Abstract
Duckweed is a valuable feedstock for bioethanol production due to its high biomass and starch accumulation. In our preliminary experiment, we found that abscisic acid (ABA) could simultaneously increase starch and biomass accumulation of duckweed, but the mechanisms are still unclear. The results showed that the biomass production of duckweed reached up to 59.70 and 63.93 g m−2 in 6 days, respectively, with an increase of 7% (P < 0.05) compared to the control. The starch percentage increased from 2.29% up to 46.18% after 14 days of treatment, with a total of starch level 2.6-fold higher than that of the control. Moreover, the level of endogenous ABA, zeatin-riboside (ZR) and indole-3-acetic acid (IAA) increased, while gibberellins (GAs) decreased. Notably, ABA content in treated samples reached 336.5 mg/kg (fresh weight), which was 7.5-fold greater than that of the control. Importantly, the enzyme activities involved in starch biosynthesis increased while those catalyzing starch degradation decreased after ABA application. Taken together, these results indicated that ABA can promote biomass and starch accumulation by regulating endogenous hormone levels and the activity of starch metabolism related key enzymes. These results will provide an operable method for high starch accumulation in duckweed for biofuels production.
Similar content being viewed by others
Introduction
With an ever-increasing demands for energy, energy shortages have received increasing attention worldwide. Moreover, some nations still rely on the consumption of traditional resources, such as coal and crude oil, which may cause air pollution and other environmental problems1. Renewable and clean bioenergy will be an alternative resource to solve the energy crisis and environmental pollution. Bio-ethanol is a gasoline alternative that can reduce greenhouse gas (GHG) emissions when it is blended as an additive2. Therefore, it is urgent to explore novel and sustainable feedstocks (starch sources) for bio-ethanol production.
Duckweed, as the smallest flowering plants in the world, grows faster than most other higher plants3. Under ideal conditions, duckweed can double its biomass in 16–48 hours. The growth rate of duckweed can reach 12.4 g/m2/day dry weight, and its yield has been documented to reach 55 tons/hectare/year dry weight4. Duckweed can absorb nutrients such as nitrogen and phosphorus from wastewater, assimilating CO2 through photosynthesis and converting it into high-quality raw materials. Moreover, duckweed can accumulate starch up to 64.9% dry weight5. High starch accumulation but low lignin content has allowed successful conversion to bioethanol in recent years6. Therefore, duckweed is a promising new non-grain feedstock with starch that is expected to partially solve the problem that the fuel ethanol industry faces due to the lack of starchy materials.
The rapid accumulation of high-quality and high-quantity duckweed is considered the key step in its conversion to bio-ethanol. Previous studies have reported that many factors can affect duckweed biomass and starch accumulation, such as nutritional conditions (starvation stress)7, CO2 concentration8, plant hormones and light intensity9,10,11. High starch content can be realized under nutrient starvation stress, but cannot be used to treat sewage. It has been reported that elevated CO2 can also promote starch accumulation in duckweed. However, it is difficult to achieve large-scale outdoor production8. In addition, plant-growth regulators (PRGs) play an important role in plant growth and yield. In particular, ABA is a universal phytohormone involved in various physiological processes of plants, including seed development, improved growth and abiotic stress tolerance, and increasing carbohydrate accumulation in higher plants12. For example, the dry biomass yield was increased up to 2.1-fold, and saturated fatty acid content was promoted 11.17% compared with the control in Scenedesmus quadricauda after ABA supplement13. ABA seems to play an essential role in starch accumulation, but few studies have reported the application of ABA in duckweed. Wang14 has reported using ABA (added to cultures) to induce turion formation in Spirodela polyrhiza. However, turion is a special structure of Spirodela polyrhiza with high starch content, found at the bottom of water bodies, and would be difficult to harvest in future applications. Importantly, through a large number of pre-experiments, high starch accumulation of Landoltia punctata was obtained after spraying ABA on the surface of fronds.
This study shows for the first time that foliar spraying of ABA on the surface of Landoltia punctate increases starch accumulation in fronds. The objectives of this research were to determine the effect of ABA on the growth and the carbohydrate metabolism of duckweed and the underlying mechanism. The biomass of duckweed, starch accumulation, endogenous hormone content and key enzymes of carbohydrate metabolism were discussed. This work provides biochemical and physiological insights into L. punctata high-starch accumulation after ABA treatment. It also shows the dual benefits of duckweed: clean-up of sewage and production of potentially high-quality feedstock for biofuels.
Results
Effect of ABA on biomass accumulation of L. punctata
Application of ABA significantly affected biomass accumulation of L. punctata. The results showed that ABA can promote biomass yield compared with the control samples (Fig. 1). The dry weight of duckweed was 29.69 and 33.39 g m−2, with an increase of 12% in ABA treatment samples compared with controls on the 1st day (P < 0.05). By the 6th day, the biomass production of duckweed reached up to 59.70 and 63.93 g m−2, respectively, with an increase of 7% (P < 0.05) compared to that of the control (P < 0.05). At 14th day, the dry weight has reached a steady-state condition, with the dry weight of 79.51 and 79.98 g m−2, respectively.
Impact of ABA on starch accumulation of L. punctata
Besides biomass production, starch accumulation is another key consideration for duckweed application in biofuel production. As shown in Fig. 2, the results demonstrated that the starch content was 2.29% (DW) at 0 day, but reached 11.20% on the 1st day after ABA treatment. This content was 3.6-fold higher than the control sample, with a content of 3.12% (P < 0.05). The starch content reached 19.26% (DW) by the 3rd day and finally reached 46.18% (DW) after ABA treatment on the 14th day. However, in the control samples, the starch content remained steady at 4.78% by the 3rd day and 17.76% on the 14th day. On the last day, the content of treatment samples was 2.6-fold higher than that of the control (P < 0.05).
Effect of ABA on L. punctata chlorophyll content and photosynthesis
The effects of ABA application on photosynthetic pigments were also investigated in this study. As shown in the Fig. 3, chlorophyll a content decreased from an initial value of 1.27 to 0.96 mg/g (FW) following treatment, but there was little change in the control sample, which was 1.27 mg/g (FW) at day 0, and maintained at 1.26 mg/g (FW) at day 7. Similarly, chlorophyll b content decreased from an initial value of 0.46 to 0.33 mg/g (FW) following treatment. There was no change in the control samples.
To investigate the effect of ABA on photosynthesis, net photosynthesis rates were also measured, as shown in Fig. 4. The results showed that the net photosynthesis rate slightly increased from 8.54 to 9.38 μmol CO2/m2/s and decreased to 2.44 CO2/m2/s in the control and the ABA treatment samples, respectively. During this period, ABA treatment obviously decreased the net photosynthesis rate of duckweed by 4.5-fold when compared with the control.
Alteration of endogenous ABA, GAs, ZR and IAA levels in L. punctata
In this study, to analyze the response of endogenous hormones after ABA treatment, the levels of endogenous phytohormones, including ABA, GA1 + 3, ZR and IAA, were measured.
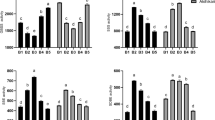
Endogenous ABA content rapidly rose from 36.3 to 262.0 mg/kg (FW) after ABA application. On the other hand, ABA levels were 37.0 mg/kg (FW) in control samples during the 1st day (Fig. 5A). By the 7th day, ABA content reached 336.5 mg/kg (FW) after ABA application, while there was little change in the control samples. Thus, endogenous ABA levels were 7.5-fold higher after ABA treatment than in the control samples. However, GA content decreased from 6.3 to 4.1 mg/kg (FW) following treatment, compared with 6.2 mg/kg (FW) in the control sample after 7 days of growth (Fig. 5B).
ZR (zeatin-riboside) content increased from 6.1 to 8.5 and 9.6 mg/kg (FW) in the control and ABA treatment samples, respectively (Fig. 5C). The level of ZR was much higher in the treatment sample than that of the control. As described in Fig. 5D, IAA content increased from 21.9 to 107.6 mg/kg (FW) after ABA application, which was 1.6-fold more than the control, with a content of 68.1 mg/kg (FW).
Effect of ABA application on activities of enzymes involved in starch metabolism
Several enzymes participate in the process of starch accumulation, and the activity of these enzymes seriously affects the accumulation of starch in plant. The key enzymes involved in starch biosynthesis (ADP-glucose pyrophosphorylase (AGPase) and soluble starch synthase (SSS)) and key enzymes (alpha-amylase (α-amylase) and beta-amylase (β-amylase)) related to starch catabolism were assayed in this study (Fig. 6).
The activity of AGPase increased from 8.37 to 10.98 U/mgprot (units per milligram protein) in the first 4 days in the control sample. It immediately rose from 8.37 to 27.53 U/mgprot after ABA application, which is a 2.5-fold increase compared with control samples (Fig. 6A). The activity of AGPase decreased slightly to 15.62 and 10.95 U/mgprot in ABA the treatment and control samples, respectively. The rising trend of SSS activity was similar to that of AGPase. SSS activity increased from the initial 8.46 to 30.50 U/mgprot in the first 4 days and then decreased to 18.47 U/mgprot by day 7 after ABA application. Meanwhile, SSS activity did not change much in the control samples (Fig. 6B).
Figure 6C,D showed the variation of α-amylase and β-amylase activities in different treatments. The activity of α-amylase gradually decreased after ABA application, while there was little change in the control samples. The activity of α-amylase decreased from 10.36 to 6.02 U/mgprot by the 7th day in the treatment samples. The activity of α-amylase reached 10.16 U/mgprot by the 7th day, which is 1.7-fold higher than that of the treatment samples (Fig. 6C). Additionally, the activities of β-amylase remarkably decreased from 33.98 to 3.31 U/mgprot by the 7th day, the final activity was 1/10 of the original activity. The activity of β-amylase maintained a stable level from 33.98 to 33.07 U/mgprot by day 7, which is 10-fold that of the ABA treatment samples (Fig. 6D).
Discussion
Biomass production and starch accumulation are two key consideration in duckweed biofuel production. High-starch duckweed is a valuable feedstock for biofuel production. In this study, after culturing for 14 days, the starch content reached 46.18% in the treated samples and 17.76% in the control samples from an initial content of 2.30% (Fig. 2). As a result, combined with the biomass production the total starch content in the treated samples was 2.6 times higher than that in the control samples. The results are consistent with those obtained from other studies. For example, reports have shown that ABA can promote starch biosynthesis in rice culture cells15. In this study, both the biomass yield and starch accumulation were increased by ABA application compared to the control. Therefore, ABA application may be regarded as an effective method to increase starch and biomass accumulation for industrial duckweed cultivation.
Chlorophyll(Chl) is the most important photosynthetic pigment in the chloroplast of plants, which plays an essential role in absorbing light and transferring light energy in the antenna systems16. In this study, the results indicated that the chlorophyll content of duckweed decreased, triggered by exogenous ABA, and similar results were observed in other reports. ABA plays a positive role in the process of leaf senescence, and exogenously applied ABA can accelerate Chl degradation in Arabidopsis17. Moreover, it has long been known that exogenously applied ABA can induce the expression of senescence-associated mRNAs and can reduce chlorophyll content in detached leaves18.
Exogenous ABA application also decreased the net photosynthetic rate (Pn) in this study. These results are in agreement with those obtained by Li et al.19, who reported that the net photosynthesis rates decreased from 13.8 to 2.2 μmol CO2/m2/s after ABA treatment compared to the control of 13.5 μmol CO2/m2/s. In this study, the change of the net photosynthetic rate is consistent with the decrease of chlorophyll content.
Exogenous ABA can promote starch accumulation and affect the levels of endogenous phytohormones in plants. In this study, the level of endogenous ABA, ZR and IAA increased, while GAs decreased by ABA treatment (Fig. 5). The similar observations were also reported in other studies. Reports have shown that the expression of genes encoding starch degradation enzymes such as a-amylases and proteases were induced by GA but suppressed by ABA20. ABA promoted starch accumulation in rice cell cultures, and the gene expression of large subunits of ADPase was also up-regulated after ABA treatment15. Moreover, Wang21 investigated the physiological responses of kiwifruit plants to exogenous ABA under drought conditions and showed that ABA induced higher endogenous ABA content than the control after ABA treatment. Importantly, in this study, the increase in ABA and decrease in GAs also supported starch accumulation in duckweed. Therefore, ABA treatment might enhance starch accumulation by blocking synthesis of GAs and enhancing ABA biosynthesis in duckweed.
Cytokinins are a group of phytohormones that promote cell division and cell differentiation in plant roots and shoots but that also affect biomass and starch accumulation22. Hence, in this study, increased ZR promoted the biomass accumulation of duckweed by regulating cell division and differentiation. Moreover, a report has shown that ZR plays an important role in regulating starch accumulation patterns and consequently influences starch percentage23. The increase of ZR plays a role in promoting biomass yield and starch accumulation in duckweed. Indole-3-acetic acid (IAA) is considered to be the primary auxin in plants and stimulates growth through cell elongation and plant growth. In this study, the levels of IAA remarkably increased from 21.9 to 107.6 mg/kg (FW) after ABA application. The increase of ZR and IAA may be another important factor that influence duckweed biomass accumulation.
The alteration of the endogenous ABA, GAs, ZR and IAA co-regulates starch metabolism in L. punctata. The endogenous hormone content changed dramatically following ABA application (Fig. 6). In this study, the results showed that ABA treatment can promote AGPase and SSS activity. ADPglucose pyrophosphorylase (EC: 2.7.7.27) and soluble starch synthase (SSS, EC 2.4.1.21) are two of the most important key enzyme involved in starch synthesis. High activity of AGPase and SSS were beneficial to starch accumulation in duckweed. 4 genes encoding starch metabolism related enzymes were selected for qRT-PCR analysis (Supplementary Figure 1), and the primers of these genes were list in the Supplementary Table 1. The results showed that the expression patterns of these genes were consistent with the activities of enzyme activity data in this study. Moreover, some reports also showed that exogenous ABA application increased starch content by temporally regulating the expression levels of the starch synthesis enzyme genes, which are involved in starch biosynthesis, and their results supported the findings of this research24,25. For example, Wang et al.24 investigated the expression of ADP-glucose pyrophosphorylase (key enzyme involved in starch synthesis) during turion formation under ABA treatment in Spirodela polyrhiza. The results showed that the expression of SpAPL2 and SpAPL3 (subunits of ADP-glucose pyrophosphorylase) was dramatically increased by 2-and 10-fold in 1–3 days and leveling off after 5 days by ABA treatment, respectively. Thus, it is probably true in Landoltia punctata as well given that our data indicate that ABA treatment increased the activities of the enzymes involved in starch biosynthesis. Therefore, the increase of ABA content plays a pivotal role in starch biosynthesis by regulating the activities of key enzymes involved in starch biosynthesis. Therefore, the increase of ABA content plays a pivotal role in starch biosynthesis by regulating the activities of key enzymes involved in starch biosynthesis.
A-amylase and β-amylase involved in the process of starch degradation, which can dramatically affect starch content. A-amylase (EC 3.2.1.1) is a-1,4 endoglycolytic enzyme, responsible for the mobilization of stored starch reserves by initiating the degradation process. These genes encoding α-amylase have been shown to be differentially expressed at various developmental stages and environmental conditions through the action of plant hormones such as ABA. The expression levels of a-amylase is down-regulated by plant hormone, abscisic acid (ABA), during the process of starch metabolism26,27. Moreover, in this study, the activities of α-amylase and β-amylase were decreased dramatically by ABA treatment (Fig. 6). Reports showed that ABA can suppress the expression of genes encoding amylases, and β-amylase activity was negatively correlated with ABA concentration during grain development in barley28,29. Moreover, GAs can decrease starch synthesis and accumulation by inducing or activating α-amylase and other hydrolases30. Thus, the starch content in ABA treatment samples was much higher than that in the control sample, possibly due to an increase in the activity of starch biosynthesis enzymes and a drop in the activity of starch-degrading enzymes regulated by endogenous hormones in duckweed.
Conclusion
In this study, high biomass yield and starch accumulation of L. punctata was achieved by ABA treatment. The increase of endogenous ZR and IAA enhanced the biomass yield of duckweed, and high levels of ABA promoted starch accumulation. Moreover, alterations of endogenous hormone levels also regulated the activity of enzymes involved in starch biosynthesis and catabolism in duckweed. These results evidenced the dual benefits of duckweed: clean-up of sewage and production of potentially high-quality feedstock for biofuels. It also provide an operable method for high starch accumulation in duckweed for biofuels production in the future.
Materials and Methods
Duckweed cultivation and ABA treatments
L. punctata, a widely distributed duckweed species with great potential to accumulate enormous amounts of starch, originally isolated from Sichuan Province, China, was cultivated in standard 1/6 Hoagland’s E + solution in illuminating incubator (Guangzhi Corp. China) for 3 to 5 days (16 h 130 μmol/m2/s 25 °C: 8 h 0 μmol/m2/s 15 °C). Then 9 g of fronds were transferred into 1,400 mL 1/6 Hoagland E + culture plastic containers (25*18*4.5 cm) for further cultivation over a period of 14 days treatment. 5 mL solution of 0.5 mM ABA (Sigma-Aldrich, St Louis, USA) was sprayed evenly on the surface of fronds. For the control group, there was no plant hormones application on the surface of the fronds. Three biological replicates were performed in the experiment, and the experiment lasted for 14 days.
Determination of fresh and dry weights
To measure the fresh weight (FW), the fronds were measured with a balance using Bergmann’s method31. To determine the dry weight (DW), the samples were dried at 60 °C until the weight was constant.
Determination of starch content
The starch content was determined according to previously published methods32. Dry duckweed powder (0.03 g) was hydrolyzed with 2.6 mL 1.2 M HCl in a boiling water bath for 2 h. Then adjusting the pH to 7.0 with NaOH, adding PbAc to precipitate protein and diluting the solution with deionized water to a final volume of 10 mL. Thereafter, the solution was filtered and treated with a C18 extraction column. Finally, the hydrolyzate was analyzed by HPLC (Thermo 2795, Thermo Corp.) with an Evaporative Lightscattering Detector (All-Tech ELSD2000, All-tech., Corp.). The starch content was determined using the total sugar content (starch content = glucose content × 0.909).
Total photosynthetic pigment analysis
The chlorophyll content was estimated according to Aron’s method33. Weigh 0.5 g of duckweed sample in a 10 ml plug seal centrifuge tube, 10 ml of 80% acetone was added and kept it 72 h at room temperature in dark condition. After the incubation, the supernatant was collected and then optical density was measured at 645 and 663 nm for chlorophyll a and chlorophyll b respectively.The chlorophyll content was expressed as chlorophyll mass on a fresh weight (FW) basis (mg/g FW).
The net photosynthesis rate was measured by Li-6400xt (LI-COR, Inc., St. Lincoln, NE, USA) using the whole chamber (6400–17) and the RGB light source (6400–18).
Endogenous level of plant hormones
Duckweed was rinsed with distilled water using a strainer for three times. After free water stopped dripping, duckweed was blotted dry with paper towels, and then measured with a balance. The extraction, purification, and determination of endogenous levels of ABA, GA1 + 3, ZR and IAA by an indirect ELISA technique were performed as described by Wang34 and Yang35 with appropriate improvements. 0.5 g of fresh duckweed samples were homogenized in 2 ml of 80% methanol (containing 40 mg l−1 butylated hydroxytoluene as an antioxidant). The extract was incubated at 4 °C for 48 h, and then centrifuged at 4000 rpm for 15 min at 4 °C. The supernatant was passed through C18 Sep-Pak cartridges (Waters Corp., Millford, MA, USA), and the phytohormone fraction was eluted with 10 ml of 100% (v/v) methanol and then 10 ml of ether. The eluate was dried down by pure N2 at 20 °C, and then stored at −20 °C until use for endogenous hormone assay.
Carbohydrate metabolism enzyme activity assay
For the assays of enzymes, 1 g fresh weight duckweed was homogenized with a ceramic pestle in an ice-cold mortar in 5 ml of 50 mmol/L HEPES-NaOH (pH = 7.6), 5 mmol/L DL-Dithiothreitol, 8 mmol/L MgCl2, 2 mmol/L EDTA, 2%(w/v) polyvinylpyrrolidone-40, 12.5% (w/v) glycerol. The homogenate was centrifuged at 10,000 g for 5 min. The supernatant extract was used as crude enzyme solution stored at minus 20 °C. All the procedures were carried out at 0–4 °C. Then, the supernatant was used to measure the enzyme’s activity. The activities of ADP-glucose pyrophosphorylase (AGPase, EC 2.7.7.27), soluble starch synthase (SSS, EC 2.4.1.21), alpha-amylase (α-amy, EC 3.2.1.1) and beta-amylase (β-amy, EC 3.2.1.2) were analyzed using the method from a previous report36,37,38. Activity was expressed as units per milligram protein (U/mgprot). Accordingly, one unit of AGPase is defined as the amount of enzyme that causes the increase of 0.01 OD at 340 nm of the finally reacted solution per minute. The AGP units are then divided by the total protein amount. One unit of amylase is defined as the amount of enzyme in the presence of excess thermostable a-glucosidase required to release 1 μmol of p-nitrophenol from non-reducing-end blocked p-nitrophenyl maltoheptaoside (BPNPG7) per min under the defined assay conditions. One unit of SSS is defined as the amount of enzyme that causes the increase in absorbance at 340 nm of the finally reacted solution per minute.
Calculations and statistics
All samples were investigated in triplicate. All the results are presented as means ± standard error in the Figures. Statistical significance (p < 0.05) was analyzed by one-way analysis of variance39 using SPSS software (Version 15.0, SPSS Inc., Chicago, IL, USA).
References
Searchinger, T. et al. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319, 1238–1240 (2008).
Aditiya, H. et al. Second generation bioethanol potential from selected Malaysia’s biodiversity biomasses: A review. Waste Management 47, 46–61 (2016).
Hillman, W. S. & Culley, D. D. The uses of duckweed: The rapid growth, nutritional value, and high biomass productivity of these floating plants suggest their use in water treatment, as feed crops, and in energy-efficient farming. American Scientist 66, 442–451 (1978).
Xu, J., Cui, W., Cheng, J. J. & Stomp, A.-M. Production of high-starch duckweed and its conversion to bioethanol. Biosystems engineering 110, 67–72 (2011).
Xu, J. & Shen, G. Growing duckweed in swine wastewater for nutrient recovery and biomass production. Bioresource Technology 102, 848–853 (2011).
Cui, W., Xu, J., Cheng, J. & Stomp, A. Starch accumulation in duckweed for bioethanol production. Biol Eng 3, 187–197 (2011).
Tao, X. et al. Comparative transcriptome analysis to investigate the high starch accumulation of duckweed (Landoltia punctata) under nutrient starvation. Biotechnology for biofuels 6, 72 (2013).
Mohedano, R. A., Costa, R. H. & Belli Filho, P. Effects of CO2 concentration on nutrient uptake and starch accumulation by duckweed used for wastewater treatment and bioethanol production. Revista Latinoamericana de Biotecnología Ambiental y Algal 7, 1–12 (2016).
Liu, Y. et al. Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) II: transcriptome alterations of pathways involved in carbohydrate metabolism and endogenous hormone crosstalk. Biotechnology for biofuels 8, 64 (2015).
Yin, Y. et al. The influence of light intensity and photoperiod on duckweed biomass and starch accumulation for bioethanol production. Bioresource technology 187, 84–90 (2015).
Liu, Y. et al. Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) I: transcriptome analysis of the effects of uniconazole on chlorophyll and endogenous hormone biosynthesis. Biotechnology for biofuels 8, 57 (2015).
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R. & Abrams, S. R. Abscisic acid: emergence of a core signaling network. Annual review of plant biology 61, 651–679 (2010).
Sulochana, S. B. & Arumugam, M. Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresource technology 213, 198–203 (2016).
Wang, W. & Messing, J. Analysis of ADP-glucose pyrophosphorylase expression during turion formation induced by abscisic acid in Spirodela polyrhiza (greater duckweed). BMC plant biology 12, 5 (2012).
Akihiro, T., Mizuno, K. & Fujimura, T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant and Cell Physiology 46, 937–946 (2005).
Fromme, P., Melkozernov, A., Jordan, P. & Krauss, N. Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. Febs Letters 555, 40–44 (2003).
Gao, S. et al. ABF2, ABF3, and ABF4 Promote ABA-Mediated Chlorophyll Degradation and Leaf Senescence by Transcriptional Activation of Chlorophyll Catabolic Genes and Senescence-Associated Genes in Arabidopsis. Molecular Plant 9, 1272–1285 (2016).
Yang, J., Zhang, J., Wang, Z., Zhu, Q. & Liu, L. Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 215, 645 (2002).
Li, H., Wang, Y., Xiao, J. & Xu, K. Reduced photosynthetic dark reaction triggered by ABA application increases intercellular CO2 concentration, generates H2O2 and promotes closure of stomata in ginger leaves. Environmental and Experimental Botany 113, 11–17 (2015).
Gómez-Cadenas, A., Zentella, R., Walker-Simmons, M. K. & Ho, T.-H. D. Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. The Plant Cell 13, 667–679 (2001).
Wang, Y., Ma, F., Li, M., Liang, D. & Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regulation 64, 63–74 (2011).
Sakai, A., Yashiro, K., Kawano, S. & Kuroiwa, T. Amyloplast formation in cultured tobacco cells; effects of plant hormones on multiplication, size, and starch content. Plant cell reports 15, 601–605 (1996).
Yang, J. et al. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant growth regulation 30, 261–270 (2000).
Wang, W. & Joachim, M. Analysis of ADP-glucose pyrophosphorylase expression during turion formation induced by abscisic acid inSpirodela polyrhiza(greater duckweed). Bmc Plant Biology 12, 1–14 (2012).
Aurelio Gómez-Cadenas, R. Z., Walker-Simmons, M. K. & Ho, T.-H. D. Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 13, 667–679 (2001).
Thomas, B. R. & Rodriguez, R. L. Metabolite Signals Regulate Gene Expression and Source/Sink Relations in Cereal Seedlings. Plant Physiology 106, 1235–1239 (1994).
Zentella, R., Yamauchi, D. & Ho, T. H. Molecular dissection of the gibberellin/abscisic acid signaling pathways by transiently expressed RNA interference in barley aleurone cells. Plant Cell 14, 2289 (2002).
Wei, K. et al. The effect of H2O2 and abscisic acid (ABA) interaction on β-amylase activity under osmotic stress during grain development in barley. Plant Physiology and Biochemistry 47, 778–784 (2009).
Hubbard, K. E., Nishimura, N., Hitomi, K., Getzoff, E. D. & Schroeder, J. I. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes & Development 24, 1695–1708 (2010).
Rentzsch, S. et al. Dose-and tissue-specific interaction of monoterpenes with the gibberellin-mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta 235, 137–151 (2012).
Bergmann, B., Cheng, J., Classen, J. & Stomp, A.-M. In vitro selection of duckweed geographical isolates for potential use in swine lagoon effluent renovation. Bioresource Technology 73, 13–20 (2000).
Zhang, L. et al. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresource Technology 102, 4573–4579 (2011).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology 24, 1 (1949).
Wang, Y. et al. Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. Journal of experimental botany 63, 5887–5901 (2012).
You-Ming, Y., Chu-Nian, X., Bao-Min, W. & Jun-Zhen, J. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regulation 35, 233–237 (2001).
Cu, S. T., March, T. J., Degner, S. & Eglinton, J. K. Identification of novel alleles from wild barley for the improvement of alpha‐amylase and related malt quality traits. Plant Breeding 135, 663–670 (2016).
Nakamura, Y., Yuki, K., Park, S.-Y. & Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant and Cell Physiology 30, 833–839 (1989).
Tárrago, J. F. & Nicolás, G. Starch degradation in the cotyledons of germinating lentils. Plant physiology 58, 618–621 (1976).
Pangallo, D. et al. Disclosing a crypt: Microbial diversity and degradation activity of the microflora isolated from funeral clothes of Cardinal Peter Pazmany. Microbiological Research 168, 289–299, https://doi.org/10.1016/j.micres.2012.12.001 (2013).
Acknowledgements
The authors acknowledge the financial support received from the Young Talent Program of Chengdu University (NO. 2081915053); Scientific and Technological Research Program of Chongqing Municipal Education Commission (No: KJ1601108), the Research Program of Yongchuan District Water Authority of Chongqing Municipal (No: 2016-04), and the Research Program of Chongqing University of Arts and Sciences (No: R2015CH10); Key Laboratory of Environmental and Applied Microbiology, Chengdu Institute of Biology, Chinese Academy of Sciences (No. KLEAMCAS201501).
Author information
Authors and Affiliations
Contributions
Y.L. carried out biochemical assays, the data analysis, drafted and revised the manuscript. X.Y.C., W.X.H., Y.F. and M.J.H. participated in the design of the study, data analysis and revised the manuscript. L.G. and Y.Z. participated in revise the manuscript. H.Z. conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Chen, X., Wang, X. et al. Improving biomass and starch accumulation of bioenergy crop duckweed (Landoltia punctata) by abscisic acid application. Sci Rep 8, 9544 (2018). https://doi.org/10.1038/s41598-018-27944-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27944-7
This article is cited by
-
Exogenous abscisic acid (ABA) improves the filling process of maize grains at different ear positions by promoting starch accumulation and regulating hormone levels under high planting density
BMC Plant Biology (2024)
-
Valorization of Spirodela polyrrhiza biomass for the production of biofuels for distributed energy
Scientific Reports (2023)
-
Frond architecture of the rootless duckweed Wolffia globosa
BMC Plant Biology (2021)
-
Biosynthesis of the starch is improved by the supplement of nickel (Ni2+) in duckweed (Landoltia punctata)
Journal of Plant Research (2020)
-
Differential effects of synthetic media on long-term growth, starch accumulation and transcription of ADP-glucosepyrophosphorylase subunit genes in Landoltia punctata
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.