with the MPMV transfer vector, SJ2, to test their ability to package the MPMV transfer vector RNA expressed from SJ238. To monitor transient transfection efficiencies, a secreted alkaline phosphatase (SEAP) expression plasmid (pSEAP) was also included in the transfection DNA cocktail.
Two-plasmid genetic complementation assay to test the ability of MPMV virus like particles (VLPs) to package transfer vector viral RNA following Pr78Gag-His6-tag expression in eukaryotic cells. (a) Graphical representation of MPMV full-length Gag expression plasmids with and without His6-tag (FN7 and FN9 respectively) and MPMV transfer vector, SJ238 which provides the substrate for packageable RNA. (b) Design and rationale of the MPMV 2-plasmid genetic complementation assay. VLPs produced by the eukaryotic Gag-expression plasmids (FN7 and FN9) should allow packaging of the transfer vector RNA expressed from SJ2 due to the presence of the packaging signal (Ψ) on its RNA. The 293T cells were co-transfected with the either of the two Gag-expression plasmids along with SJ2 and fractionated into nuclear and cytoplasmic fractions. The cytoplasmic fractions were analyzed for transfer vector (SJ2) RNA expression, while the virus particles were tested for their ability to package SJ2 RNA using RT- PCR. (c) MPMV full-length Gag expression plasmids with (FN7) and without His6-tag (FN9) were transfected into 293T cells along with SJ2 and western blots were performed on cell lysates to detect Gag proteins using anti-Pr78Gag polyserum (panel I) and β-actin proteins using a monoclonal antibody as a control (panel II). Western blots on ultracentrifuged virus particles using anti-Pr78Gag polyserum (panel III). RT-PCR using MPMV transfer vector (SJ2)-specific primers (OTR1161 and OTR1163) amplifying a 530 bp fragment from the cDNAs prepared from the cytoplasmic (panel IV) and virion RNAs amplified for 30 (panel V) and 50 (panel VI) cycles, respectively. Experiments were conducted multiple times with reproducible results and representative blots are shown. Blots/gels have been cropped to show the relevant parts only and full images are provided in Supplementary Data.
As can be seen in Fig. 8, the transfected 293T cells revealed successful expression of the full-length MPMV Pr78Gag proteins using anti-Pr78Gag polyserum and anti-β-Actin monoclonal antibody as a control (Fig. 8(c); panels I and II, respectively). Western blot analysis of virus particles isolated from transfected culture supernatants further confirmed VLP production by both the His(+) and His(−) Gag-expression plasmids, FN7 and FN9, respectively (Fig. 8(c); panel III). These results clearly demonstrate that the presence of His6-tag at the C-terminus of MPMV full-length Pr78Gag did not interfere with the expression of recombinant full-length MPMV Pr78Gag-His6-tag fusion protein or its ability to form Gag VLPs in 293T cells.
Next, we tested the ability of VLPs produced by the recombinant MPMV full-length Pr78Gag in eukaryotic cells to package MPMV transfer vector RNA. Towards this end, RNA was extracted from the cytoplasmic fractions as well as the pelleted viral particles and DNase-treated to deplete any contaminating plasmid DNA from the transfected cultures. PCR conducted on the DNased-RNAs using transfer vector RNA-specific primers (OTR 1161 and OTR 1163) confirmed the absence of any contaminating plasmid DNA in the RNA preparations (see Supplementary Data) and this was followed by their conversion into cDNAs. We also ensured that our fractionation technique was not compromised and there was no leakage of RNA from the nucleus to the cytoplasm by testing for the absence of unspliced actin mRNA in our cytoplasmic fractions during this process (data not shown), as described by our group earlier38,39,72,73,74. RT-PCR amplification of transfer vector (SJ2)-specific RNA revealed its successful nuclear transport and expression in the cytoplasm (Fig. 8(c); panel IV). Amplification of the SJ2-specific cDNAs isolated from Gag VLPs formed by either the MPMV Pr78Gag-His6-tag fusion protein (FN7) or without His6-tag (FN9) revealed that transfer vector RNA was efficiently packaged by both types of VLPs (Fig. 8(c); panels V and VI). Interestingly, the VLPs formed by the C-terminally His6-tagged version of the Gag protein seemed slightly more efficient in encapsidating the transfer vector RNA than its untagged version (Fig. 8(c); panels V and VI). Since the Gag NC is thought to be the main domain involved in the interaction with the genomic RNA using its positively-charged zinc finger domains, addition of the His6-tag may have increased the basic nature of the polyprotein and stabilized its interaction with the RNA further. Therefore, the slight increase in packaging could be attributed to the increased positive charge added to the full-length MPMV Pr78Gag due to the presence of positively charged His6-tag, as has recently been suggested for HIV-1 Pr55Gag- nucleic acid interaction in vitro63.
Conclusions
Together, this study reveals that the recombinant MPMV Pr78Gag- His6-tag fusion protein has the ability to form VLPs in both prokaryotic and eukaryotic cells. Furthermore, Gag VLPs formed in the eukaryotic cells have the capability of encapsidating transfer vector RNA, one of the important biological roles of Gag. The availability of relatively large amounts of bacterially expressed, biologically active, full-length MPMV Pr78Gag protein, as described here, offers a range of opportunities to study RNA-protein interactions that take place during MPMV gRNA packaging and viral assembly. One of the interesting research areas that can now be explored are co-crystallization experiments with Pr78Gag and viral genomic RNA. Having crystal structures of viral RNA-Gag complexes would allow for confirmation of proposed MPMV RNA secondary and tertiary structures, as well as proposed Gag-RNA interactions. Such refinement in the understanding of these crucial steps in retroviral life cycle is essential if MPMV-based vectors are to be used for gene therapy.
Methods
Nucleotide numbering system
Nucleotide numbers in this study refers to the MPMV genome with the Genbank accession number M1234946.
Construction of Prokaryotic MPMV Gag Expression Plasmids
MPMV gag gene (Pr78Gag) harboring sequences spanning nucleotides 890 to 2863 was commercially synthesized as a double-stranded DNA fragment (Macrogen, South Korea). Two restriction sites, NcoI and XhoI, were incorporated at either ends of the gene to facilitate cloning into the prokaryotic expression vector pET28b(+) (Fig. 2(a)). Use of the NcoI site pET28b(+) vector kept the gag open reading frame (ORF) intact and allowed the introduction of a hexa-histidine (His6-tag) at the C-terminus of the Pr78Gag, allowing purification of the expressed recombinant Gag polyprotein using IMAC63,64. Since the MPMV gag gene contained an additional internal NcoI site, a silent mutation was introduced into the internal NcoI site at the gene synthesis step at nt 2462, changing CCATGG to CTATGG, which resulted in the loss of the NcoI site; while maintaining the same amino acid (Fig. 2(a and c)). The resultant clone FN1 was sequenced to ensure that the MPMV gag gene did not contain any point mutations (Fig. 2(b)). Such an expression plasmid should produce a recombinant fusion protein comprising of full-length MPMV Pr78Gag and a LEHHHHHH tag at the C-terminus (Pr78Gag-His6-tagged), with a predicted molecular weight of 74101 daltons. Employing a similar strategy, another bacterial expression plasmid (FN1A) was constructed expressing full-length MPMV Pr78Gag without the His6-tag (Fig. 1(b)).
Construction of Eukaryotic MPMV Gag Expression Plasmids
The full length Pr78Gag sequences both with and without the His6-tag were also cloned into the eukaryotic expression vector pcDNA3. Towards this end, the FN1 plasmid was used as a template for amplification of gag gene sequences using the forward primer, OTR1330 and the reverse primers, OTR1331 or OTR1320. OTR1330 (5′ CCG CTC GAG GCC GCC ACC ATG GGG CAA GAA TTA AGC CAG G 3′) introduced an XhoI restriction site (italicized) followed by Kozak sequence (underlined) at the 5′ end of the gag gene to enhance gene expression. OTR1331 (5′ CAA GGT GGA GGG TGT GTC ATA GTG GTG GTG GTG GTG GTG ATT GAG CTC GCC 3′) on the other hand, created a His6 tag (underlined) just upstream of the gag stop codon, followed by the XhoI restriction site (italicized). To create a clone without His6 tag, the reverse primer OTR1320 (5′ CCG CTC GAG TTA ATA CTG TGT GGG AG 3′) was used, which did not contain the His6 tag sequences but did contain an XhoI restriction site (italicized). Polymerase chain reaction (PCR) was performed using an initial denaturation at 98 °C for 30 seconds, then 15 cycles of denaturation at 98 °C for 10 seconds, primer annealing at 62 °C for 30 seconds, followed by primer extension at 72 °C for 30 seconds. A final extension step at 72 °C for 10 minutes was also included. The PCR-amplified products were cleaved with XhoI endonuclease and cloned into pCDNA3 which had previously been cleaved with the same restriction endonuclease to create clones FN3 and FN5, with and without His6-tag, respectively. Finally, to facilitate efficient nuclear export of gag mRNA, a PCR-amplified fragment containing the MPMV CTE34 with flanking XbaI sites was cloned into FN3 and FN5 which had already been cleaved with XbaI, resulting in clones FN7 and FN9, respectively (Fig. 8(a)). All clones were confirmed by sequencing.
Bacterial Strains and Media
During the course of cloning, all transformations were performed in the DH5α strain of E. coli using standard heat shock protocol and allowed to grow on Luria-Bertani (LB) agar plates in the presence of appropriate antibiotics (kanamycin at 50 µg/ml and ampicillin at 100 µg/ml), depending on the clones. To monitor the recombinant protein expression in bacteria, the prokaryotic expression clones were transformed into T7 Express (New England Bio Labs, USA), a BL21(DE3) strain of E. coli. For bacterial expression studies, cells were cultured in LB medium [1% (w/v) peptone, 0.5% (w/v) yeast extract, and 0.5% NaCl] in the presence of kanamycin (50 µg/ml) antibiotic.
Large Scale Expression of Recombinant Pr78Gag-His6-tagged Protein
Large scale recombinant Pr78Gag-His6-tagged protein expression was performed by inoculating a single colony into 25 ml LB media containing kanamycin antibiotic (50 µg/ml), and cultured at 37 °C, overnight with shaking at 200 rounds per minute (rpm). The overnight culture was used to inoculate 500 ml LB media containing kanamycin (50 µg/ml) in a 2-liter baffled Erlenmeyer flask. The culture was grown at 28 °C until an OD600 nm of approximately 0.6 was reached. Protein expression was induced with the addition of 0.4 mM IPTG, and the cells were grown for a further 4 h at 28 °C. Cells were harvested by centrifugation (6,300 g) at 4 °C for 15 minutes and cell pellets were stored frozen at −80 °C.
IMAC Protein Purification and Size Exclusion Chromatography
Recombinant Pr78Gag-His6-tag protein was purified, as has been described previously19,63,64. Briefly, IPTG-induced bacterial pellets were lysed in cold CelLytic B (Sigma-Aldrich) supplemented with 500 units of Benzonase (Merck), 0.2 mg/ml lysozyme (Sigma-Aldrich) and 1X concentration of EDTA-free protease inhibitor tablet (Roche). The soluble fraction was collected after centrifugation (48,000 g for 1 hour) at 4 °C and the supernatant was diluted with 4X binding buffer (0.2 M Tris-HCl (pH 8.0), 4.0 M NaCl, 40 mM ß-mercaptoethanol, 10 mM dithiothreitol, 100 mM imidazole, 0.4% (w/v) Tween-20) to a final concentration of 1X. The lysate was then filtered through a 0.4 µm Polyethersulfone (PES) syringe filter and loaded onto a 5 ml HisTRAPTM FF (fast flow) cartridge (GE Healthcare) that had been equilibrated with equilibration buffer (50 mM Tris-HCl (pH 8.0), 1.0 M NaCl, 10 mM ßmercaptoethanol, 2.5 mM dithiothreitol, 25 mM imidazole, 0.1% (w/v) Tween-20, and 10% (v/v) glycerol). After loading the filtrate, the column was washed with the same equilibration buffer except it contained 50 mM imidazole and the bound proteins were eluted with equilibration buffer containing 250 mM imidazole.
Following HisTRAPTM column elution, Pr78Gag-His6-tag protein was concentrated using Amicon® Ultra 15 (30,000 molecular weight cut-off membrane) and was further fractionated by gel filtration/size exclusion chromatography using a Superdex 200 10/300 GL column (GE Healthcare) previously equilibrated with 50 mM Tris-HCl (pH 8.0) and 1.0 M NaCl. Following SDS-PAGE analysis, peak fractions containing Pr78Gag-His6-tag protein were pooled and stored for long term usage in 2 µg/µl aliquots at −80 °C.
SDS-polyacrylamide Gel Electrophoresis and Western Blotting
Expression and purification of recombinant Pr78Gag-His6-tag protein was monitored by SDS-PAGE and western blotting. Briefly, protein samples were mixed with 6X SDS dye, boiled for 5 minutes before loading onto a 4–12% ExpressPlusTM PAGE gel (GenScript), electrophoresed under reducing conditions using MOPS buffer (GenScript), followed by their staining with Coomassie Brilliant Blue. Recombinant Pr78Gag expression and purification was further monitored by transferring non-stained gels onto a nitrocellulose membrane and blotting with anti-rabbit MPMV Gag/Pol Pr78 polyserum (kindly provided by Dr. Eric Hunter, Emory University, Atlanta, GA) and with an anti-His6 monoclonal antibody-HRP conjugate (Sigma-Aldrich).
Eukaryotic Expression of Recombinant Pr78Gag-His6-tag Protein
Transient transfections of the expression vectors (4 micrograms (µg) of full-length Gag eukaryotic expression plasmids (FN7 and FN9) along with 2 µg of MPMV-based transfer vector SJ238 were carried out in HEK 293T cells in triplicates using a calcium phosphate kit (Invitrogen) following manufacturer’s recommendations. The resulting supernatants from the transfected cultures containing virus particles were subjected to low-speed centrifugation (bench-top centrifuge, 3,700 g for 10 minutes) to clear cellular debris. Next, supernatants were filtered using 0.2 μm surfactant free cellulose acetate (SFCA) syringe filters and subjected to ultracentrifugation at 70,000 g to pellet virus like particles (VLPs) using a 20% (w/v) sucrose cushion. The pelleted VLPs were resuspended in TN buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl) and processed for RNA extraction and western blotting. RNA isolation was performed using TRIzol®.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Viral RNA preparations were treated with Turbo DNase (Invitrogen) and amplified using transfer vector (SJ2)-specific primers, OTR1161 (5′ GAT CAG AAC ACT GTC TTG TC 3′) and OTR1163 (5′ CTT TCT TAT CTA TCA ATT CTT TAA 3′), to ensure that the RNA preparations were not contaminated with any plasmid DNA that may have been carried over from the transfected cultures. Next, the DNAsed-RNAs were converted into cDNAs using random hexamers (5′ NNNNNN 3′) and MMLV reverse transcriptase (Promega, USA) as described previously75,76. cDNAs were amplified using the same vector-specific primers (OTR1161 and OTR1163) to monitor the ability of Pr78Gag VLPs to package transfer vector (SJ2) RNA, as described previously38.
VLP Production in Prokaryotic Cells and Transmission Electron Microscopy (TEM)
To monitor the formation of VLPs by recombinant Pr78Gag-His6-tag protein in bacterial cells (following induction with IPTG), cells were pelleted, washed with 0.1 M PBS and fixed in Karnovsky’s fixative. Next, cell pellets were stained with 1% osmium tetroxide and subjected to dehydration using graded ethanol solutions. Finally, cell pellets were fixed in epoxy resin (agar 100). Ultrathin sections on 200 mesh copper (Cu) grids were negatively stained with 1% uranyl acetate followed by lead citrate double stain and analyzed using a FEI Tecnai Biotwin Spirit G2 transmission electron microscope.
In Vitro Assembly of Recombinant Pr78Gag-His6-tag Protein to Form VLPs
To observe in vitro assembly of VLPs, purified recombinant Pr78Gag-His6-tag protein (in 20 mM Tris (pH 7.4) containing 1.0 M NaCl and 10 mM dithiothreitol) was mixed with yeast tRNA at a nucleic acid to protein ratio of 4% (w/w), placed in a Slide-A-Lyzer® 10 KDa dialysis cassette G2 (Thermo Scientific), and dialyzed against 20 mM Tris (pH 7.4) containing 150 mM NaCl and 10 mM dithiothreitol overnight at 4 °C. Following dialysis, ~400 µl was recovered that was concentrated using Amicon® Ultra 15 (30,000 molecular weight cut-off membrane) to a final volume of ~250 µl of which 8–10 µl was spotted on a carbon coated formvar gr id (Proscitech, Australia), air dried, and stained with 1% uranyl acetate for TEM observation.
References
D’Souza, V. & Summers, M. F. How retroviruses select their genomes. Nat. Rev. Microbiol. 3, 643 (2005).
Lever, A. M. L. HIV-1 RNA packaging. Adv. Pharmacol. San Diego Calif 55, 1–32 (2007).
Johnson, S. F. & Telesnitsky, A. Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why. PLOS Pathog. 6, e1001007 (2010).
Ali, L. M., Rizvi, T. A. & Mustafa, F. Cross- and Co-Packaging of Retroviral RNAs and Their Consequences. Viruses 8, 276 (2016).
Comas-Garcia, M., Davis, S. R. & Rein, A. On the Selective Packaging of Genomic RNA by HIV-1. Viruses 8, 246 (2016).
Kaddis Maldonado, R. J. & Parent, L. J. Orchestrating the Selection and Packaging of Genomic RNA by Retroviruses: An Ensemble of Viral and Host Factors. Viruses 8 (2016).
Dubois, N., Marquet, R., Paillart, J.-C. & Bernacchi, S. Retroviral RNA Dimerization: From Structure to Functions. Front. Microbiol. 9, 527 (2018).
Mailler, E. et al. The Life-Cycle of the HIV-1 Gag-RNA Complex. Viruses 8 (2016).
Jewell, N. A. & Mansky, L. M. In the beginning: genome recognition, RNA encapsidation and the initiation of complex retrovirus assembly. J. Gen. Virol. 81, 1889–1899 (2000).
Aldovini, A. & Young, R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J. Virol. 64, 1920–1926 (1990).
Dorfman, T., Luban, J., Goff, S. P., Haseltine, W. A. & Göttlinger, H. G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol. 67, 6159–6169 (1993).
Gorelick, R. J., Henderson, L. E., Hanser, J. P. & Rein, A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a ‘zinc finger-like’ protein sequence. Proc. Natl. Acad. Sci. 85, 8420–8424 (1988).
Méric, C., Gouilloud, E. & Spahr, P. F. Mutations in Rous sarcoma virus nucleocapsid proteinp12 (NC): deletions of Cys-His boxes. J. Virol. 62, 3328–3333 (1988).
Poon, D. T., Wu, J. & Aldovini, A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J. Virol. 70, 6607–6616 (1996).
Lu, K. et al. NMR detection of structures in the HIV-1 5′-leader RNA that regulate genome packaging. Science 334, 242–245 (2011).
Kaye, J. F. & Lever, A. M. L. Nonreciprocal Packaging of Human Immunodeficiency Virus Type 1 and Type 2 RNA: a Possible Role for the p2 Domain of Gag in RNA Encapsidation. J. Virol. 72, 5877–5885 (1998).
Roy, B. B., Russell, R. S., Turner, D. & Liang, C. The T12I mutation within the SP1 region of Gag restricts packaging of spliced viral RNA into human immunodeficiency virus type 1 with mutated RNA packaging signals and mutated nucleocapsid sequence. Virology 344, 304–314 (2006).
Russell, R. S. et al. Effects of a Single Amino Acid Substitution within the p2 Region of Human Immunodeficiency Virus Type 1 on Packaging of Spliced Viral RNA. J. Virol. 77, 12986–12995 (2003).
Tanwar, H. S. et al. The thermodynamics of Pr55Gag-RNA interaction regulate the assembly of HIV. PLOS Pathog. 13, e1006221 (2017).
D’Souza, V. & Summers, M. F. Structural basis for packaging the dimeric genome of Moloney murine leukaemia virus. Nature 431, 586 (2004).
Miyazaki, Y. et al. An RNA Structural Switch Regulates Diploid Genome Packaging by Moloney Murine Leukemia Virus. J. Mol. Biol. 396, 141–152 (2010).
Ferrer, M. et al. Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies. Nucleic Acids Res. 44, 7922–7934 (2016).
Abd El-Wahab, E. W. et al. Specific recognition of the HIV-1 genomic RNA by the Gag precursor. Nat. Commun. 5, 4304 (2014).
Bernacchi, S. et al. HIV-1 Pr55 Gag binds genomic and spliced RNAs with different affinity and stoichiometry. RNA Biol. 14, 90–103 (2017).
Smyth, R. P. et al. In cell mutational interference mapping experiment (in cell MIME) identifies the 5′ polyadenylation signal as a dual regulator of HIV-1 genomic RNA production and packaging. Nucleic Acids Res. https://doi.org/10.1093/nar/gky152 (2018).
Smyth, R. P. et al. Mutational interference mapping experiment (MIME) for studying RNA structure and function. Nat. Methods 12, 866–872 (2015).
Didierlaurent, L. et al. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res. 39, 8915–8927 (2011).
Kuzembayeva, M., Dilley, K., Sardo, L. & Hu, W.-S. Life of psi: How full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 454–455, 362–370 (2014).
Bryant, M. L. et al. Immunodeficiency in Rhesus Monkeys Associated With the Original Mason-Pfizer Monkey Virus. JNCI J. Natl. Cancer Inst. 77, 957–965 (1986).
Fine, D. L. et al. Responses of infant rhesus monkeys to inoculation with Mason-Pfizer monkey virus materials. J. Natl. Cancer Inst. 54, 651–658 (1975).
Aktar, S. J. et al. SHAPE analysis of the 5′ end of the Mason-Pfizer monkey virus (MPMV) genomic RNA reveals structural elements required for genome dimerization. RNA 19, 1648–1658 (2013).
Bharat, T. A. M. et al. Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy. Nature 487, 385–389 (2012).
Bohl, C. R., Brown, S. M. & Weldon, R. A. The pp24 phosphoprotein of Mason-Pfizer monkey virus contributes to viral genome packaging. Retrovirology 2, 68 (2005).
Bray, M. et al. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA 91, 1256–1260 (1994).
Füzik, T. et al. Nucleic Acid Binding by Mason-Pfizer Monkey Virus CA Promotes Virus Assembly and Genome Packaging. J. Virol. 90, 4593–4603 (2016).
Guesdon, F. M. J. et al. Sequences in the 5′ Leader of Mason-Pfizer Monkey Virus Which Affect Viral Particle Production and Genomic RNA Packaging: Development of MPMV Packaging Cell Lines. Virology 288, 81–88 (2001).
Harrison, G. P., Hunter, E. & Lever, A. M. Secondary structure model of the Mason-Pfizer monkey virus 5′ leader sequence: identification of a structural motif common to a variety of retroviruses. J. Virol. 69, 2175–2186 (1995).
Jaballah, S. A. et al. A G–C-Rich Palindromic Structural Motif and a Stretch of Single-Stranded Purines Are Required for Optimal Packaging of Mason–Pfizer Monkey Virus (MPMV) Genomic RNA. J. Mol. Biol. 401, 996–1014 (2010).
Kalloush, R. M. et al. Packaging of Mason-Pfizer monkey virus (MPMV) genomic RNA depends upon conserved long-range interactions (LRIs) between U5 and gag sequences. RNA 22, 905–919 (2016).
Kroupa, T. et al. Membrane Interactions of the Mason-Pfizer Monkey Virus Matrix Protein and Its Budding Deficient Mutants. J. Mol. Biol. 428, 4708–4722 (2016).
Montiel, N. A. REVIEW ARTICLE: An updated review of simian betaretrovirus (SRV) in macaque hosts. J. Med. Primatol. 39, 303–314 (2010).
Mustafa, F., Lew, K. A., Schmidt, R. D., Browning, M. T. & Rizvi, T. A. Mutational analysis of the predicted secondary RNA structure of the Mason-Pfizer monkey virus packaging signal. Virus Res. 99, 35–46 (2004).
Prchal, J., Kroupa, T., Ruml, T. & Hrabal, R. Interaction of Mason-Pfizer monkey virus matrix protein with plasma membrane. Front. Microbiol. 4, 423 (2013).
Schmidt, R. D., Mustafa, F., Lew, K. A., Browning, M. T. & Rizvi, T. A. Sequences within both the 5′ untranslated region and the gag gene are important for efficient encapsidation of Mason-Pfizer monkey virus RNA. Virology 309, 166–178 (2003).
Schur, F. K. M. et al. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 Å resolution. Nature 517, 505 (2015).
Sonigo, P., Barker, C., Hunter, E. & Wain-Hobson, S. Nucleotide sequence of Mason-Pfizer monkey virus: An immunosuppressive D-type retrovirus. Cell 45, 375–385 (1986).
Vile, R. G., Ali, M., Hunter, E. & Mcclure, M. O. Identification of a generalised packaging sequence for D-type retroviruses and generation of a D-type retroviral vector. Virology 189, 786–791 (1992).
Rizvi, T. A., Schmidt, R. D. & Lew, K. A. Mason–Pfizer Monkey Virus (MPMV) Constitutive Transport Element (CTE) Functions in a Position-Dependent Manner. Virology 236, 118–129 (1997).
Rizvi, T. A., Schmidt, R. D., Lew, K. A. & Keeling, M. E. Rev/RRE-Independent Mason–Pfizer Monkey Virus Constitutive Transport Element-Dependent Propagation of SIVmac239 Vectors Using a Single Round of Replication Assay. Virology 222, 457–463 (1996).
Rizvi, T. A., Lew, K. A., Murphy, E. C. & Schmidt, R. D. Role of Mason-Pfizer monkey virus (MPMV) constitutive transport element (CTE) in the propagation of MPMV vectors by genetic complementation using homologous/heterologous env genes. Virology 224, 517–532 (1996).
Barajas, B. C. et al. Identifying the assembly intermediate in which Gag first associates with unspliced HIV-1 RNA suggests a novel model for HIV-1 RNA packaging. PLoS Pathog. 14, e1006977 (2018).
Becker, J. T. & Sherer, N. M. Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly. J. Virol. 91, e02315–16 (2017).
Behrens, R. T., Aligeti, M., Pocock, G. M., Higgins, C. A. & Sherer, N. M. Nuclear Export Signal Masking Regulates HIV-1 Rev Trafficking and Viral RNA Nuclear Export. J. Virol. 91 (2017).
Brandt, S. et al. Rev Proteins of Human and Simian Immunodeficiency Virus Enhance RNA Encapsidation. PLOS Pathog. 3, e54 (2007).
Jouvenet, N., Lainé, S., Pessel-Vivares, L. & Mougel, M. Cell biology of retroviral RNA packaging. RNA Biol. 8, 572–580 (2011).
Lingappa, J. R. et al. HIV-1 initiates genomic RNA packaging in a unique subset of host RNA granules. bioRxiv 183855 (2017).
Moore, M. D. et al. Probing the HIV-1 Genomic RNA Trafficking Pathway and Dimerization by Genetic Recombination and Single Virion Analyses. PLOS Pathog. 5, e1000627 (2009).
Bradac, J. & Hunter, E. Polypeptides of Mason-Pfizer monkey virus I. Synthesis and processing of the gag-gene products. Virology 138, 260–275 (1984).
Henderson, L. E., Sowder, R., Smythers, G., Benveniste, R. E. & Oroszlan, S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J. Virol. 55, 778–787 (1985).
Hrusková-Heidingsfeldová, O. et al. Cloning, Bacterial Expression, and Characterization of the Mason-Pfizer Monkey Virus Proteinase. J. Biol. Chem. 270, 15053–15058 (1995).
Rhee, S. S. & Hunter, E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell 63, 77–86 (1990).
Klikova, M., Rhee, S. S., Hunter, E. & Ruml, T. Efficient in vivo and in vitro assembly of retroviral capsids from Gag precursor proteins expressed in bacteria. J. Virol. 69, 1093–1098 (1995).
Bewley, M. C. et al. A non-cleavable hexahistidine affinity tag at the carboxyl-terminus of the HIV-1 Pr55Gag polyprotein alters nucleic acid binding properties. Protein Expr. Purif. 130, 137–145 (2017).
McKinstry, W. J. et al. Expression and purification of soluble recombinant full length HIV-1 Pr55Gag protein in Escherichia coli. Protein Expr. Purif. 100, 10–18 (2014).
Sakalian, M., Parker, S. D., Weldon, R. A. & Hunter, E. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J. Virol. 70, 3706–3715 (1996).
Sakalian, M. & Hunter, E. Separate Assembly and Transport Domains within the Gag Precursor of Mason-Pfizer Monkey Virus. J. Virol. 73, 8073–8082 (1999).
Campbell, S. & Vogt, V. M. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J. Virol. 71, 4425–4435 (1997).
Ehrlich, L. S., Agresta, B. E. & Carter, C. A. Assembly of recombinant human immunodeficiency virus type 1 capsid protein in vitro. J. Virol. 66, 4874–4883 (1992).
Affranchino, J. L. & González, S. A. In vitro assembly of the feline immunodeficiency virus Gag polyprotein. Virus Res. 150, 153–157 (2010).
Campbell, S. & Rein, A. In Vitro Assembly Properties of Human Immunodeficiency Virus Type 1 Gag Protein Lacking the p6 Domain. J. Virol. 73, 2270–2279 (1999).
Campbell, S. et al. Modulation of HIV-like particle assembly in vitro by inositol phosphates. Proc. Natl. Acad. Sci. 98, 10875–10879 (2001).
Aktar, S. J. et al. Structural basis of genomic RNA (gRNA) dimerization and packaging determinants of mouse mammary tumor virus (MMTV). Retrovirology 11, 96 (2014).
Mustafa, F. et al. Sequences within Both the 5′ UTR and Gag Are Required for Optimal In Vivo Packaging and Propagation of Mouse Mammary Tumor Virus (MMTV) Genomic RNA. PLOS ONE 7, e47088 (2012).
Rizvi, T. A. et al. Optimal packaging of FIV genomic RNA depends upon a conserved long-range interaction and a palindromic sequence within gag. J. Mol. Biol. 403, 103–119 (2010).
Ghazawi, A. et al. Both the 5′ and 3′ LTRs of FIV contain minor RNA encapsidation determinants compared to the two core packaging determinants within the 5′ untranslated region and gag. Microbes Infect. 8, 767–778 (2006).
Mustafa, F. et al. Relative activity of the feline immunodeficiency virus promoter in feline and primate cell lines. Microbes Infect. 7, 233–239 (2005).
Acknowledgements
This research was funded primarily by a grant from the United Arab Emirates University (UAEU) Program for Advanced Research-UPAR (UPAR-31M233) and in part by a grant from the College of Medicine and Health Sciences (31M280) to TAR. FNNP and AC were supported by UPAR-31M233 and UAE University Zayed Bin Sultan Center for Health Sciences (UCBR-31R123) grants, respectively. The authors would like to express their sincere thanks and appreciation to Dr. Mustafa T. Ardah and Dr. Syed Tariq for their help in gel filtration chromatography and electron microscopy, respectively.
Author information
Authors and Affiliations
Contributions
T.A.R., R.M., and F.M. conceived and designed the experiments. F.N.N.P., L.A., V.N.P., and A.C. performed the experiments. S.S.A. contributed reagents/materials/analysis tools. T.A.R., R.M., and F.M. analyzed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pitchai, F.N.N., Ali, L., Pillai, V.N. et al. Expression, purification, and characterization of biologically active full-length Mason-Pfizer monkey virus (MPMV) Pr78Gag. Sci Rep 8, 11793 (2018). https://doi.org/10.1038/s41598-018-30142-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30142-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.