Abstract
Marine organisms especially sessile invertebrates, such as soft corals, gorgonians and sponges, can survive in the competitive environment mainly relying on their second metabolites with chemoecological effects including allelopathy and algal growth inhibition. It is well known that the microscale models are urgently needed in marine chemoecology assessment to evaluate the algal growth inhibition activity of trace quantity natural products. In this work, a microalgal growth inhibition model was established for microalgal inhibition evaluation of marine natural products with 96-well microplate by automatic fluorescence observation using microplate reader. Subsequently, this model was applied to bioassay-guided isolation and preliminary bioactivity screening of the secondary metabolites from soft corals, gorgonians, sponges and their symbiotic microbes collected from the South China Sea. As a result, fifteen compounds (1‒15) were found to exhibit microalgal growth inhibition activities against at least one of marine microalgae, Karenia mikimotoi, Isochrysis galbana, and Heterosigma akashiwo. Specifically, altersolanol C (13) demonstrated potent activity against K. mikimotoi with the 96h-EC50 value of 1.16 µg/mL, more than four times stronger than that of the positive control K2Cr2O7. It was suggested that the microalgal growth inhibition microscale model is suitable for bioassay-guided isolation and preliminary bioactivity screening of marine natural products.
Similar content being viewed by others
Introduction
In marine ecosystem, marine organisms especially sessile invertebrates, such as soft corals, gorgonians and sponges, can survive in the competitive environment mainly relying on their second metabolites with chemical ecology functions. The secondary metabolites from marine invertebrates and their symbiotic microbes have been found to serve as defensive and allelopathic substances possessing various chemical ecology effects, such as algal growth inhibition, antifouling, allelopathy, ichthyotoxicity, insecticidal, antipredatory and antimicrobial activities1,2,3,4,5,6,7. To evaluate the chemoecological effects of secondary metabolites isolated from marine organisms, many models have been established and applied in the field of marine chemical ecology3,5,6. It has been concerned that the microscale models for marine chemoecology assessment are urgently needed for trace quantity of marine natural products.
Since 1971, microalgal models have been used to test the microalgal growth-inhibition activities8,9,10,11,12. Marine microalgae, as the dominant primary producers, are considered as an ecologically important group playing an essential role in the marine ecosystem13. Marine microalgae have been testified as the basic link in aquatic food chains and a key functional group of marine organisms14. In recent years microalgal blooms have caused serious problems in eutrophic water bodies by destroying the ecological balance15,16. The public health and economic problems caused by harmful algal blooms appear to have increased in frequency, intensity, and geographic distribution over the past decades17. Microalgae were reported to have greater sensitivity than invertebrates and fish to natural products as well as municipal and industrial effluents18,19,20,21,22,23,24. Based on these facts, microalgae have been widely used in the microalgal models to estimate the ecotoxicological character of environmental samples. Specifically, microalgal models have been used as a microscale model in the fields of environment ecological evaluation, including herbicides, pestcides, industrial wastes and sediment, as well as the heavy metal toxicity assessment in marine and aquatic systems10,25,26,27,28,29.
Traditionally, the classic models for microalgal growth-inhibitory activity were conducted with conical glass flasks, and a large test volume, e.g. 50 mL to 500 mL, is normally used in practical experiments30. In order to improve the efficiency of standard test procedures, the test vessels were necessary to be miniaturized, and the miniature methods with 96-well microplates (250 μL/well) were proposed and applied10. The traditional 250 mL Erlenmeyer flask vessels were substituted by microplates requiring only 2 mL or less test volume10,31,32. There have been several approaches to develop a small-scale microplate toxicity test, together with the ways of quantifying microalgal growth such as cell counting with electronic particle counting optical density (O.D.) measurement31,32,33,34, fluorescence measurement26,35, and ATP quantification36.
It should be noted that the microalgal microscale models have been seldom reported for the chemoecological assessment of marine natural products. Due to the fact that marine natural products exist in the original organisms with usually micro- or trace-scale (ppm, or even ppb), it is necessary to build a microscale model for microalgal inhibition evaluation. Based on the above consideration, in the present study, we modified a microalgal growth inhibition microscale model by using 96-well microplates with three microalgae and measuring the chlorophyll fluorescence based on microalgal biomass. This improved model would be suitable for the assessment of the microalgal inhibition activities, specifically for micro- or trace marine natural products. The model was applied to microalgal inhibition activity assessment for the secondary metabolites isolated from corals, gorgonians, sponges and their symbiotic microorganisms. Herein, we report how to evaluate the microalgal inhibition activity of the micro- or trace marine natural products in a convenient and efficient way.
Materials and Methods
General experimental procedures
Microplate reader SpetraMax M5 (Molecular Instrument Company, USA) was used to estimate the microalgal biomass through chlorophyll fluorescence measurement26,35. Inverted fluorescence microscope DMI 6000B (Leica Microsystems Company, Germany) was used for the observation of microalgal fluorescence. 96-Well microplates (Beijing Siqi Biological Technology Company, China) were used for the microalgal growth inhibition tests. Intelligent illumination incubator GXZ (Ningbo Jiangnan Park New Science and Technology Company, China) was used for cultivation of marine microalgae. Potassium dichromate (K2Cr2O7) (Tianjin Guangcheng Chemical Company, China, purity ≥99.9%) was used as a reference substance and a positive control. Dimethyl sulfoxide (DMSO) (Tianjin Guancheng Chemical Reagent Company, China, purity ≥99.9%) was used as cosolvent to improve the solubility of the tested compounds. Considering of the dissolvability for the most of tested marine natural products and almost no observed inhibitory effect on microalgae, 0.5% DMSO was chosen as the cosolvent and negative control. The nutritive medium composed of natural seawater and a supply of nutrients and vitamins according to the f/2 (half strength f medium) nutritive medium37. Natural seawater obtained from Qingdao offshore was used to configure the f/2 culture medium after using 0.45 μm microporous membrane filter.
Microalgal species and culture condition selection
The marine microalgal species Karenia mikimotoi, Isochrysis galbana and Heterosigma akashiwo were supplied by the Laboratory of Ecological, Ocean University of China. These microalgae were maintained and precultured according to ISO 869238, with f/2 medium kept at 25 °C, pH 8.0 ± 0.1 under a 12: 12 light: dark cycle using a photon flux density of 80 μmol photon/m2·s and were shaken three times daily. In order to determine the exponential growth phases of three microalgae, we investigated the growth patterns of these three microalgae in 250 mL Erlenmeyer flasks with three replicates (n = 3). The initial concentrations of K. mikimotoi, I. galbana, and H. akashiwo were 5 × 104 cells/mL, 1 × 105 cells/mL, and 5 × 104 cells/mL, respectively.
Chlorophyll fluorescence value measurement
Microplate reader SpectraMax M5 was used to acquire the fluorometric data, with excitation wavelength of 485 nm and emission wavelength of 680 nm. As transparent polystyrene microplates cannot be used for fluorometric measurement, 96-well nontransparent polystyrene microplates with transparent bottom were used. At the time point of 96 h, the microalgal culture was transported from 96-well transparent microplates to 96-well nontransparent polystyrene microplates with transparent bottom. Then the fluorometric data were recorded using microplate reader. The 96 h half maximal effective concentration (96h-EC50) value of each compound was estimated with different concentrations at 96 h10.
Statistical ananlysis
The intensity fluorescence values were obtained from microplate reader. These data were first processed by Excel, the initial and endpoint measurement were used to calculate the percentage of microalgal growth inhibition corresponding each concentration of tested substances. Then the 96h-EC50 values were determined by applying a PROBIT analysis39 with the aid of the statistical software SPSS 17.0.
Microalgal growth inhibition bioassay
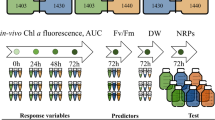
The procedures of the bioassay could be summarized as follows. In order to reduce evaporation, sterile water was added to the exterior ring wells of 96-well transparent microplate. Precultured exponentially-growing microalgae of K. mikimotoi (6th day, 5 × 104 cells/mL), I. galbana (21st day, 1 × 105 cells/mL), and H. akashiwo (15th day, 5 × 104 cells/mL) were moved to the wells of 96-well transparent microplate using a pipette. Each well was injected with 199 μL of microalgal suspension and 1 μL of test solution containing the tested compound with different concentration prepared in advance to make a final volume of 200 μL. To measure the microalgal growth inhibition activity, the maximum amount required for each compound was 200 μg. The tested compounds were dissolved in 0.5% (v/v) DMSO firstly30, and then diluted with 0.5% DMSO to obtain five test concentrations. The final concentrations of the tested compounds in each well of 200 μL culture system were 50 μg/mL, 10 μg/mL, 2 μg/mL, 0.4 μg/mL, and 0.08 μg/mL, respectively. For the blank control, positive control and negative control, 1 μL H2O, 1 μL K2Cr2O7 (2 μg/mL) and 1 μL 0.5% DMSO were added into microalgal suspensions instead of the test compound solutions, respectively. Each sample was tested in three replicates. The 96-well transparent microplates were incubated at 25 °C, pH 8.0 ± 0.1 under a 12 h light/12 h dark photoperiod using a photon flux density of 80 μmol photon/m2·s and were shaken thrice daily. In the course of the whole experiment, microalgal culture conditions remained consistent. The fluorometric values reflecting microalgal biomass were measured by microplate reader. The measured initial and endpoint fluorescence values representing the microalgal biomass were used to calculate the percentage of growth inhibition, then the 96h-EC50 values were calculated. The above procedures were summarized in Fig. 1.
In order to investigate the reproducibility and feasibility of the established microplate microalgal biotest model, three repeated tests were conducted with six replicates per concentration of the positive control K2Cr2O7. The final concentrations of positive control K2Cr2O7 in each well of 96-well microplate (200 μL) were 16, 8, 4, 2, 1, and 0.5 μg/mL, respectively.
Bioassay-guided isolation for secondary metabolites from marine organisms
The secondary metabolites were isolated under the guidance of microalgal microscale model from marine organisms, including corals, gorgonians, sponges and their symbiotic microorganisms collected from the South China Sea. To assess the microalgal growth inhibition activity, a maximum amount of 100 µg for each extract or fraction was required. All of the compounds were isolated by using chromatographic techniques including column chromatography and semi-preparative HPLC. Taking a soft coral-derived fungus, Alternaria sp., as an example, the procedures of the bioassay-guided isolation for secondary metabolites from this fungus were as follows. The ethyl acetate extract of the fungal fermentation broth was tested for its microalgal growth inhibition activity. The active extract was then subjected to silica gel column chromatography using gradient elution with petroleum ether/ethyl acetate mixtures of increasing polarity. The fractions and subfractions were obtained and tested for their microalgal growth inhibition activities. Trough further bioassay-guided isolation by column chromatography and semi-preparative HPLC, pure compounds were obtained eventually. The similar approaches of bioassay-guided isolation were applied to the isolation of the secondary metabolites from other marine species. The structures of all the isolated compounds were elucidated by comprehensive analysis of spectroscopic data, including IR, UV, NMR, MS, and X-ray40,41,42.
Result and Discussions
Establishment of microalgal growth inhibition microscale model
In this study, the microalgal growth inhibition model was applied based on microalgal biomass. Different from the previous reported microalgal model with 96-well microplates32, the model in present study was operated with three marine microalgal species, K. mikimotoi, I. galbana and H. akashiwo because they are sensitive to tested substances and could be isolated and cultured easily in laboratory. K. mikimotoi and H. akashiwo were harmful red tide algae, while I. galbana was frequently used in microalgal growth inhibition tests as a standard microalga11. Through pre-experiments, the exponential growth phases of three microalgae were found to be in the periods of 5‒27, 3‒25, and 3‒23 days, respectively (see Supplementary Fig. S1). Subsequently, the growth curves for populations of these three microalgae in 96-well transparent microplates during 96 hours were inspected. During the period of 96 h, three microalgae grew with a huge variation and I. galbana grew fastest among these three microalgae (Fig. 2). It has been revealed that the tested sensitivity increased with decreasing initial microalgal cellular concentration43,44,45. Considering the maximum sensitivity of the bioassay and sufficient cells to determine cellular concentration changes over the test duration, most test protocols recommended an initial cellular concentration of 104 to 105 cells/mL46. In our study, the initial concentration of K. mikimotoi and H. akashiwo was chosen as 5 × 104 cells/mL, while that of I. galbana was twice. Based on the exponential growth phases, we choose the three microalgae at 6th day for K. mikimotoi, 21st day for I. galbana, and 15th day for H. akashiwo, to ensure the microalgal cells in exponential growth status and good cellular viability. Then, for the bioassay, we diluted and inoculated the microalgae to the wells of 96-well transparent microplate separately according to the designed initial microalgal cellular concentration above.
In order to define an appropriate measurement on microalgal growth status, we investigated the chlorophyll fluorescence assay. It is well known that the biomass of microalgae, in vivo chlorophyll a (Chl a) fluorescence has been widely used and the Chl a fluorescence values can be measured accurately, fast, and repeatedly in microplates using microplate fluorometers3,47. In our experiments, the measurement for Chl a fluorometric values was selected at the excitation wavelength of 485 nm and the emission wavelength of 680 nm. Nevertheless, when microalgal cell death occurs, the light absorption properties and Chl a fluorescence yield of their pigment antenna are modified resulting in fluorescence quenching47,48,49. It has been reported that a good linear relationship was observed between the Chl a concentration and intensity of chlorophyll fluorescence when the Chl a concentration was within the range of 0.1 μg/mL to 5.0 μg/mL26. Consequently, we investigated the correlations between microalgal cellular concentrations and fluorometric values. Based on the regression analyses, it could be found that the microalgal cellular concentrations and fluorometric values of the three microalgae exhibited linear relationships, respectively (Fig. 3). According to the microalgal cellular concentration at 96 h (Fig. 2), it could be found that the observed Chl a fluorescence values and the corresponding microalgal cellular densities were in the linear range. The above analysis spoke well for the feasibility of using automated fluorometric values to quantify the microalgal growth. Ultimately, we determined to quantify the microalgal growth through measuring the chlorophyll fluorescence values by a microplate reader. Furthermore, the observed endpoint was determined at 96 h according to the growth curves of the tested microalgae (Fig. 2) and the linear regression analyses between microalgal cellular concentrations and fluorometric values (Fig. 3). It should be pointed that considerable test deviations occurred when we calculated the microalgal growth inhibition rates at the time points of 48 h and 72 h.
By repeated experiments and observations based on the above consideration, a microalgal growth inhibition microscale model was finally established. It should be noted that the previous reported microalgal microscale models were aimed at detecting environmental toxic samples as well as pollution chemicals26,32. Differing from the previous reports, we modified the microalgal microscale model with optimized conditions in the aspects of target marine microalgae, microalgal tested period, cosolvent, excitation wavelength, and calculation method. Therefore, the established model is specifically suitable for the assessment of the chemoecological effects of marine natural products with small molecular and micro- or trace quantity from marine organisms.
The positive control and method validation
In our microalgal growth inhibition model, K2Cr2O7 was used as a positive control. To the best of our knowledge, there have not been any data available for the 96h-EC50 value of the K2Cr2O7 towards the three microalgae, K. mikimotoi, I. galbana, and H. akashiwo in microplates. These microalgae were widely used in tests in many fields, but there has been no report to evaluate their growth inhibition activities tested in Erlenmeyer flasks or microplates by using K2Cr2O7 as a positive control. For example, I. galbana was extensively used in testing the ecotoxicity of products such as petroleum in water, linear alkylbenzene sulfonate (LAS) and mental contaminant11,27,34. Whereas we noticed that K2Cr2O7 was used as a tested compound to evaluate the ecotoxicity towards other algal species, such as to Desmodesmus subspicatus with an EC50 value of 0.67‒0.80 µg/mL in 96-well microplate32. In the present study, the 96h-EC50 values were measured in 96-well microplate by fluorometric tests. The 96h-EC50 values of the positive control K2Cr2O7 towards the three microalgae, K. mikimotoi, I. galbana, and H. akashiwo were tested as 4.90‒5.29, 4.79‒5.24, 3.72‒4.02 µg/mL, respectively (Table 1). The reproducibility and feasibility of the established microplate microalgal biotest model were investigated. It was found that the relative standard deviation (RSD) of 96h-EC50 values of the positive control were 3.86% for K. mikimotoi, 4.51% for I. galbana, and 4.07% for H. akashiwo, respectively (Table 1), suggesting that the established model was feasible and stable. Specifically, the differences in microalgal biomass could be visually and qualitatively described with the aid of inverted fluorescence microscope. It could be clearly observed that the growth of three microalgal species was inhibited by K2Cr2O7, which is reflected in the cell viability and fluorescence intensity (Fig. 4).
Application of microalgal growth inhibition microscale model to marine natural products
The established model was applied to test the microalgal growth inhibition activities of the extracts, fractions, and scendary metabolites from soft corals, gorgonians, sponges and their symbiotic microorganisms collected from the South China Sea. As for Alternaria sp., a soft coral-derived fungus, the ethyl acetate extract of its fermentation broth showed inhibitory activity to K. mikimotoi with a growth inhibition rate of 76% at 50 μg/mL. The extract (30.2 g) was subjected to silica gel column chromatography to yield nine fractions (Fr. 1−Fr. 9). Two fractions, Fr. 6 and Fr. 7, were found to display microalgal growth inhibitory activities. Fr.6 exhibited the activity with the inhibition rates of 56% and 32% at the concentrations of 50 μg/mL and 10 μg/mL respectively, while Fr.7 with 96% and 84% at the same concentrations. Through further bioassay-guided isolation, two active compounds, tetrahydroaltersolanol E (6.0 mg, from Fr. 6) and altersolanol C (60 mg, from Fr.7), were obtained. By the same approaches as described for the soft coral-derived fungus Alternaria sp., other compounds were also isolated from corals, gorgonians, sponges and their symbiotic microorganisms under the guidance of microalgal growth inhibition model.
All of the isolated secondary metabolites were tested for their microalgal growth inhibition activities with the established microalgal microscale model. Preliminary screening of 116 isolated compounds from marine organisms resulted in the discovery of 15 active compounds, including four steroids (1‒4), six bisabolane sesquiterpenoids (5‒10), one scalarane sesterterpene (11), three anthraquinoids (12‒14) and one difurano-sesterterpene (15) (Fig. 5). These compounds were characterized by comprehensive spectroscopic data and identified as follows: muristeroid G (1)50 was isolated from gorgonian Anthogorgia caerulea; numersterol A (2)51 from soft coral Sinularia sp.; saringosterol (3)52 and suberoretisteroid C (4)3 from gorgonian Dichotella gemmacea; expansol B (5)53, anhydrowaraterpols A and B (6 and 7)54, (S)-(+)-sydonol (8)40, waraterpol (9)54 and disydonol B (10)40 from sponge-derived fungus Aspergillus sp.; cateriofenone A (11)41 from sponge Carteriospongia foliascens; tetrahydroaltersolanol E (12)42 and altersolanol C (13)42 from soft coral-derived fungus Alternaria sp.; rhodoptilometrin (14)55 and 12,13-didehydrofurospongin-1 (15)56 from sponge C. foliascens (see Supplementary Table S1). All of these active compounds exhibited growth inhibition activity against at least one of three marine microalgal species (Table 2). Specifically, altersolanol C (13) demonstrated potent activity against K. mikimotoi with the 96h-EC50 value of 1.16 µg/mL, which was more than 4 times stronger than that of the positive control K2Cr2O7 for K. mikimotoi. Eight compounds (1‒5, 7, 8, 14) showed more significant impact than other compounds on I. galbana with the 96h-EC50 values lower than or comparable to that of K2Cr2O7. For H. akashiwo, ten compounds (2, 4‒7, 9‒11, 14, 15) exhibited strong inhibitory activities with the 96h-EC50 values ranging from 2.74 to 10.3 µg/mL. Among these ten compounds, Expansol B (5) was the most powerful inhibitor against H. akashiwo with a EC50 value of 2.74 µg/mL, stronger than that of the positive control K2Cr2O7 (3.72 µg/mL) (Table 2). The above results demonstrated that different microalgal species displayed different sensitivities to the same compounds. Herein, it should be mentioned that some compounds were also found to be active on other chemoecological models. For instance, saringosterol (3) also showed lethal activity towards brine shrimp Artemia salina40, suberoretisteroid C (4) displayed antifouling activity against the larval settlement of barnacle Balanus amphitrite3, and suberoretisteroid C (4) and altersolanol C (13) exhibited ichthyotoxicity on the embryo of zebrafish Danio rerio5.
The above results revealed that the established model requires only very small amounts of the tested compounds, which is efficient for saving time and space. This model is suitable for the assessment of marine microalgal growth-inhibitory activity of marine natural products with micro- or trace quantity and could also be used in the bioactive screening and bioassay-guided isolation for the extracts and fractions from marine organisms.
Conclusion
In this study, a convenient and operable microalgal growth inhibition microscale model was established by modifying the microalgal microscale model with optimized conditions involving in target marine microalgae, microalgal tested period, cosolvent, excitation wavelength for fluorescence observation, and calculation method. The microaglal growth inhibition activity was tested by using marine microalgae, 96-well microplate, together with fluorescence measurement to quantify microaglal biomass. The practicality and feasibility of the established model were validated by application of this model to the evaluation of microalgal growth inhibition activity of marine natural products derived from marine organisms. Fifteen compounds isolated from soft corals, gorgonians, sponges and their symbiotic microorganisms collected from the South China Sea were found to display microalgal growth inhibition activity. In conclusion, this method could be applied to evaluation of microaglal growth inhibition activity of marine natural products. Admittedly, this method could also be applicable for a range of toxicant testing. It could be prospected that more abundant marine natural products with microalgal growth-inhibitory activities would be discovered by using this model, facilitating in-depth understanding the chemoecological effects of marine chemical defensive substances.
References
Reverter, M., Perez, T., Ereskovsky, A. V. & Banaigs, B. Secondary metabolome variability and inducible chemical defenses in the Mediterranean sponge Aplysina cavernicola. J Chem Ecol. 42, 60–70 (2016).
Wang, C. Y. et al. Chemical defensive substances of soft corals and gorgonians. Acta Ecol Sin. 28, 2320–2328 (2008).
Li, Y. X. et al. Antifouling activity of secondary metabolites isolated from Chinese marine organisms. Mar Biotechnol. 15, 552–558 (2013).
Lavrentyev, P. J., Franzè, G., Pierson, J. J. & Stoecker, D. K. The effect of dissolved polyunsaturated aldehydes on microzooplankton growth rates in the Chesapeake Bayand Atlantic coastal waters. Mar Drugs. 13, 2834–2856 (2015).
Bai, H. et al. Zebrafish embryo toxicity microscale model for ichthyotoxicity evaluation of marine natural products. Mar Biotechnol. 18, 264–270 (2016).
Epifanio, R. D. A., Maia, L. F. & Fenical, W. Chemical defenses of the endemic Brazilian gorgonian Lophogorgia violacea Pallas (Octocorallia, Gorgonacea). J Braz Chem Soc. 11, 584–591 (2000).
Qin, X. Y., Yang, K. L., Li, J., Wang, C. Y. & Shao, C. L. Phylogenetic diversity and antibacterial activity of culturable fungi derived from the zoanthid Palythoa haddoni in the South China Sea. Mar Biotechnol. 17, 99–109 (2015).
Greene, J. C., Miller, W. E., Shiroyama, T. & Maloney, T. E. Utilization of algal assays to assess the effects of municipal, industrial, and agricultural waste water effluents upon phytoplankton production in the Snake River system. Water Air Siol Poll. 4, 415–434 (1975).
Blanck, H., Wallin, G. & Wängberg, S. Å. Species-dependent variation in algal sensitivity to chemical compounds. Ecotox Environ Safe. 8, 339–351 (1984).
Blaise, C., Legault, R., Bermingham, N., Van Collie, R. & Vasseur, P. A simple microplate algal assay technique for aquatic toxicity assessment. Tox Assess. 1, 261–281 (1986).
Tsvetnenko, Y. & Evans, L. Improved approaches to ecotoxicity testing of petroleum products. Mar Pollut Bull. 45, 148–156 (2002).
Jiang, W., Akagi, T., Suzuki, H., Takimoto, A. & Nagai, H. A new diatom growth inhibition assay using the XTT colorimetric method. Comp Biochem Physiol C. 185, 13–19 (2016).
Geis, S. W. et al. Modifications to the growth inhibition test for use as a regulatory assay. Environ Toxicol Chem. 19, 36–41 (2000).
Burkiewicz, K., Synak, R. & Tukaj, Z. Toxicity of three insecticides in a standard algal growth inhibition test with Scenedesmus subspicatus. Bull Environ Contam Toxicol. 74, 1192–1198 (2005).
Smith, V. H. & Schindler, D. W. Eutrophication science: where do we go from here? Trends Ecol Evol. 24, 201–207 (2008).
Wu, L. et al. Bacilysin from bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. Appl Environ Microb. 80, 7512–7520 (2014).
Paerl, H. W. & Paul, V. J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 46, 1349–1363 (2012).
Lewis, M. A. Are laboratory-derived toxicity data for freshwater algae worth the effort? Environ Toxicol Chem. 9, 1279–1284 (1990).
Lewis, M. A. Use of freshwater plants for phytotoxicity testing: a review. Environ Pollut. 87, 319–336 (1995).
Walsh, G. E., Bahner, L. H. & Horning, W. B. Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans, and fishes. Environ Pollut. 21, 169–179 (1980).
Walsh, G. E., Duke, K. M. & Foster, R. B. Algae and crustaceans as indicators of bioactivity of industrial wastes. Water Res. 16, 879–883 (1982).
Walsh, G. E. & Garnas, R. L. Determination of bioactivity of chemical fractions of liquid wastes using freshwater and saltwater algae and crustaceans. Environ Sci Technol. 17, 180–182 (1983).
Blinova, I. The perspective of microbiotests application to surface water monitoring and effluents control in Estonia. Environ Toxicol. 15, 385–389 (2000).
Blinova, I. Use of freshwater algae and duck weeds for phytotoxicity testing. Environ Toxicol. 19, 425–428 (2004).
Gilbert, F., Galgani, F. & Cadiou, Y. Rapid assessment of metabolic activity in marine microalgae: application in ecotoxicological tests and evaluation of water quality. Mar Biol. 112, 199–205 (1992).
Satoh, A., Vudikaria, L. Q., Kurano, N. & Miyachi, S. Evaluation of the sensitivity of marine microalgal strains to the heavy metals, Cu, As, Sb, Pb and Cd. Environ Int. 31, 713–722 (2005).
Levy, J. L., Stauber, J. L. & Jolley, D. F. Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ. 387, 141–154 (2007).
Araújo, C. V. et al. Sediment integrative assessment of the Bay of Cádiz (Spain): an ecotoxicological and chemical approach. Environ Int. 35, 831–841 (2009).
Araújo, C. V., Diz, F. R., Lubián, L. M., Blasco, J. & Moreno-Garrido, I. Sensitivity of Cylindrotheca closterium to copper: Influence of three test endpoints and two test methods. Sci Total Environ. 408, 3696–3703 (2010).
OECD 201. Alga growth inhibition test. Test Guideline No. 201. OECD Guidelines for Testing of chemicals. Organization for Economic Cooperation and Development. Paris, France (1984).
Rojíčková, R., Dvořáková, D. & Maršálek, B. The use of miniaturized algal bioassays in comparison to the standard flask assay. Environ Toxicol Water. 13, 235–241 (1998).
Eisentraeger, A., Dott, W., Klein, J. & Hahn, S. Comparative studies on algal toxicity testing using fluorometric microplate and Erlenmeyer flask growth-inhibition assays. Ecotoxicol Environ Saf. 54, 346–354 (2003).
Arensberg, P., Hemmingsen, V. H. & Nyholm, N. A miniscale algal toxicity test. Chemosphere. 30, 2103–2115 (1995).
Garrido-Perez, M. C., Perales-VargasMachuca, J. A., Nebot-Sanz, E. & Sales-Márquez, D. Effect of the test media and toxicity of LAS on the growth of Isochrysis galbana. Ecotoxicology. 17, 738–746 (2008).
Hutchinson, T. H. et al. Extending the environmental risk assessment for oseltamivir (Tamiflu®) under pandemic use conditions to the coastal marine compartment. Environ Int. 35, 931–936 (2009).
Tzovenis, I. et al. Cryopreservation of marine microalgae and potential toxicity of cryoprotectants to the primary steps of the aquaculture food chain. Aquaculture. 230, 457–473 (2004).
Guillard, R. R. & Ryther, J. H. Studies on marine planktonic diatoms I. Cyclotella nana Hustedt, and Dentonula confervaceae (Cleve) Gran. Can J Microbiol. 8, 229–239 (1962).
ISO 8692. Water Quality-Freshwater Algal Growth Inhibition Test with Unicellular Green algae. International Organization for Standardization. Geneva, Switzerland (2004).
Sheng, X. M., Xiong, L. & Tang, H. F. The toxicity of two newly-synthesized compounds on scenedesmus obliquus kutz. Shuisheng Shengwu Xuebao. 30(6), 676–679 (2006).
Sun, L. L. et al. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria. Bioorg Med Chem Lett. 22, 1326–1329 (2012).
Cao, F. et al. Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia foliascens. Org Biomol Chem. 13, 4016–4024 (2015).
Zheng, C. J. et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J Nat Prod. 75, 189–197 (2012).
Vasseur, P., Pandard, P. & Burnel, D. Influence of some experimental factors on metal toxicity to Selenastrum capricornutum. Environ Toxicol. 3, 331–343 (1988).
Stratton, G. W. & Giles, J. Importance of bioassay volume in toxicity tests using algae and aquatic invertebrates. Bull Environ Contam Toxicol. 44, 420–427 (1990).
Moreno-Garrido, I., Lubián, L. M. & Soares, A. M. V. M. Influence of cellular density on determination of EC50 in microalgal growth inhibition tests. Ecotoxicol Environ Saf. 47, 112–116 (2000).
Stauber, J. L. Toxicity testing using marine and freshwater unicellular algae. Aust J Ecotoxicol. 1, 15–24 (1995).
Falkowski, P. G. & Raven, J. A. Aquatic Photosynthesis (2nd ed.). USA, Princeton (2013).
Lavaud, J., Rousseau, B. & Etienne, A. L. In diatoms, a transthylakoid proton gradient alone is not sufficient for non-photochemical fluorescence quenching. Febs Lett. 523, 163–166 (2002).
Lavaud, J., Rousseau, B., Van Gorkom, H. J. & Etienne, A. L. Influence in the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiol. 129, 1398–1406 (2002).
Sun, L. L. et al. New polyhydroxylated steroid from the South China Sea gorgonian Anthogorgia caerulea. Nat Prod Res. 27, 1159–1166 (2013).
Sheu, J. H., Chang, K. C. & Duh, C. Y. A cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J Nat Prod. 63, 149–151 (2000).
Cao, F., Liu, H. Y., Zhao, J., Fang, Y. C. & Wang, C. Y. New 19-oxygenated steroid from the South China Sea gorgonian Dichotella gemmacea. Nat Prod Res. 29, 169–173 (2015).
Wang, J. F. et al. Tetramic acid derivatives and polyphenols from sponge-derived fungus and their biological evaluation. Nat Prod Res 29, 1761–1765 (2015).
Henne, P. et al. Waraterpols, new penicillium metabolites and their derivatives. Eur J Org Chem. Liebigs Ann Chem. 22, 565–571 (1993).
Fain, V. Y., Zaitsev, B. E. & Ryabov, M. A. Tautomerism of the natural anthraquinones physcion and emodin and their analogs. Chem Nat Compd. 41, 501–507 (2005).
Schmitz, F. J. & Chang, J. C. Systerterpenes from a pacific sponge. Carteriospongia Flabellifera. J Nat Prod. 51, 745–748 (1988).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 41130858; 41322037; U1406403); the Fundamental Research Funds for the Central Universities (No. 201762017); the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02); and the Taishan Scholars Program, China.
Author information
Authors and Affiliations
Contributions
C.Y.W., C.L.S., X.X.T., Q.Z. and A.N.C. conceived the ideas and designed the methodology; Q.Z. and A.N.C. performed the fieldwork; Q.Z., A.N.C. and Q.L. collected the data; C.Y.W., Q.Z., A.N.C. and S.X.H. analysed the data; C.Y.W., Q.Z., A.N.C., M.C. and L.L. led the writing of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Q., Chen, AN., Hu, SX. et al. Microalgal Microscale Model for Microalgal Growth Inhibition Evaluation of Marine Natural Products. Sci Rep 8, 10541 (2018). https://doi.org/10.1038/s41598-018-28980-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28980-z
This article is cited by
-
Strong and widespread cycloheximide resistance in Stichococcus-like eukaryotic algal taxa
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.