Abstract
Certain plant cells synthesize secondary cell walls besides primary cell walls. This biosynthesis is strictly controlled by an array of transcription factors. Here, we show that SND1, a regulator of cell-wall biosynthesis, regulates abscisic acid (ABA) biosynthesis to ensure optimal plant growth. In Arabidopsis, the lack of SND1 and its homolog NST1 leads to the deficiency of secondary cell walls, preventing snd1nst1 double mutant seedlings from growing upright. Compared to wild type seedlings, the snd1 knockout mutant seedlings accumulated less anthocyanin and exhibited low tolerance to salt stress. Compared to wild type seedlings, the snd1 knockout seedlings were more sensitive to salt stress. Although SND1 can bind to the promoter of Myb46, we observed that SND1 binds directly to the promoter of the ABI4 gene, thereby reducing ABA levels under normal growth conditions. Thus, plants adjust secondary cell wall thickening and growth via SND1. SND1 has a dual function: it activates the Myb46 pathway, fostering lignin biosynthesis to produce sufficient cell wall components for growth, while maintaining a low ABA concentration, as it inhibits growth. This dual function of SND1 may help plants modulate their growth efficiently.
Similar content being viewed by others
Introduction
In addition to primary cell walls, plant cells also have secondary walls, composed of cellulose, lignin, and other molecules1. Because only certain types of plant cells can deposit secondary cell wall materials, including phenylpropanoid, during specific developmental phases, phenylpropanoid biosynthesis is strictly controlled by an array of genes2,3,4,5, which have been targeted to modify lignin content in order to manipulate biomass composition, as well as plant tolerance to abiotic stress6,7,8. Diverse transcription factors (TF) modulate various compounds in the phenylpropanoid biosynthesis pathway. AtMyb46 and its homologs AtMyb83, AtMyb58 and AtMyb63, play crucial roles in cell-wall biosynthesis9. Furthermore, NAC (NAM, ATAF1/2, and CUC2)-domain TFs are also xylem-associated, and 105 NAC genes with numerous functions exist in the genome of Arabidopsis thaliana10. Among these, the secondary wall-associated NAC domain protein 1 (SND1) is expressed in fibre-associated cells and plays a central role in fibre thickening3.
Complex and multifaceted signalling cascades, accompanied by various cellular responses, are activated in plants to ensure their survival under conditions of abiotic stress. Plant cells produce osmoprotectants to compensate for the water loss caused by salt, drought, or cold stress11. High salinity and drought may cause a marked imbalance in redox homeostasis, which drives plant cells to reorganize primary as well as secondary metabolism12,13. In this process, plant hormones serve as crucial integrators that modulate complex developmental and stress signalling pathways. Among them, abscisic acid (ABA) enables Arabidopsis to adapt to detrimental conditions imposed by abiotic stress and often triggers the inhibition of plant growth, thereby re-directing nutrients for successful withstanding of the specific stress conditions14.
Anthocyanins are recognized as part of the defence mechanism that plants use when challenged by stress. Indeed, they often accumulate in response to stress7. We aimed to verify whether SND1, the master controller of cell-wall biosynthesis, has any role under plant stress, as expression of SND1 is known to affect the accumulation of lignins which are produced from the same precursor of anthocyanins. Herein we report that SND1 directly regulates ABA biosynthesis to procure best possible plant growth under salinity stress. Furthermore, we show that SND1 binds directly to the promoter of the ABI4 gene, leading to low levels of ABA under saline conditions. Our observations suggest that plants can adjust secondary cell-wall thickening and growth performance via this SND1 regulatory effect, which displays a dual function by thickening secondary walls, while concomitantly reducing ABA content when environmental conditions are favourable plant growth.
Results
Altered anthocyanin content in the snd1ko mutant and SND1-overexpressing line and SND1 was induced by abiotic stresses
In our previous study, we showed that several genes involved in flavonoid biosynthesis participate in plant abiotic stress tolerance7,8. Plants accumulate a wide variety of flavonoids via phenylalanine through elaborate regulatory mechanisms15. There are several junctions in this pathway, leading to the synthesis of different types of flavonoid compounds. For example, coumaroyl CoA, which is utilized to produce anthocyanins via various enzymes including chalcone synthase (CHS)16, can be converted into lignins by hydroxycinnamoyl transferase (HCT). Thus, the synthesis of anthocyanin likely affects the synthesis of lignin, which belongs to the flavonoid family. SND1 is essential for the synthesis of lignin, and thereby for the formation of secondary cell walls3.
To determine whether the changes in lignin accumulation due to the changes in SND1 expression affect anthocyanin synthesis, we obtained seeds of the snd1ko mutant from TAIR and examined abiotic stress tolerance of this line. We then measured the anthocyanin content in the snd1ko mutant and in the SND1-overexpressing (SND1-OE) (Fig. S1). Notably, the anthocyanin content in four-day-old seedlings was lower in the mutant and higher in the SND1-overexpressing line than in the Col-0 wild type (WT). Under normal conditions, SND1 serves as a positive regulator of lignin synthesis. Therefore, overexpression of SND1 should theoretically increase lignin accumulation and decrease anthocyanin accumulation. In contrast, lignin content is expected to decrease and anthocyanin content to increase in the mutant. A similar observation has been previously reported, whereby the overexpression of SND1 reduced lignin biosynthesis3. These results indicate that SND1 is positively involved in the accumulation of anthocyanin. The expression of most flavonoid-related genes increased in the SND1-overexpressing line, but decreased in the snd1nst1 double mutant (Fig. S2). NST1 is a homologue of SND11,17, and marked effects on secondary wall biosynthesis are observed when both are deleted. Furthermore, we observed a decrease in the expression of PAP1 in the snd1nst1 double mutant (Fig. S2), which specifically activates the expression of genes associated with flavonoid synthesis. These results show that SND1 plays a positive role in the expression of genes associated with flavonoid biosynthesis, thereby increasing anthocyanin accumulation.
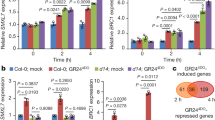
Anthocyanin is a part of the plant defence mechanism. In fact, anthocyanin often accumulates in response to stress7. We verified whether SND1 has a role in plant stress responses, as the expression of SND1 is known to affect the accumulation of anthocyanin. Thus, the transcript level of SND1 was measured in Col-0 by the qRT-PCR, upon treatment with different plant hormones and stresses. In the presence of salt or mannitol, the expression of SND1 increased. Similar results were observed with abscisic acid (ABA) treatment (Fig. 1). In particular, the expression of SND1 was significantly higher under salinity stress than under other stress conditions tested. These results indicate that SND1 is related to osmotic stress, especially in response to salinity stress, as well as to secondary cell wall synthesis.
Relative expression of the SND1 transcript in plants subjected to different hormone treatments or abiotic stresses. The relative expression of SND1 transcript in Col-0 was determined by the qRT-PCR. Eight-day-old seedlings were used to extract mRNA following the treatment with 10 µM abscisic acid (ABA), 10 µM indole-3-acetic acid (IAA), 10 µM jasmonic acid (JA), 10 µM salicylic acid (SA), 300 mM sucrose (Suc), 400 mM mannitol (Man), and 300 mM NaCl for 6 h. The error bars indicate the standard error (SE) of three replicates. The values with different letters were significantly different from that of WT plants (P < 0.05).
Reduced tolerance of the snd1ko mutant under salinity stress
As an extension of the above experiments, we verified whether altered anthocyanin biosynthesis due to SND1 mutation can affect abiotic stress responses in plants. As shown in Fig. 2A and B, the snd1ko mutant exhibited a very low survival rate under salinity stress, whereas that of the SND1-overexpressing line did not significantly differ from the WT. The WT showed a 20% survival rate 7 d after being transferred to the 200 mM NaCl condition, whereas the mutant showed a maximal survival rate of 5% under similar conditions. However, the difference between the SND1-overexpressing line and WT was negligible. By the seedling-transfer method, the phenotypes were confirmed under salinity stress. The snd1ko mutant exhibited a lower survival rate than the WT, whereas the survival rate of SND1-complementation (SND1-Com) line was a similar to that of the WT (Fig. S3). These results indicate that the changes in SND1 expression affect the tolerance to salinity stress in plants.
Phenotype and survival rates of Col-0, snd1ko mutant, and SND1-overexpressing line under salinity stress. (A) The phenotype of Col-0, snd1ko mutant, and SND1-overexpressing line under salinity stress. The seeds were sown on filter paper placed on normal medium. After 2 d, the filter paper was transferred on medium supplemented with 200 mM NaCl. The figure shows seedlings 20 d after the transfer. (B) Survival was quantified by counting the green cotyledons of seedlings. The experiments included 40 seeds per sample and the error bars indicate the standard error (SE) from three replicates. The asterisks represent significant differences with respect to Col-0 (P < 0.05).
To determine whether the altered sensitivity to salt stress in the snd1ko mutant resulted from the changes in the expression of ABA-responsive genes, the expression of these genes was determined by qRT-PCR in eight-day-old seedlings. In the snd1ko mutant, the expression of most ABA-responsive genes increased, particularly that of NCED318, which encodes the major enzyme involved in ABA synthesis, and was induced under salinity stress (Fig. 3A). In the experiments conducted with mannitol and NaCl, the snd1ko mutant exhibited higher expression of NCED3, whereas the expression was lower in the SND1-overexpression line than in the WT (Fig. 3B). These results suggest that the expression of ABA-responsive genes is negatively regulated by SND1, which might affect the biosynthesis of ABA due to the changes in NCED3 expression.
Transcript level of ABA-related genes in Col-0, snd1ko mutant, and transgenic plants in response to osmotic stress. (A) By the qRT-PCR, the relative expression of ABI1, ABI2, ABI3, ABI4, ABI5, CesA8, NCED3, NHL6, and NDR1 was determined in Col-0, snd1ko mutant, SND1-overexpressing line, and Myb46-overexpressing line/snd1ko after treatment with 200 mM NaCl for 6 h. (B) The relative expression of NCED3 in Col-0, snd1ko mutant, and SND1-overexpressing line under 10 µM ABA, 300 mM NaCl, or 400 mM Mannitol for 6 h. Eight-day-old seedlings were sampled for the analysis. The error bars indicate the standard error (SE) of three replicates. The asterisks represent significant differences from that of Col-0. The values with different letters were significantly different from that of WT plants (P < 0.05).
As SND1 is known to bind directly to the Myb46 and Myb83 promoters to enhance lignin biosynthesis4, we verified whether the enhanced transcript levels of ABA-responsive genes were due to these Myb genes in the snd1ko mutant. Thus, we generated a transgenic plant overexpressing Myb46 in the background of the snd1ko mutant, resulting in Myb46-OE/snd1ko seedlings. As the expression of ABA-related genes in Myb46-OE/snd1ko seedlings was similar to that of the snd1ko mutant, we concluded that Myb46 is not associated with the function of SND1, at least in terms of salt stress response (Fig. 3A).
Enhanced ABA accumulation in the snd1ko mutant than the wild type
As NCED3 belongs to the ABA biosynthesis pathway, we determined ABA content both in eight-day-old seedlings of the snd1ko mutant and SND1-complimentation (SND1-Com) line following treatment with NaCl. As observed for gene expression, the amount of ABA in the snd1ko mutant increased under both normal and salinity conditions; however, there was no significant difference between the SND1-complimentation and the WT lines (Fig. 4A). These results indicate that SND1 is involved in ABA signalling, as well as in the expression of ABA-responsive genes and in the regulation of ABA accumulation.
Abscisic acid (ABA) contents and germination rate of Col-0, snd1ko mutant, and transgenic plants. (A) The ABA content in Col-0, snd1ko mutant, and SND1-complementation line. Eight-day-old seedlings were used to extract ABA following treatment with 200 mM NaCl for 24 h. (B) Germination rate of Col-0, snd1ko mutant, and Myb46-overexpressing line/snd1ko in response to 5 or 10 µM ABA. The appearance of roots was regarded as germination 8 d after seeding on media supplemented with 5 or 10 µM ABA. The experiments included 120 seeds per sample, and the error bars indicate the standard error (SE) of three replicates. The asterisks represent significant differences from that of Col-0 (P < 0.05).
As SND1 regulates ABA accumulation, we verified whether the snd1ko mutant exhibits altered germination rates in response to ABA. To test this, we germinated Col-0, snd1ko, and Myb46-OE/snd1ko mutants in the presence of different concentrations of ABA in the medium (Fig. 4B). The snd1ko mutant showed lower germination rate in response to ABA than that in the WT. We could not revert this rate to that of WT even with the overexpression of Myb46 in the snd1ko mutant background. This indicates that SND1 is associated with ABA sensitivity during germination, as well as stress tolerance. Moreover, Myb46 is not associated with the function of SND1 in ABA response.
Direct binding of SND1 to the ABI4 promoter
As the snd1ko mutant accumulated more ABA than that of the WT, we reasoned that SND1 may control the expression of genes associated with ABA biosynthesis. This led us to examine whether SND1 binds to the promoter of NCED3 as its transcript level was enhanced in the snd1ko mutant (Fig. 5). We then performed chromatin immunoprecipitation (ChIP) to determine if SND1 can bind directly to the promoter of the NCED3 gene. Contrary to our expectation, SND1 did not bind to the NCED3 promoter (data not shown). We further tested the promoters of other genes (ABA anabolism-related genes: ZEP, SDR1, and AAO3; and a catabolism-related gene: CYP707A3) by the ChIP assay, but found no binding activity. However, when ABA signalling-related genes, including ABI3 and ABI4, were subjected to the ChIP assay, SND1 clearly bound to the promoter of the ABI4 gene, but not to the promoter of ABI3 (Fig. 5). SND1 bound to the region encompassing −981 to −1536 bp upstream (ABI4pro #1) of the ABI4 coding DNA sequence (CDS). We further characterized the SND1 binding site in the ABI4 promoter region by dividing them into several small sections (Fig. 5A). We analyzed cis-elements of ABI4 promoter region by PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)19. By qRT-PCR, the −1386 to −1536 (q#1) and −1079 to −1179 (q#4) regions were identified to be important for SND1-ABI4 promoter interaction (Fig. 5B). To confirm this result, we conducted a traditional yeast one-hybrid assay approach in which the ABI4 promoter sequence (ABI4pro #1) was used as a bait. As shown in Fig. 5C, the SND1 protein exhibited successful binding to the ABI4 promoter, whereas empty vector did not show any interaction. Moreover, the interaction between SND1 and ABI4 promoter was confirmed via the electrophoretic mobility shift assay (EMSA). We generated the protein of the SND1 NAC domain containing 191 amino acids, and conducted EMSA with the probe of ABI4 promoter. The ABI4 promoter q#1, which includes ABA-responsive element (ABRE), was used for the EMSA. In this experiment, we also found that the SND1 NAC domain binds directly to the ABI4 promoter, especially to the part of q#1 (−1386 to −1536 upstream from coding DNA sequence) (Fig. 5D). These results indicate that SND1 directly binds to ABI4 promoter in Arabidopsis.
Structure of the ABI4 promoter and chromatin immunoprecipitation (ChIP) using a SND1-GFP fusion protein. (A) cis-elements in the ABI4 promoter region. ABRE is an abscisic acid (ABA)-response element; GT1-motif and GAG-motif are light-response elements; CAAT-Box is a common cis-acting element. The PlantCARE program was used for ABI4 promoter analysis (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)19. ABI4pro#1, #1–1, and #1–2 show the size and location of the expected PCR products from the DNA template of ChIP. ABI4pro q#1, q#2, q#3, q#4, and q#5 present the size and location of the expected products of qRT-PCR products from the DNA template of ChIP. (B) The fold-enrichment values were calculated by dividing the ChIP signals obtained via the qRT-PCR by the signals obtained for the mock. The error bars indicate the standard error (SE). The asterisks represent significant differences among values (P < 0.05). (C) The yeast one-hybrid assay between SND1 and ABI4 promoter. Full-length SND1 was fused with GAL4 AD, and ABI4pro#1 was used as a bait. The ABI4pro-AbAi/pGADT7 yeast served as the negative control. Yeasts were cultured on SD/-Leu or SD/-Leu with 50 ng/mL aureobasidin A (AbA). (D) The electrophoretic mobility shift assay (EMSA) showed that the SND1 NAC domain (SND1 BD-His MBP) can bind to the biotin-labelled ABI4 promoter q#1.
Discussion
To actively cope with environmental threats, plants have developed various physiological and molecular mechanisms20. We have long been studying the responses of plants to environmental stress and have found that flavonoids play an important role in these resistance mechanisms8. In the present study, our findings reveal new functions of SND1 in ABA signalling. We hypothesized that reducing the lignin content via a knockout mutation of its master controller SND1 might enhance abiotic stress tolerance. Contrary to our expectation, we observed that the snd1ko mutation lowered the anthocyanin content, whereas it increased in the SND1 overexpression line (Fig. S1). As shown in Fig. S2, we observed altered expression of a variety of anthocyanin biosynthesis genes in both snd1ko and SND1-OE plants. These results suggest that SND1 plays a role in anthocyanin accumulation, besides it role in the synthesis of lignin3. However, the role of SND1 in anthocyanin biosynthesis has not been previously studied.
We investigated how the expression of SND1 varies upon various treatments with hormones under different growing conditions. The results revealed that the most significant change in SND1 was caused by salinity stress (Fig. 1). This change in the expression of SND1 in response to salinity stress may correlate with the reduced survival rate of snd1ko plants in the presence of salt at high concentration in the medium (Fig. 2). One possibility is that the snd1ko plants accumulate less anthocyanins, which serve as antioxidants. Another possibility is that SND1 functions as a transcriptional regulator of other genes, such as some of the ABA signalling genes. To evaluate this hypothesis, we screened a number of genes by the qRT-PCR to identify genes with altered transcript level in snd1ko, in response to salinity (Fig. 3A). In this analysis, the Myb46-OE/snd1ko line was included, as we wanted to know if Myb46, one of the target genes of SND1 in the lignin biosynthesis pathway21, can recover the altered phenotype of snd1ko when it is overexpressed in the snd1ko background. The results revealed that Myb46 is not involved in the SND1-driven alterations in terms of ABA related stress responses (Figs 3A and 4B). It has been reported that double knockout mutations of MYB46 and MYB83 result in secondary cell wall deficiency, thereby limiting plant growth21. Thus, the finding of the present study imply that SND1 likely drives different sets of genes to control ABA signalling and ABA biosynthesis.
In the search for SND1 target genes, we initially focused on the NCED3 gene, based on the qRT-PCR results (Fig. 3B). The NCED genes encode 9-cis-epoxycarotenoid dioxygenase, which catalyzes the cleavage of 9-cis-epoxycarotenoid to xanthoxin in the key regulatory ABA biosynthesis step22. NCED3 is important for ABA biosynthesis during drought23, as evidenced by the fact that nced3 mutants exhibited increased water loss and low ABA content23. Thus, the snd1ko plants may accumulate more ABA than WT, as the NCED3 transcript level was found to be higher in this mutant (Fig. 3B). In accordance with this result, we observed that the snd1ko plants accumulated more ABA than WT (Fig. 4A). The SND1-complementation line exhibited the recovery of snd1ko in terms of ABA content. Moreover, enhanced ABA content in the snd1ko plants seems to confer increased germination sensitivity to ABA (Fig. 4B). However, by the ChIP analysis, we found that SND1 does not bind to the promoter of NCED3 (data not shown). Thus, we performed the ChIP analysis with other possible candidate genes, which revealed that the ABI4 gene is an SND1 target. As shown in Fig. 3A, the ABI4 transcript level was enhanced in the snd1ko mutant. Our detailed ChIP analysis and yeast one hybrid assay clearly demonstrated that SND1 directly bound to the ABI4 promoter (Fig. 5). Moreover, the result of EMSA showed that SND1 interacts with promoter fragment of −1386 to −1536 from ABI4 coding DNA sequence. ABI4 also has been shown to positively regulate seed dormancy through ABA biosynthesis by directly binding to the promoters of CYP707A1 and CYP707A2, thereby, repressing their expression24. Arabidopsis CYP707A encodes ABA 8′-hydroxylase, which plays key roles in ABA catabolism25, as indicated by the observation that the enhanced expression level of CYP707A1 and CYP707A2 in the abi4 ko mutant reduced ABA content24. However, we did not observe any difference in the transcript levels of CYP707A1 and CYP707A2 between in WT and the snd1ko mutant (data not shown). In contrast, ABI4 is known to increase the expression of CHO1 which is an activator of NCED326. Hence, the increase in ABA content in the snd1ko mutant was not caused by the suppression of CYP707A1/2, but by the genes CHO1 and NCED3.
Abscisic acid frequently plays a central role in adaptation of plants to environmental stress27,28. In the present study, we demonstrated that SND1 has more than one function; it can reduce ABA biosynthesis via the repression of ABI4 (Figs 3 and 5), and it can also enhance lignin biosynthesis through Myb46 activation21. Furthermore, ABA has been shown to reprogram transcriptional schemes for improved adaptation to abiotic stress29. In this pathway, ABA bound to PYR/PYL/RCAR (PYRABACTIN RESISTANCE1/PYR1-LIKE/REGULATORY COMPONENTS OF ABA RECEPTORS) receptors, and inhibits ABI1, ABI2, HAB1, and PP2CA protein phosphatases, thus activating SnRK2 protein kinases18. In addition to triggering the stress defence pathways and closing of stomata, one important function of ABA is to inhibit seedling growth. A transcriptional mechanism associated with the inhibition of the cell cycle30 and metabolism31 has been proposed in association with ABA function. Moreover, ABA modulates the function of PM H+-ATPase32 and nutrient transporters to adjust of plant growth33. On the basis of our results, we propose a working model of the dual function of SND1 (Fig. 6). Under normal conditions, plants can allocate all available resources to the growing points; however, they have to redirect them under growth-limiting conditions, of one kind or another. Therefore, it is plausible to reason that SND1 activates Myb46, thereby fostering the biosynthesis of lignins to produce sufficient cell wall components needed for plant growth, while maintaining a low concentration of ABA, a growth inhibitor. Such dual function of SND1 may help plants to modulate their growth patterns to suit specific growth environmental conditions and, thus, thrive in the best possible way under such circumstances.
Working model of SND1, negatively regulating ABI4 in the ABA biosynthesis pathway. SND1 is a master switch for the formation of secondary cell walls through the induction of Myb46 under normal conditions. For a strong and successful plant growth, SND1 directly binds to the ABI4 promoter and negatively regulates the expression of the ABI4 transcript, inhibiting of the ABA biosynthesis pathway. SND1 is induced by osmotic stress and inhibits continuous ABA production under these conditions.
Methods
Plant material and growth conditions
The seed coat of all experimental materials was sterilized. After 3 d at 4 °C in dark, the seeds were sown on half-strength Murashige and Skoog (MS) medium supplemented with 2% sucrose (pH 5.7), as the normal growing condition. The seedlings were grown in a growth chamber at 23 °C and 60% relative humidity under long-day conditions (light 16 h, dark 8 h) for all experiments. Arabidopsis thaliana ecotype Colombia-0 was used as the wild type (WT). The snd1 knockout mutant (SALK_015495.54.50.x) was obtained from the Arabidopsis Biological Resource Center (ABRC).
DNA construction and generation of transgenic plants
Coding DNA sequences (CDS) of SND1 and Myb46 were amplified by the RT-PCR and cloned into the TOPO vector (pCR™8/GW/TOPO® TA Cloning Kit, Invitrogen). The SND1-TOPO vector construct was subcloned into the pMDC32 vector34 to generate the SND1 overexpressing lines, and the pMDC83 vector34 for SND1-GFP fusion. The Myb46-TOPO vector construct was subcloned into the pMDC32 vector. For SND1 complementation, a DNA construct including the sequence −1000 bp upstream to the coding DNA sequence (CDS) was cloned into the TOPO vector. The vector was subcloned into the pMDC100 vector34 to generate the SND1 complementation line. The subcloning constructs were transformed into Agrobacterium tumefaciens (GV3101) by electroporation. The floral dipping method was used for plant transformation35. Background plant of the SND1-overexpressing line and the SND1 GFP line was Col-0. The Myb46-OE/snd1ko and SND1-complementation lines were generated in a snd1 knockout mutant background.
RNA isolation and quantitative real-time RT-PCR
The total RNA was isolated from eight-day-old seedlings following treatment with 10 µM ABA, 10 µM Indole-3-acetic acid (IAA), 10 µM Jasmonic acid (JA), 10 µM Salicylic acid (SA), 200 mM NaCl, 300 mM NaCl, 400 mM mannitol, or 200 mM sucrose for 6 h. The cDNA was synthesized using the total mRNA samples from eight-day-old seedlings and a cDNA synthesis kit (RevertAid First Strand cDNA Synthesis Kit, ThermoScientific). For quantitative real-time PCR (qRT-PCR), the cDNA was amplified using the EvaGreen MasterMix (BrightGreen qPCR MasterMix, Abm). Actin2 was used as an internal control; gene primers for the qRT-PCR are listed in Table S1.
Salinity stress phenotype analysis
The seeds were sown on filter paper placed on half-strength MS medium supplemented with 2% sucrose (pH 5.7). Two days after seeding, the filter papers with seedlings were moved onto a medium supplemented with 200 mM NaCl.
Determination of ABA content
The content of ABA was measured as previously described36. The samples of eight-day-old seedlings were used to extract ABA after treatment with 200 mM NaCl for 24 h. The content of ABA was measured using the Phytodetek Elisa kit according the instructions of the manufacturer.
Analysis of cis-regulatory elements and chromatin immunoprecipitation
The cis-elements in ABI4 promoter were analyzed by PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)19. The chromatin immunoprecipitation (ChIP) was performed with modifications to the method originally reported37. Fourteen-day-old SND1GFP seedlings were used to extract nuclear content with cross-link. An antibody against GFP (G1544, Sigma, https://www.sigmaaldrich.com/) was used for immunoprecipitation. The purified DNA was quantified by the qRT-PCR using the specific primers listed in Table S1.
Yeast one-hybrid assay
Full-length SND1 was fused in frame with the GAL4 activation domain of pGADT7 AD vector. The bait construct of ABI4 promoter region (ABI4pro Sac1 F: GAGCTCTAGTTTTTACTTATGTCCAAAAATATGA, and ABI4pro Kpn1 R: GGTACCTAGTAAAAGATCTAAATGCATTTTTAAT) was cloned into the pAbAi vector. The recombinant plasmid pGADT7-SND1 and ABI4 promoter bait plasmid were co-transformed into the yeast strain Y1HGold (Clontech) as described in the instruction of the manufacturer (Matchmaker® Gold Yeast One-Hybrid Library Screening System, Clontech). The yeast containing pGADT7-empty vector and ABI4 promoter bait were used as control. The transformants were cultured in SD/-Leu medium, and then transferred to SD/-Leu medium supplemented with 50 ng/mL aureobasidin A (AbA).
Protein purification and electrophoretic mobility shift assay
To obtain recombinant protein for the electrophoretic mobility shift assay (EMSA), the DNA fragment of SND1 NAC domain was subcloned into pMAL-His-C2X vector, which is a recombinant pMAL-C2X vector (NEW ENGLAND BioLabs). The 6x-histidine (His) tag was fused in-frame with the C-terminus of maltose-binding protein (MBP) of pMAL-His-C2X vector. The primer for SND1 NAC domain cloning is listed in Table S1. The construct obtained was transformed into E-coli strain BL21 (DE3). To express the recombinant protein, the recombinant E-coli was grown in LB liquid medium at 37 °C until the OD (600) reached 0.7, and then 0.1 mM isopropyl-b-D-thiogalactopyranoside was added to the medium and the cells were grown again at 18 °C for 18 h. After centrifugation at 3500 rpm for 1 h, the supernatant was removed and the bacteria were stored at −20 °C until the isolation of protein. The Ni-His tag column was used to purify the recombinant protein. The AIB4 promoter fragment was amplified by the PCR with both 5′ and 3′ biotin-labelled primers. The primer sequences for the ABI4 promoter fragment were similar to those of the ABI4pro q#1 primer for the ChIP assay. The EMSA assay was conducted according to the instructions provided in the LightShift® Chemiluminescent EMSA kit insert (Thermo Scientific).
Germination rate analysis
The seeds were sown on half-strength MS medium supplemented with 2% sucrose (pH 5.7) or ABA-supplemented medium. Eight days after seeding, the roots were counted to analyse germination rates. One-hundred seeds were used for the analysis and three independent experiments were conducted.
Statistical analyses
Each experiment was replicated at least thrice. The statistical analyses were performed by the one-way ANOVA, followed by Tukey’s test for comparison of means at 95% confidence level.
Gene accession number
The accession numbers of the genes used in this study are as follows: ABF1 (AT1G49720), ABF2 (AT1G45249), ABF3 (AT4G34000), ABF4 (AT3G19290), ABI1 (AT4G26080), ABI2 (AT5G57050), ABI3 (AT3G24650), ABI4 (AT2G40220), ABI5 (AT2G36270), CesA8 (AT4G18780), COR15a (AT2G42540), CHI (AT3G55120), CHS (AT5G13930), DFR (AT5G42800), F3H (AT3G51240), F3′H (AT3G51240), FLS1 (AT5G08640), LDOX (AT4G22880), Myb46 (AT5G12870), NCED3 (AT3G14440), NDR1 (AT3G20600), NHL6 (AT1G65690), NST1 (AT2G46770), PAP1 (AT1G56650), RD29a (AT5G52310), RD29b (AT5G52300), SND1 (AT1G32770).
References
Mitsuda, N., Seki, M., Shinozaki, K. & Ohme-Takagi, M. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17, 2993–3006 (2005).
Boerjan, W., Ralph, J. & Baucher, M. Lignin biosynthesis. Ann. Rev. Plant. Biol. 54, 519–546 (2003).
Zhong, R., Demura, T. & Ye, Z. H. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibres of Arabidopsis. Plant Cell 18, 3158–3170 (2006).
Zhong, R., Richardson, E. A. & Ye, Z. H. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19, 2776–2792 (2007).
Nguyen, N. H. et al. MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J. 84, 1192–1205 (2015).
Sattler, S. E., Funnell-Harris, D. L. & Pedersen, J. F. Brown midrib mutations and their importance to the utilization of maize, sorghum, and pearl millet lignocellulosic tissues. Plant Sci. 178, 229–238 (2010).
Lee, W. J. et al. Drastic anthocyanin increase in response to PAP1 overexpression in fls1 knockout mutant confers enhanced osmotic stress tolerance in Arabidopsis thaliana. Plant Cell Rep. 35, 2369–2379 (2016).
Kim, J. et al. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 36, 1215–1224 (2017).
Zhou, J., Lee, C., Zhong, R. & Ye, Z. H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 21, 248–266 (2009).
Olson, A. N., Ernst, H. A., Leggio, L. L. & Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 10, 79–87 (2005).
Hasegawa, P. M., Bressan, R. A., Zhu, J. K. & Bohnert, H. J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 51, 463–499 (2000).
Joo, J. et al. Intergenic transformation of AtMYB44 confers drought stress tolerance in rice seedlings. Appl. Biol. Chem. 60, 447–455 (2017).
Miller, G., Suzuki, N., Ciftci-Yilmaz, S. & Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467 (2010).
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R. & Abrams, S. R. Abscisic acid: emergence of a core signalling network. Annu. Rev. Plant Biol. 61, 651–679 (2010).
Petroni, K. & Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 181, 219–229 (2011).
Wang, G. F. & Balint-Kurti, P. J. Maize homologs of CCoAOMT and HCT, two key enzymes in lignin biosynthesis, form complexes with the NLR Rp1 protein to modulate the defence response. Plant Physiol. 171, 2166–2177 (2016).
Mitsuda, N. et al. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19, 270–280 (2007).
Barrero, J. M. et al. The ABA1 gene and carotenoid biosynthesis are required for late skotomorphogenic growth in Arabidopsis thaliana. Plant Cell Environ. 31, 227–234 (2008).
Lescot, M. et al. PlantCARE a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327 (2002).
Fujii, H. et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature 462, 660–664 (2009).
McCarthy, R. L., Zhong, R. & Ye, Z. H. MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 50, 1950–1964 (2009).
Lefebvre, V. et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 45, 309–319 (2006).
Iuchi, S. et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 27, 325–333 (2001).
Shu, K. et al. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. Plos Genet. 9, e1003577 (2013).
Kushiro, T. et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23, 1647–1656 (2004).
Yamagishi, K. et al. CHOTTO1, a double AP2 domain protein of Arabidopsis thaliana, regulates germination and seedling growth under excess supply of glucose and nitrate. Plant Cell Physiol. 50, 330–340 (2009).
Li, Y. et al. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res. 16, 414–427 (2006).
Hey, S. J., Byrne, E. & Halford, N. G. The interface between metabolic and stress signalling. Ann. Bot. 105, 197–203 (2010).
Planes, M. D. et al. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 66, 813–825 (2015).
Wang, H. et al. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 15, 501–510 (1998).
Penfield, S., Li, Y., Gilday, A. D., Graham, S. & Graham, I. A. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18, 1887–1899 (2006).
Haruta, M. & Sussman, M. R. The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol. 158, 1158–1171 (2012).
Sano, T. et al. Plant cells must pass a K+ threshold to re-enter the cell cycle. Plant J. 50, 401–413 (2007).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Liu, N., Ding, Y., Fromm, M. & Avramova, Z. Endogenous ABA extraction and measurement from Arabidopsis leaves. Bio.Protoc. 4, e1257 (2014).
Saleh, A., Alvarez-Venegas, R. & Avramova, Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 3, 1018–1025 (2008).
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (to Hojoung Lee, 2017; grant NRF-2017R1A2B4008706).
Author information
Authors and Affiliations
Contributions
Chan Young Jeong: qRT-PCR and ChIP assay, EMSA, Yeast one-hybrid assay, and MS writing. Won Je Lee: Anthocyanin detection. Hai An Truong and Cao Sơn Trịnh: Complementation assay. Joo Yeon Jin: Abiotic stress test. Sulhee Kim and Kwang Yeon Hwang: Protein purification. Chon-Sik Kang: ABA extraction. Joon-Kwan Moon: Sodium contents determination. Suk-Whan Hong and Hojoung Lee: Experiment designs and MS writing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, C.Y., Lee, W.J., Truong, H.A. et al. Dual role of SND1 facilitates efficient communication between abiotic stress signalling and normal growth in Arabidopsis. Sci Rep 8, 10114 (2018). https://doi.org/10.1038/s41598-018-28413-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28413-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.