Abstract
The prevalence of obstructive sleep apnea (OSA) during pregnancy is rising. OSA during pregnancy has been associated with hypertensive disorders of pregnancy and gestational diabetes. The effect of maternal OSA on the fetus, particularly on fetal growth, is less apparent. Most of the currently available human data is based on non-objective assessment of OSA and includes heterogeneous populations with inadequate control on confounders, such as maternal obesity and pregnancy complications. Using objective tools in non-obese women with uncomplicated pregnancies, we aimed to investigate the association between maternal OSA and fetal growth. A total of 155 non-obese pregnant women were recruited. Birth-weight percentile of the newborns of women with mild OSA was significantly higher compared with the newborns of non-OSA controls (72% vs. 57%, respectively, P < 0.01). Birth-length and triceps thickness measurements were significantly higher among the newborns of women with OSA compared with controls (P = 0.02 for both). The proportion of large for gestational age (LGA) newborns was higher among women with OSA compared with controls (28% vs. 8%, respectively, P = 0.04). Our results suggest that maternal OSA during the third trimester of pregnancy - even in a mild form -is associated with accelerated fetal growth.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of upper airway obstruction during sleep, resulting in disruption of ventilation, hypoxemia, and sleep fragmentation. OSA has a significant impact on public health, and may lead to daytime sleepiness, reduced neurocognitive functions, accidents, and metabolic and cardiovascular consequences if left untreated1. Various intermediary mechanisms link OSA to these morbidities including sympathetic activation, endothelial dysfunction, oxidative stress, inflammation and metabolic dysregulation2. Over the past several decades, the prevalence of OSA has been continuously rising in the general population, most likely due to the growing obesity epidemic1. Accumulating evidence shows that habitual snoring, the hallmark symptom of OSA, increases in frequency during pregnancy and affects up to one-third of women by the third trimester3,4,5,6,7,8,9. Similar to the general population, overweight and obesity increase the risk of OSA in pregnant women10,11,12, and the prevalence of maternal OSA is rising, with an annual increase of 24%13,14. OSA during pregnancy has been associated with adverse maternal outcomes, including hypertensive disorders of pregnancy9,13,14,15,16,17,18,19,20,21 and gestational diabetes15,16,17,18,19,20,21,22.
The effect of maternal OSA on the fetus, particularly on fetal growth, is less apparent. Animal studies results are inconsistent, with some demonstrating that maternal intermittent hypoxia, a model of OSA, leads to low birthweight of the pups23,24,25. A recent publication has shown that late gestational intermittent hypoxia induces metabolic dysfunction, as reflected by increased body weight and adiposity index in male mice offspring when they reach adulthood26, suggesting long-term metabolic dysregulation in offspring exposed to intermittent hypoxia in pregnancy. Indeed, several downstream effects of OSA may affect placental function and fetal growth and not always in the same direction.
Numerous human studies have shown an association between maternal OSA and adverse fetal outcomes, such as intrauterine growth restriction, low Apgar scores, preterm births and neonatal intensive care unit admissions17,27,28,29. Studies on the association between maternal OSA and birthweight, however, yielded conflicting results3,27,30,31,32,33,34,35,36,37. Taken together, most of the currently available human data were reported in studies that used non-objective tools for the assessment of OSA and included heterogenous populations with inadequate control on confounders, such as maternal obesity and adverse maternal pregnancy complications that could potentially affect fetal growth. We conducted a prospective cohort study for assessing the association between maternal OSA and fetal growth with good control on maternal obesity and pregnancy complications.
Methods
Women in the third trimester of a singleton, uncomplicated pregnancy who attended a low-risk obstetric surveillance outpatient clinic at the Noy Women Health Care Center or the outpatient clinics at the Lis Maternity Hospital were recruited between June 2008 and December 2010. At enrollment (gestational week 25–27), all participants completed a questionnaire on smoking exposure history, medical and obstetric history and current pregnancy complications. They also responded to questions on the presence of habitual snoring before and during pregnancy, including the Berlin Questionnaire38 and the Epworth sleepiness scale (ESS)39. All participants underwent an ambulatory overnight sleep study between 33 to 36 weeks of gestation in which the watch-PAT 200 device (Itamar Medical; Israel) was used. The device had been validated in pregnancy and shown to correlate well with polysomnography40.
Women with an apnea hypopnea index (AHI) >5 per hour of sleep were considered to have OSA. We used the standard adult criteria for OSA, since there is no definitive threshold for OSA in pregnancy, and decided to restrict our analysis to the mild form of OSA. A medical records review was conducted by one of the researchers who was unaware of the sleep study results. Pertinent demographic (gender, gestational age, birthweight), and clinical information (mode of delivery, Apgar scores at 1 and 5 minutes, and any perinatal complications) was collected. The neonatal birthweight percentile was calculated according to gender and gestational age using a birth centile curves reference standard from Israel41. Small for gestational age (SGA) was defined as a birth percentile <10th percentile, and large for gestational age (LGA) was defined as a birth percentile >90th percentile for age and gender.
Body length, head circumference and adiposity (skinfold thickness measurements of the subscapular and triceps areas) were measured at birth in a subset of newborns. Recumbent crown-heel length was measured on a length board (O’Leary Premie Length Board, Ellard Instrumentation Ltd., Monroe, WA). Fronto-occipital head circumference was measured using a standard 1-cm wide measuring tape. Skinfold thickness measurements were performed using Holtain calipers (Holtain, UK). Each measurement was recorded twice and the average was used for analysis42,43.
The study was approved by the institutional review board of the Tel Aviv Medical Center. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants and/or their legal guardians.
Statistical analysis
Descriptive statistics, chi-square tests, logistic and linear regression analysis were used to compare the distributions of maternal and newborn characteristics in non-obese women with mild OSA (AHI within a range of 5–14/h) compared with controls (AHI < 5). We examined the association between mild maternal OSA exposure (using an AHI < 5 as the referent group) and newborn size (first defined as birthweight percentile [continuous measure) and then as a binary variable) by classifying newborns with a birthweight percentile >90 as LGA vs. appropriate for gestational age newborns (birthweight percentile ≤90). We examined these associations by constructing bivariate and adjusted linear and logistic regression models controlled for parity and pre-pregnancy body mass index (BMI). Since common pregnancy complications that are known predictors of newborn birthweight percentiles, i.e., gestational diabetes and gestational hypertensive disorders of pregnancy, were absent in our sample, they were not included as covariates in our regression models. SAS 9.4 (Cary, NC) was used for these analyses.
Results
A total of 169 women were recruited, of whom 11 had a pre-pregnancy BMI >30 kg/m2 and were excluded from the study. Of the remaining 158, only three women had moderate-to-severe OSA (an AHI >15/h) and therefore were also excluded. The final analytic sample included 155 non-obese pregnant women with mild OSA (Fig. 1) of whom 26 (17%) had mild OSA. None of the participants developed hypertension, preeclampsia or gestational diabetes mellitus throughout the entire pregnancy. There were no pre-term deliveries in the study group, and one delivery at a gestational age of 36 weeks in the control group.
The maternal and pregnancy characteristics of the women with mild OSA and the controls are presented in Table 1. They were similar for the two groups with the exception of the mean pre-pregnancy BMI, which was significantly higher in the women with OSA compared with the controls, although the mean weight gain rate (kg/week) was similar. As expected, significantly more women with OSA had a positive Berlin score. No differences in ESS scores were found between the two groups.
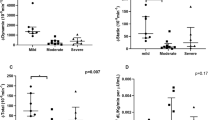
The delivery and newborn characteristics are presented in Table 2. The mean birthweight percentile of newborns of women with mild OSA was significantly higher compared with the newborns of the controls (72% vs. 57%, respectively, P < 0.01, Fig. 2A). The proportion of LGA newborns was significantly higher among the women with OSA compared with the controls (28% vs. 8%, respectively, P = 0.04). The percentage of SGA among women with OSA did not differ from that of the controls. Moreover, in a subset of 80 newborns, the mean birth length and mean triceps thickness measurements were significantly higher among the newborns of women with OSA compared with controls (P = 0.02 and P = 0.02, Fig. 2B,C, respectively). The mean 1-minute Apgar score was significantly lower among newborns of women with mild OSA (P < 0.01), and the proportion of Apgar score <7 at 1 minute was significantly higher (23% vs. 5%, respectively, P < 0.01).
(A) Birth-weight percentile of newborns of mothers with mild OSA (n = 26) compared to newborns of controls (n = 129), (p < 0.01). (B) Birth-length of newborns of mothers with mild OSA (n = 26) compared to newborns of controls (n = 129), (p = 0.02). (C) Triceps thickness of newborns of mothers with mild OSA (n = 26) compared to newborns of controls (n = 129), (p = 0.02).
Bivariate and multivariate linear regression analyses were conducted with the birthweight percentile as the dependent variable, and the results are shown in Table 3. After adjustment for parity and pre-pregnancy BMI, the birthweight percentile of newborns of the women with mild OSA was 16.5 units higher compared with newborns of the controls. Bivariate and multivariate logistic regression analyses were conducted with LGA as the dependent variable and the findings are shown in Table 4. After adjustment for parity and pre-pregnancy BMI, the women with mild OSA had increased odds for an LGA newborn compared with the controls (OR = 5.1, 95% CI 1.3, 20.0).
Discussion
The mild form is the most frequent presentation of OSA in non-obese otherwise healthy pregnant women. An association between mild maternal OSA in these women and fetal growth has not been investigated before. The present study is the first to demonstrate that mild maternal OSA in non-obese pregnant women with no gestational diabetes or hypertensive disorders of pregnancy is associated with accelerated fetal growth that is expressed in several dimensions of growth such as weight, length and adiposity and that it increases the risk of LGA newborns. This cohort limited to non-obese women with no pregnancy complications enabled us to examine the associations of the mild form of OSA on fetal growth and avoid potential confounders, such as maternal obesity and adverse maternal pregnancy complications that could potentially affect fetal growth.
OSA in the general population is associated with a variety of metabolic disturbances, including glucose intolerance, insulin resistance and lipid dysregulation44. While it is well established that normal pregnancy is associated with physiological alterations in glucose and lipid metabolism45,46,47, and that maternal OSA may be associated with an increased risk of gestational diabetes mellitus15,16,17,18,19,20,21,22, little information is available on the metabolic consequences of OSA during pregnancy and their effect on fetal growth. Indeed, several downstream effects of OSA, such as intermittent hypoxia, increased sympathetic activity, repeated arousals, repeated episodes of elevated blood pressure and peripheral vasoconstriction, potentiation of inflammatory cascades and metabolic alteration, may affect placental function and fetal growth in directions yet to be established.
The effect of maternal OSA on fetal growth has been studied, but the results were conflicting. The results of the present study support previous publications that have also shown association between maternal OSA and LGA infants35,36,37. However, most of the currently available human data were based on studies that used non-objective tools for the assessment of OSA and included heterogenous populations with inadequate control of the above-cited confounders. Those factors alone may explain the conflicting results. It is also possible that different components of OSA (intermittent hypoxia/sleep fragmentation) or the severity of OSA (mild vs. moderate-severe) affect placental function and fetal growth in different ways.
Our study results are in contrast to those recently published by Pamidi et al. who also included a relatively healthy non-obese cohort of pregnant women34. Their study showed that maternal OSA was associated with an increased risk of delivering an SGA baby. Similar to our cohort, most of their OSA cases in the third trimester were mild and characterized by sleep fragmentation rather than by oxygen desaturations, as has been demonstrated previously in pregnancy5,8,48,49. However, the main disparity between their study and ours that could explain the different results is in the different study designs. Pamidi et al. focused solely on OSA and SGA and recruited only women with a third-trimester ultrasound that showed <75th centile predicted weight in order to minimize the number of LGA babies in their cohort, thus excluding the possibility of examining a link between OSA and accelerated fetal growth. By not being restricted by any fetal growth criteria, our study design could better reflect the effect of maternal OSA on fetal growth. Moreover, our findings on increased birth length and adiposity in a subset of newborns supported our observation of accelerated fetal growth in women with mild OSA.
Since parity and maternal BMI are known risk factors for accelerated fetal growth and LGA, we controlled for both variables and found that mild maternal OSA is an independent predictor of increased birthweight and LGA.
Several mechanisms may explain our results, among them maternal lipids levels, fetal insulin and placental function. It is possible that the metabolic consequences associated with even mild OSA contribute to or exaggerate the normal metabolic alterations present in late gestation, thus creating a metabolic milieu favorable for accelerated fetal growth.
All our participants were non-obese women, however, the ones who had a higher BMI (still within the normal range) tended to develop OSA during pregnancy, although the rate of their weight gain did not differ from that of the controls. Also, similar to the general population, smoking tended to be more prevalent among women with OSA, although not to a level of significance. In support of a previous publication50, our results also indicated that the Berlin questionnaire and the Epworth sleepiness scale perform poorly in the setting of pregnancy.
Finally, the mean 1-minute Apgar score was significantly lower among newborns of women with OSA compared with controls and, similar to previous publications, the proportion of low Apgar scores (Apgar score <7) was higher among women with OSA3,16.
The strengths of our study include its prospective nature and relatively large number of participants in the third trimester of pregnancy who underwent objective sleep studies within a narrow window of pregnancy (weeks 33–36). Further strengths include a homogeneous population of non-obese pregnant women with no pregnancy complications and the addition of an analysis of other measures of fetal growth, such as head circumference, birth length and adiposity. The major limitation of this study is the usage of an ambulatory sleep study rather than polysomnography, which enables better detection of flow limitation and sleep fragmentation. Another limitation is the lack of information regarding paternal anthropometry which was found to be associated with fetal growth51,52.
In summary, birthweight is a marker of fetal outcome and a surrogate indicator of conditions occurring in the intrauterine environment. It has been suggested that the impact of birthweight echoes throughout the course of life and may influence the risk of cardiovascular disease, diabetes, and obesity (“fetal programming”). Our results suggest that maternal OSA in pregnancy — even in a mild form — is associated with accelerated fetal growth that is expressed in several dimensions of growth such as weight, length and adiposity. These findings have significant clinical implications since accelerated fetal growth may affect the health of both the mother and the baby and may predispose the baby to abnormal growth later in life. Studies exploring the mechanisms underlying this association are warranted. Since OSA is a treatable condition, interventional trials involving the treatment of maternal OSA are also recommended.
References
Garvey, J. F., Pengo, M. F., Drakatos, P. & Kent, B. D. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 7, 920–9 (2005).
Carneiro, G. & Zanella, M. T. Obesity metabolic and hormocal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. Ahead of print (2018).
Franklin, K. A. et al. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 117, 137–41 (2000).
Izci, B. et al. Sleep complaints: snoring and daytime sleepiness in pregnant and pre-eclamptic women. Sleep Med 6, 163–9 (2005).
Pien, G. W., Fife, D., Pack, A. I., Nkwuo, J. E. & Schwab, R. J. Changes in symptoms of sleep disordered breathing during pregnancy. Sleep. 28, 1299–1305 (2005).
Izci, B. et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 167, 137–40 (2003).
Izci, B. et al. Sleep disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J. 27, 321–27 (2006).
Bourjeily, G. et al. Airflow limitations in pregnant women suspected of sleep disordered breathing. Sleep Med. 15, 550–55 (2014).
O’Brien, L. M. et al. Pregnancy-onset habitual snoring, gestational hypertension and pre-eclampsia: prospective cohort study. Am J Obstet Gynecol. 207(487), e1–9 (2012).
Facco, F. L., Kramer, J., Ho, K. H., Zee, P. C. & Grobman, W. A. Sleep disturbances in pregnancy. Obstet Gynecol. 115, 77–83 (2010).
Maasilta, P., Bachour, A., Teramo, K., Polo, O. & Laitinen, L. A. Sleep related disordered breathing during pregnancy in obese women. Chest. 120, 1448–54 (2001).
Ursavas, A., Karadag, M., Nalci, N., Ercan, I. & Gozu, R. O. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 76, 33–9 (2008).
Spence, D. L. et al. Association of obstructive sleep apnea and adverse pregnancy-related outcomes in military hospitals. Eur J Obstet Gynecol Reprod Biol. 210, 166–72 (2017).
Louis, J. M., Mogos, M. F., Salemi, J. L., Redline, S. & Salihu, H. M. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep. 37, 843–49 (2014).
Pamidi, S. et al. Maternal sleep disordered breathing and adverse pregnancy outcomes: a systemic review and metaanalysis. Am J Obstet Gynecol. 210, 52 e1–52 e14 (2014).
Ding, X. X. et al. A systemic review and quantitative assessment of sleep disordered breathing during pregnancy and perinatal outcomes. Sleep Breath. 18, 703–13 (2014).
Chen, Y. H. et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 206, 136 e1–135 (2012).
Carnelio, S., Morton, A. & McIntyre, D. Sleep disordered breathing in pregnancy: the maternal and fetal implications. J Obstet Gynecol. 37, 170–8 (2017).
Facco, F. L. et al. Association between sleep disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 129, 31–41 (2017).
Dunietz, G. L., Chervin, R. D. & O’Brien, L. M. Sleep disordered breathing during pregnancy: future implications for cardiovascular health. Obstet Gynecol Surv. 69, 164–76 (2014).
Facco, F. L. et al. Implications of sleep disordered breathing in pregnancy. Am J Obstet Gynecol. 210, 559 e1–556 (2014).
Bourjeily, G., Raker, C. A., Chalhoub, M. & Miller, M. A. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 36, 849–55 (2010).
Gozal, D., Gozal, E., Reeves, S. R. & Lipton, A. J. Gasping and autoresuscitation in the developing rat: effect of antecedent intermittent hypoxia. J Appl Physiol. 92, 1141–44 (2002).
Schwartz, J. E., Kovach, A., Meyer, J., McConnell, C. & Iwamoto, H. S. Brief, intermittent hypoxia restricts fetal growth in Sprague-Dawley rats. Biol Neonate. 73, 313–19 (1998).
Iqbal, W. & Ciriello, J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol. 209, 564e1–9 (2013).
Khalyfa, A. et al. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 595, 551–68 (2017).
Fung, A. M. et al. Effects of maternal obstructive sleep apnea on fetal growth: a prospective cohort study. PLOS One. 8, e68057 (2013).
Izci-Balserak, B. & Pien, G. W. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 16, 574–82 (2010).
Sahin, F. K. et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynecol Obstet. 100, 141–6 (2008).
Loube, D. I. Self-reported snoring in pregnancy. Association with fetal outcome. Chest. 109, 885–9 (1996).
Tauman, R., Sivan, Y., Katsav, S., Greenfeld, M. & Many, A. Maternal snoring during pregnancy is not associated with fetal growth restriction. J Matern Fetal Neonatal Med. 25, 1983–86 (2012).
Ayrim, A. et al. Influence of self-reported snoring and witnessed sleep apnea on gestational hypertension and fetal outcome in pregnancy. Arch Gynecol Obstet. 283, 195–9 (2011).
Micheli, K. et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 22, 738–44 (2011).
Pamidi, S. et al. Maternal sleep disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 71, 719–25 (2016).
Bin, Y. S., Cistulli, P. A. & Ford, J. B. Population based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 12, 871–877 (2016).
Higgins, N. et al. The Berlin quiestionnaire for assessment of sleep disordered breathing risk in parturients and non-pregnant women. Int J Obstet Anest. 20, 22–25 (2011).
Antony, K. M. et al. Association of adverse perinatal outcomes with screening measures of obstructive sleep apnea. J Perinatol. 34, 441–448 (2014).
Netzer, N. C., Stoohs, R. A., Netzer, C. M., Clark, K. & Strohl, K. P. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 131, 485–91 (1999).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 14, 540–45 (1991).
O’Brien, L. M. et al. Validation of watch PAT- 200 against polysomnography during pregnancy. J Clin Sleep Med. 8, 287–94 (2012).
Dollberg, S., Haklai, Z., Mimouni, F. B., Gorfein, I. & Gordon, E. S. Birth weight standards in the live-born population in Israel. Isr Med Assoc J. 7, 311–14 (2005).
Addo, O. Y. & Himes, J. H. Reference curves for triceps and subcapsular skinfold thickness in US children and adolescents. Am J Clin Nutr. 91, 635–42 (2010).
Crume, E. L. et al. Long term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 34, 641–5 (2011).
Tasali, E. & Ip, M. S. Obstructive sleep apnea and metabolic syndrome, alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 5, 207–17 (2008).
Catalano, P. M., Tyzbir, E. D., Roman, N. M., Amini, S. B. & Sims, E. A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 165, 1667–72 (1991).
Desoye, G., Schweditsch, M. O., Pfeiffer, K. P., Zechner, R. & Kostner, G. M. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab. 64, 704–12 (1987).
Arcos, F., Castelo-Branco, C., Casals, E., Sanllehy, C. & Cararach, V. Normal and gestational diabetic pregnancies- lipids, lipoproteins and apolipoproteins. J Reprod Med. 43, 144–48 (1998).
Connolly, G. et al. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 18, 672–6 (2001).
Pien, G. W. et al. Risk factors for sleep disordered breathing in pregnancy. Thorax. 69, 371–7 (2014).
Tantrakul, V., et al. Performance of screening questionnaires for obstructive sleep apnea during pregnancy: a systemic review and meta-analysis. Sleep Med Rev. S1087-079230133-2 (2016).
Pomeroy, E., Wells, J. C., Cole, T. J., O’Callaghan, M. & Stock, J. T. Relationship of maternal and paternal anthropometry with neonatal body size, proportions and adiposity in an Australian cohort. Am J Phys Antropol. 156, 625–36 (2015).
Sorensen, T. I. et al. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7y assessed in the Danish National Birth Cohort. Am J Clin Nutr. 104, 389–96 (2016).
Acknowledgements
We wish to thank the pregnant women and their children who participated in this study. This research was supported by the Israel Science Foundation (grant No. 707/12).
Author information
Authors and Affiliations
Contributions
Ayana Telerant had substantial contribution to the acquisition of data, analysis and interpretation of data and drafting the article. She approved the final version of the manuscript. Galit Levi Dunietz had substantial contribution to the analysis and interpretation of data and drafting the article She has revised the manuscript for important intellectual content and approved its final version. Ariel Many had substantial contribution to the conception and design, acquisition of data, interpretation of data. He has revised the manuscript for important intellectual content and approved its final version. Riva Tauman had substantial contribution to the acquisition of data, analysis and interpretation of data and drafting the article. She has revised the manuscript for important intellectual content and approved its final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Telerant, A., Dunietz, G.L., Many, A. et al. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci Rep 8, 10768 (2018). https://doi.org/10.1038/s41598-018-29052-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29052-y
This article is cited by
-
Birth weight influences differently on systolic and diastolic blood pressure in children and adolescents aged 8–15
BMC Pediatrics (2022)
-
Fetal Heart Rate Decelerations in Women with Sleep-Disordered Breathing
Reproductive Sciences (2021)
-
Mild maternal sleep-disordered breathing during pregnancy and offspring growth and adiposity in the first 3 years of life
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.