Abstract
Myo-inositol hexaphosphate (phytate; IP6) is a natural compound that is abundant in cereals, legumes, and nuts and it has the ability to chelate metal cations. The binding of IP6 to transition metals suggests that it could be used for the treatment of metal-catalyzed protein glycation, which appears to trigger diabetes-related diseases. Our in vitro studies showed that IP6 reduced the formation of Fe3+-catalyzed advanced glycation end-products (AGEs). This led us to perform a randomized cross-over trial to investigate the impact of the daily consumption IP6 on protein glycation in patients with type 2 diabetes mellitus (T2DM; n = 33). Thus, we measured AGEs, glycated hemoglobin (HbA1c), several vascular risk factors, and urinary IP6 at baseline and at the end of the intervention period. Patients who consumed IP6 supplements for 3 months had lower levels of circulating AGEs and HbA1c than those who did not consume IP6. This is the first report to show that consumption of IP6 inhibits protein glycation in patients with T2DM. Considering that AGEs contribute to microvascular and macrovascular complications in T2DM, our data indicates that dietary supplementation with IP6 should be considered as a therapy to prevent the formation of AGEs and therefore, the development of diabetes-related diseases in patients with T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a common endocrine disorder, in which chronic insulin resistance and progressive failure of β-cells leads to hyperglycemia1. Worldwide, 382 million people are currently diagnosed of T2DM, and this number is expected to reach 600 million in 20352.
Hyperglycemia induces the development of diabetes-related disorders, such as nephropathy3, retinopathy4, hypertension5 or dyslipidemia6. Moreover, recent studies reported positive correlations of T2DM with Alzheimer’s disease7,8 and with Parkinson’s disease9,10, although the detailed molecular mechanisms that follow from hyperglycemia to these diseases are uncertain. However, one of the main triggering factors seems to be protein glycation and the consequent formation of advanced glycation end-products (AGEs)11,12. It is assumed that the accumulation of AGEs modifies the protein structure causing loss-of-function, which may lead to development of diabetes-related diseases13,14. However, we recently demonstrated that protein glycation does not necessary involve structural modifications15,16, in agreement with other contributions17,18.
Protein glycation starts with the reaction between reducing sugars and the protein Lys side chains, a process enhanced in patients with T2DM due to their increased levels of serum glucose (>7 mM;19). This reaction initially yields a Schiff base that rearranges into an Amadori product, which is the key factor that enables the final formation of AGEs through a metal-catalyzed mechanism. AGEs are a heterogeneous family of compounds with different chemical features, which are mainly formed on protein Lys, Arg, and Cys side chains. Hence, their chemical structure depends on the glycating compound, the protein target, the chemical environment of each glycation hot-spot, and other factors. Therefore, a diverse set of AGEs can be formed in vivo on a single amino-acid residue (non-crosslinking AGEs) and/or between different amino-acid residues (crosslinking AGEs)20,21,22.
Several mechanisms may explain the relationship between AGEs and diabetes complications: (i) accumulation of AGEs in the extracellular matrix, causing crosslinking and decreased vessel elasticity (increased arterial stiffness);23 (ii) binding to specific AGEs receptors (RAGEs) that activate certain cell signaling pathways, such as NF-κB pathway, and modulate gene expression in different cell types;24 (iii) alteration of protein structure and function;25 and (iv) quenching of intracellular nitric oxide and disrupting the function of certain growth factors26.
The role of AGEs in the development of diabetes-related diseases has focused therapeutic investigations towards the search of compounds that can inhibit protein glycation. Several pharmacological approaches can inhibit AGEs formation: (i) trapping of reactive carbonyl compounds; (ii) chelation of metal cations that are important in the catalysis of AGEs formation; (iii) blocking of RAGEs; (iv) scavenging of free radicals formed during protein glycation; (v) cleavage of crosslinking AGEs; and (vi) reducing serum glucose27. Thus, several compounds (e.g. pyridoxamine, benfotiamine, aminoguanidine, ALT-711, etc.) have potential as anti-glycation agents because they act through one or several of these mechanisms27,28. Among them, pyridoxamine stands out because it scavenges carbonyl compounds, traps reactive oxygen species, and inhibits oxalate biosynthesis29. Pyridoxamine has also the ability to strongly chelate metal cations30, thus inhibit the formation of AGEs directly arising from Amadori compounds31. Because metal-catalyzed reactions play a major role in the formation of AGEs32,33,34,35, a therapeutic approach based on chelation of metal cations, such as Cu2+ or Fe3+, has emerged as potential therapy against protein glycation.
Myo-inositol hexaphosphate (phytate, IP6) is a natural compound present in seeds (e.g. cereals, legumes, and nuts) as a calcium-magnesium salt (phytin)36, that can chelate most divalent cations37,38. IP6 has also a high affinity towards Fe3+, being even greater than that of EDTA, deferoxamine (DFOA) or ATP39, thus suggesting that IP6 consumption could reduce mineral bioavailability40,41. Nevertheless, several studies have indicated that dietary phytin intake of 2 g/day, as part of a balanced diet, had no adverse effects on mineral bioavailability42,43,44,45,46. In addition, we have also shown that IP6 prevents the formation of pathological calcifications in vivo, such as renal calculi47,48, dental calculi49, and cardiovascular calcification50,51,52, and protects against osteoporosis53. Moreover, IP6 may also provide protection against cancer54 and Parkinson’s disease55.
The remarkable ability of IP6 to chelate Fe3+, together with the potential of this cation as a catalyst of the AGEs formation processes56,57,58, motivated us to study whether IP6 could act as inhibitor of the metal-catalyzed glycation. Thus, the specific aims of this paper are: (i) study the ability of IP6 to inhibit protein glycation in vitro; (ii) and investigate the impact of daily IP6 consumption on metabolic and glycemic control, and on the in vivo formation of AGEs in patients with T2DM.
Results
Effect of IP6 on the formation of AGEs in vitro
IP6 is a powerful Fe3+ chelator39, so we hypothesized that it could block the steps along the glycation process that are sensitive to Fe3+ catalysis. Thus, we initially used fluorescence spectroscopy to measure the effect of IP6 concentration (0 to 2 µM) on the time-dependent formation of Fe3+-catalyzed AGEs. These studies were carried out on a solution containing Nα-acetyl-lysine (Ac-Lys), Nα-acetyl-arginine (Ac-Arg), ribose and Fe3+, which mimicked a protein glycation process that could occur in vivo. The obtained results showed that the fluorescent signal temporally increased over 7 days due to the formation of ribose-derived fluorescent AGEs (Fig. 1A), as we previously described15. Although IP6 concentrations of 0.5 µM and below had no effect on the AGEs formation, the fluorescence signal increase was lower in the presence of 1 and 2 µM IP6. However, the inhibitory effects of IP6 at these concentrations were only significant on day-4 and day-7 (Fig. 1B), thus suggesting that IP6 may inhibit late steps in the glycation pathway, such as post-Amadori reactions.
Effect of IP6 concentration on in vitro formation of AGEs. (A) Changes of fluorescence intensity signal (λexc 320 nm; λem 420 nm) a different incubation times of a solution containing Ac-Lys (2 mM), Ac-Arg (1 mM), ribose (0.2 M), and 2 µM Fe3+ with 0 to 2 µM IP6. (B) Percentage inhibition of AGEs formation by different concentrations of IP6 after 7 days of incubation *p < 0.05 vs. control (0 µM).
Baseline characteristics of patients
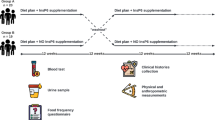
In vitro data indicating an inhibitory effect of IP6 on fluorescent AGEs formation, led us to design an interventional randomized crossover trial study to analyze whether these positive results would also be observed on T2DM patients consuming a diet rich in IP6 during 12 weeks (Fig. 2). Thirty-three patients (20 females and 13 males) completed the clinical study (Table 1). The median age was 64 years (interquartile range [IQR]: 52 to 70) and the mean duration of T2DM was 11 years (IQR: 6 to 14 years). Regarding dietary phytate consumption at baseline, the median of estimated intake was 260 mg/day (IQR: 150 to 426 mg/day). As can be expected, it was very low when compared with the IP6 consumption in a typical Mediterranean diet (1 g/day)59.
Five patients (15.2%) consumed alcohol, 4 patients smoked (12.1%), and 8 patients (24.2%) had complications associated with T2DM. Thirty patients (90.9%) were on oral antidiabetic agents, and 19 (57.6%) used insulin therapy. Twenty (60.6%) were on antihypertensive therapy and 23 (69.7%) were treated with lipid-lowering agents. During the study period, there were no changes in medication usage (Table 1). The anthropometric and laboratory analysis variables before starting both diets did not reveal differences between the two groups (Table 2).
Effect of dietary IP6 on AGE, HbA1c, and other variables
At baseline (T0, before intervention), the two groups had similar HbA1c and AGEs levels (Fig. 3). At 3 months (T1, after intervention), patients treated with the IP6 diet had significantly lowered their HbA1c (7.8 ± 0.1% to 7.5 ± 0.1%; p = 0.029) and AGEs (7.8 ± 0.4% to 5.8 ± 0.3%; p < 0.001) levels. At 6 months (T2, after follow-up period), patients who received the IP6 diet had significant increases in HbA1c and AGEs levels relative to T1 (HbA1c: 7.5 ± 0.1% to 7.7 ± 0.2%; p = 0.012; AGEs: 5.8 ± 0.3% to 7.1 ± 0.3%; p < 0.001). Patients given the non-IP6 diet had no significant changes in HbA1c and AGEs levels from T0 to T2.
Effect of a phytate diet on serum levels of HbA1c (A) and AGEs (B). Values are expressed as means ± SEs. Intra-group differences (T0 vs. T1 and T1 vs. T2) were assessed using a paired-sample Wilcoxon signed-rank test or a paired-samples t-test (a: p < 0.05 for T0 vs. T1; b: p < 0.05 for T1 vs. T2). Inter-group differences (phytate diet vs. non-phytate diet) were assessed using an analysis of covariance after adjusting for baseline levels (*p < 0.05).
The effect of IP6 intake on other risk factors was evaluated after the dietary intervention (T1 vs. T0; Table 3) and after follow-up period (T2 vs. T1; Table 4). No significant changes in the other clinical and biochemical parameters were detected. However, an unexpected but small increase in weight and BMI was observed at the end of the follow-up period (T2) in the IP6 dietary group (Table 4). There was a minor (but not statistically significant) increase of total cholesterol detected after the IP6 dietary intervention (T1) with respect to the baseline (T0). Also, a significant decrease in total cholesterol was observed after non-IP6 intervention (Table 3). Regarding iron status, no deficit was found after IP6 dietary intervention. An slight increase in iron levels was observed after IP6 intervention (median change [IQ]: 4.0 (−0.25–16.0), p = 0.029). Nevertheless, no differences were observed in iron and transferrin levels between IP6 and non-IP6 intervention after adjusting for baseline values. No changes in inflammatory markers (CRP and leukocyte count) were observed.
Effect of dietary IP6 on urinary excretion of IPs
At baseline (T0), both groups had similar urinary excretion of myo-inositol phosphates (IPs) (Fig. 4). After 3 months (T1), patients treated with the IP6 diet had a significant increase in urinary IPs (0.34 ± 0.04 to 0.50 ± 0.05 mg/g creatinine; p = 0.005). However, after the follow-up period (T2) these patients had a slight but no significant decrease in urinary IPs (0.50 ± 0.05 to 0.42 ± 0.04 mg/g creatinine; p = 0.357). Patients who received a non-IP6 diet decreased its urinary IPs at T1 (0.34 ± 0.08 to 0.26 ± 0.03 mg/g creatinine; p = 0.020), whereas it increased at T2 (0.26 ± 0.03 to 0.41 ± 0.04 mg/g creatinine; p = 0.478).
Effect of phytate diet on urinary levels of IPs. Values are expressed as medians ± SEs. Intra-group differences (T0 vs.T1 and T1 vs. T2) were assessed using a paired-sample Wilcoxon signed-rank test or a paired-samples t-test (a: p < 0.05 for T0 vs. T1). Inter-group differences (phytate diet vs. non-phytate diet) were assessed using an analysis of covariance after adjusting for baseline levels (*p < 0.05).
Safety and adverse events
All patients exhibited good tolerance to the IP6 tablets. There were no serious adverse events (death, life-threatening events, or events placing a patient in jeopardy or leading to admission) and no drop-outs related to IP6 supplementation. However, one patient presented had a severe hypoglycemic event while on the non-IP6 diet.
Discussion
This study is the first to report the effect of IP6 as inhibitor of protein glycation and therefore, on AGEs formation both in vitro and in patients suffering of T2DM.
Our in vitro studies indicated that IP6 significantly reduced the formation of fluorescent AGEs on Ac-Lys and Ac-Arg (the main protein glycation hot spots) in the presence of Fe3+ and ribose, a powerful glycating agent60 whose concentration is increased in T2DM patients61. This occurs because IP6 is able to strongly chelate Fe3+ through the three phosphate groups in positions 1, 2 and 3. These groups are flexible, and bind the Fe3+ so that all six coordination sites are occupied by hydroxyl groups, thus yielding a highly stable complex39. Once within the IP6-Fe complex, Fe3+ notably decreased its ability to catalyze glycation reactions. Accordingly, previous results indicated that IP6 had the same protective effect in blocking the formation of Fe-catalyzed free radicals via the Fenton reaction62.
The in vitro inhibitory effect of IP6 only occurred at prolonged incubation times, thus suggesting that its inhibitory effect mainly affects the post-Amadori reactions, which yields the final formation of AGEs (Fig. 1). Previous studies reported that AGEs inhibitors and AGEs breakers act through chelation of metal cations, either directly or due the action of products of their hydrolysis and/or metabolism63. Moreover, several studies have also reported that metal chelators may provide therapeutic benefits for diabetes, cardiovascular disease, and renal disease63,64,65. There is also evidence that some chelators may shift the redox potential of iron and copper, altering their catalytic activity and thereby ameliorating oxidative stress and reducing complications of diabetes. Thus, further consideration should be given to iron and copper chelators as agents for the treatment of oxidative stress and AGEs formation, which lead to major complications in patients with T2DM63.
Protein glycation leading to AGEs accumulation are thought to be one of the main triggering factors of the diabetic complications including nephropathy, retinopathy and neuropathy13,14,15,16,17,18. AGEs are not exclusively formed on proteins, but also on other endogenous key molecules such as lipids or nucleic acids. This alters the intracellular signaling and the gene expression, and release pro-inflammatory molecules and free radicals13,14,15,16,17,18. For this reason, natural compounds like IP6 that are able to inhibit protein glycation, can be crucial on the dietary management of type 2 diabetes and its related-complications.
Our in vitro results led us to analyze the effect of IP6 on AGEs formation in vivo. Thus, we designed a randomized crossover trial of patients with T2DM to investigate the potential therapeutic effects of a 3-month dietary intervention supplemented with IP6. To our knowledge, this is the first study to investigate the effects of IP6 supplementation on AGEs formation in humans with TDM2. Previous studies have used AGEs levels in serum and tissues as a biomarker of aging and longevity66, and as a predictor of decreased glomerular filtration rate67, heart failure68, and mortality in patients undergoing hemodialysis69. An increased total AGEs content is also associated with development of kidney disease70, obstructive sleep apnea71, vascular calcification in patients with kidney disease72, and cardiovascular disease in patients on peritoneal dialysis73 or with thrombosis74. In addition, an elevated AGEs levels may also adversely affect perinatal outcomes in mothers with gestational diabetes75. However, the diagnosis of T2DM and quantification of its therapeutic control focuses on the level of HbA1c76,77. Therefore, HbA1c is considered a marker of circulating lipids78, diabetic retinopathy79, nephropathy80, and neuropathy81, and is used to predict the severity of coronary artery disease82 and pathological retinal vasculature (as indicated by fractal dimension)83.
Our results indicate that a 3-month IP6 diet significantly lowered the levels of circulating AGEs (~25%) in patients with T2DM. Moreover, these patients also had a 3.8% decline in HbA1c, presumably due to a reduced overall protein glycation84. Otherwise, it is difficult to quantify or discard a possible effect of an improvement of the overall glucose metabolism due to the IP6. Although we observed no effect of IP6 intake on fasting glucose and on insulin levels, we did not measure postprandial glucose. Some previous experimental studies suggested that IP6 can decrease intestinal amylase activity, thus diminishing the postprandial glycemic excursion84.
AGEs and IP6 have both been linked to inflammation and insulin resistance85,86. However, we found that the IP6 diet did not lead to changes in hs-CRP, leukocyte count, or insulin resistance. Nevertheless, we cannot exclude a possible effect of IP6 on other modulators or molecular surrogates of inflammation.
We observed no large changes in body weight during the experimental period. However, there was a minor, but statistically significant, weight gain in patients during the follow-up period (T2) after the IP6 diet. This was surprising, and the reasons for this weight gain require further investigation. In diabetic animal models, IP6 supplementation is related to less weight gain and higher levels of leptin87. It is possible that withdrawal of IP6 from our patients led to increased caloric intake, together with a decrease in leptin.
We found that the IP6 diet did not affect serum lipid levels and the non-IP6 diet decreased total cholesterol. However, experimental studies reported that sodium IP6 reduces lipase activity, total cholesterol, low density lipoprotein, hepatic total lipids, and hepatic triglycerides, and increases high density lipoprotein88,89. In this regard, we should note that we used calcium-magnesium IP6, the naturally occurring form. There is evidence that the calcium-IP6 complex does not bind bile acids. Thus, this could reduce fecal bile excretion and increase cholesterol availability90. This data are in accordance with our results, so it was possible that the non-IP6 dietary intervention provoked a decrease in total cholesterol levels by the adherence of the patients to a dietary plan whereas the no lipid changes after IP6-intervention was explained by the modification in fecal bile excretion. In this sense, it would be interesting to investigate the clinical effects of sodium phytate as a dietary supplement.
Moreover, the iron and transferrin levels did not decrease after IP6 intervention. This observation makes IP6 suitable to be considered as a therapy since there is a great deal of controversy regarding the effects of IP6 on the metal absorption. Some studies suggest that the iron absorption might decrease ~3% in diets including low calcium, high phosphorus and high IP6 content40,41. However, other studies indicated that a dietary IP6 intake as phytin in a balanced diet (0.1%), had no adverse effects on mineral bioavailability and iron absorption42,43,44,45,46. Human trials indicated that phytin intake of 2 g/day did not affect mineral balance45,46. Interestingly, the Mediterranean diet results in consumption of approximately 1 g of phytate/day59. In any case, our data supports the idea that the dietary phytin intake of 1 g/day, as a part of a balanced diet, had no adverse effects on iron absorption.
There is strong evidence that eating a variety of whole-grain foods and legumes benefits patients with T2DM91. In fact, several studies reported improvements in glycemic control and a reduction of HbA1c following increased dietary intake of legumes and whole grains92,93. Legumes and whole grains are rich in IP6, so this could explain, at least in part, their beneficial effects. Unfortunately, experimental evidences suggest that in our Mediterranean region, the IP6 consumption is lower in patients with T2DM than in non-diabetic subjects (unpublished data). Based on the results presented here, we strongly suggest that clinicians should actively encourage patients with T2DM to consume a diet rich in IP6.
Although our study is the first to report the effects of IP6 on AGEs in vitro and in patients with T2DM, there were several limitations. In particular, we only examined 33 patients, all of whom were from a single medical center. Thus our findings may have limited generalizability. Another limitation is that IP6 supplementation was not blinded. Thus, a large multicenter and blinded placebo-controlled study is needed to strengthen our results.
In conclusion, our results show that IP6, a natural product present legumes and cereals, reduces AGEs formation in vitro, and that dietary supplementation with IP6 reduces circulating AGEs and HbA1c levels in patients with T2DM. This protective effect is likely due to IP6-mediated chelation of Fe3+ 94. Thus we suggest that a diet rich in IP6 could help to prevent complications of diabetes. Furthermore, IP6 consumption may help to prevent or minimize other AGE-related disorders. Large, long-term, and randomized prospective clinical studies must be performed to more completely assess the benefits and risks of IP6 consumption in patients with TDM2.
Methods
Reagents
All chemicals were from Sigma-Aldrich Chemical Co. unless otherwise noted.
In vitro studies of AGEs formation
The formation of Fe3+-catalyzed AGEs was monitored using fluorescence spectroscopy on a solution containing Ac-Lys, Ac-Arg, ribose, and Fe3+ with different concentrations of IP6 (0 to 2 µM). Stock solutions of Ac-Lys, Ac-Arg, D-ribose, FeCl3, and IP6 were freshly prepared in 0.2 M phosphate buffer (pH 7.4) that was previously treated with CheleXTM (70 g/L). Stock solutions were then filtered using a 0.22 μm membrane and combined to obtain mixtures containing Ac-Lys (2 mM), Ac-Arg (1 mM), ribose (0.2 M) and 2 µM Fe3+. The reaction mixtures and control samples were incubated at 37 °C for 7 days. Aliquots were collected for analysis at different incubation times. Time-dependent AGEs formation was monitored at 25 °C using a Cary Eclipse fluorescence spectrophotometer equipped with a Peltier temperature controlled cell holder (λexc 320 nm; λem 360–540 nm). The maximum emission signal was observed at 420 nm and therefore, used to calculate the percent of AGEs inhibition (PAI) after 7 days of incubation:
I0 is the fluorescence intensity in absence of IP6 and II is the fluorescence intensity in the presence of IP6. These assays were performed in triplicate, and means and standard errors are reported.
Randomized crossover study of patients with T2DM
Subjects and study design
This was a single-center, randomized, crossover, open-label study. The 35 subjects, who were consecutive T2DM patients, were prospectively enrolled from our outpatient clinic at the Endocrinology Department of Hospital Son Llàtzer, a public tertiary care center that covers 250,000 residents of urban and rural areas in the Balearic Islands (Spain).
Volunteers were eligible if they: (i) were older than 18 years; (ii) had well-controlled T2DM and stable use of medication(s) during the previous 3 months; (iii) had HbA1c levels between 6.5–9.0%; and (iv) had low dietary consumption of IP6 (estimated from dietary questionnaires). No patient had cancer or clinically significant cardiovascular, liver, or end-stage kidney disease. Two patients did not complete the dietary intervention. Thus, 33 volunteers successfully completed the study. The minimum number of patients for detecting a 20% difference in AGEs level at the end of the study, with 80% power and 95% confidence interval, was 30 patients per group.
Subjects were randomly assigned to the IP6-diet group or to the non-IP6 diet group for 12 weeks. After 12 weeks, each group was given a 12-week washout period, and then switched to the alternate diet for 12 weeks. All patients were then followed for an additional 12 weeks (Fig. 2). Patients were asked not change their use of anti-hyperglycemic medications (dose or drug) during the study period, unless they had a fasting blood glucose level above 250 mg/dL during two consecutive measurements or suffered from a severe hypoglycemic event. In addition, changes in the total daily insulin dose greater than 10% were discouraged during the trial.
Randomization sequence was created using Excel software (Microsoft Office 2010) and was stratified with a 1:1 allocation using random block of 4 by an independent researcher with no clinical involvement in the trial. After the clinicians had obtained the patient’s consent, they made contact with the independent researcher of the recruitment process for allocation consignment, enrolling and assessing participants in sequential order. After randomization, there was no blinding of study participants and researchers administering interventions, but there was blinding for researchers assessing outcomes.
Dietary intervention
Patients received an IP6 diet (diet plan with IP6 supplementation) or a non-IP6 diet (the same diet plan without IP6 supplementation). The IP6 supplementation consisted of 1 capsule of 380 mg of calcium-magnesium IP6 (Broken Laboratorios, SALVAT S.A.), and was given three times daily with each main meal.
After each intervention, all subjects received oral and written information about their diet plans. The diet plan consisted of a daily meal plan, with five meals per day. Participants were requested to follow the menu plan as much as possible, and to report any meal that differed from what was stipulated.
The diet plan for both groups included fruits, vegetables, fish, shell-fish, meat, eggs, olive oil, and low-fat dairy products. Participants in both groups were specifically advised to avoid foods rich in IP6, such as legumes, cereals, and nuts. In these cases, the main sources of carbohydrates were white bread, white rice (not basmati), pasta, white flour, potatoes and non-whole wheat semolina. This diet is in accordance with the dietary recommendations of Spain for people with T2DM, regarding macronutrient composition, dietary fiber, minerals, and vitamins.
A dietician followed each patient monthly to ensure that the dietary plan was followed. The dietician also checked for compliance (consumption of at least 80% of pills) at every visit by counting of pills.
Variable Outcomes
The main outcome measure was serum level of AGEs. The secondary outcomes were HbAc1, fasting glucose, insulin, homeostatic model assessment (HOMA) of insulin resistance, lipids, blood pressure, and weight. Changes in C-reactive protein (CRP) and leukocyte count, which are related to inflammation, were also analyzed.
Clinical histories were extracted from the electronic medical records. Furthermore, data from anamnesis, laboratory analysis, and physical examinations were prospectively collected during the trial. Physical and anthropometric measurements were carried out by qualified personnel while the subjects were barefoot and wearing light clothes. Urine and blood samples were taken before and after the dietary interventions, and after the follow-up period. Blood samples were collected in the morning (after 12 h of fasting). These samples were left to stand for 30 min at room temperature, and the serum was then separated by centrifugation. Hematimetric and biochemical analyses were performed in an automated analyzer (Cell-Dyn Sapphire and Architect ci16200, Abbott). Insulin was analyzed by chemiluminescent-immunometric assay (Advia Centaur, Siemens). Highly sensitive CRP (hs-CRP) and lipoprotein (a) (Lp [a]), were analyzed by Nephelometry (Immage 8000, Beckman Coulter). All samples were run in duplicate, and the coefficients of intra- and inter-assay variation were below 10%.
Blood pressure was measured 3 times consecutively after 5 min of rest while the subject was sitting quietly. The average of the second and third measurements was recorded. Patients using anti-hypertensive drugs and those with systolic blood pressure of 140 mmHg or more and/or diastolic blood pressure of 90 mmHg or more were categorized as having hypertension95.
Dyslipidemia was defined as the presence of one of the following: LDL cholesterol levels of 100 mg/dL or more, HDL cholesterol below 40 mg/dL (men) or 50 mg/dL (women), triglycerides of 150 mg/dL or more, or use of a lipid-lowering drug96.
Serum AGEs determination
AGEs in serum samples were measured using the Cell Biolabs’ OxiSelect™ AGEs Competitive ELISA Kit, which provides a rapid detection and quantification of circulating AGEs. Quantitation was determined by using an AGE-BSA standard curve.
Estimation of IP6 consumption
IP6 consumption was estimated as previously described51,59. Briefly, it was based on a Food Frequency Questionnaire (FFQ) which considered 10 items that were major sources of IP6 (whole cereals, legumes, and nuts), the serving size of each item, and the IP6 concentration of each item. The same day, a 2-h urine sample was collected, and the dietician interviewed each patient to confirm the answers to the 10 selected FFQ items. IP6 consumption was determined by consumption of foods in 3 major food groups: legumes, whole grains, and nuts. Low IP6 consumption was defined by the consumption of these foods fewer than three times a week. Previous studies from our laboratory indicated a good correlation between declared IP6 consumption and IP6 levels in urine and other tissues97,98. These studies indicated that consumption of foods rich in IP6 at least three times a week is sufficient to achieve a high serum level of IP6.
Measurement of urinary IPs
Urinary IPs was measured at 2 h after the first urine of the morning. For this test, 20 mL of fresh urine was acidified with HCl (1:1) to pH 3, and then diluted with 20 mL of milli-Q water. This solution was transferred to a 100 mL beaker containing 0.5 g of AG1-X8 resin (anion exchange resin), without previous conditioning. This mixture was stirred with an orbital stirrer at 160 rpm for 15 min. The resin and urine were transferred into a 20 mL solid phase extraction (SPE) tube with a frit, and urine was passed through to separate it from the resin. The resin was then washed with 120 mL of 50 mM HCl and 2 × 5 mL of deionized water. Finally, IP6 was eluted by 4 × 1 mL of 2 M NaCl, with contact between the resin and each 1 mL portion maintained for 5 min by mixing with an orbital stirrer (180 rpm). The final 4 mL of eluate were collected into a single tube, and the solution was mixed prior to IP6 quantification. IPs were determined by indirect phytate analysis of this eluate, using the aluminum-pyrocatechol violet (Al-PCV) system99. The two reagents (RI and RII) were prepared daily. RI was a mixture of 0.6 mL of 4 mM Al[NO3]3 and 4.4 mL of 1.5 M acetic acid/acetate buffer at pH 5.2; RII was a mixture of 0.6 mL of 5.6 mM PCV and 4.4 mL of deionized water. IP6 standards in the range 1–10 µM were prepared in 2 M NaCl. The assays were performed in 96-well plates, with each well containing 30 µL RI, 290 µL of a standard or eluate, and 30 µL of RII. After incubation for 15 min, absorbance was measured at 570 nm. All samples were assayed in duplicate. This method does not discriminate between IP6 and other IPs, so the measured parameter is considered to be ‘phytic acid equivalents’. We carried out the determination of the urinary IP6 to have an analytical parameter to confirm the adherence of patients to the dietary interventions.
Statistical analysis
Data are presented as means (standard deviations), medians (interquartile ranges), or numbers (percentages). Intergroup comparisons at baseline (T0, before intervention) were analyzed using the independent-samples t-test or the Mann-Whitney U test for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. Intragroup differences (before [T0] vs. after intervention [T1]; after intervention [T1] vs. after follow-up [T2]) were evaluated using a paired samples t-test or Wilcoxon’s signed-rank paired test for continuous variables, and the McNemar test for dichotomized variables. Intergroup comparisons (after the intervention and after the follow-up period) were assessed using analysis of covariance and Fisher’s exact test, with adjustment for changes in categorical and continuous variables according to baseline values. Bivariate associations were evaluated by Pearson’s or Spearman’s correlation coefficients.
A two-tailed p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
Ethical considerations
The study design was approved by the Research Committee of Hospital Son Llàtzer and Research Ethics Committee of Balearic Islands [CEI-IB] (IB1933/12 PI). The clinical trial number is EudraCT n° 2017-003609-16, which was validated on 22/02/2018. The Universal Trial Number (UTN) is U1111-1201-5736. All patients provided written informed consent before participation. All experiments were performed in accordance with relevant guidelines and regulations.
References
Lin, Y. & Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 204, 1–11 (2010).
Kharroubi, A. T. & Darwish, H. M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 6, 850–867 (2015).
Chan, G. C. & Tang, S. C. Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol. Dial. Transplant. 31, 359–368 (2016).
Wong, T. Y., Cheung, C. M., Larsen, M., Sharma, S. & Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers. 2, 16012 (2016).
Colosia, A. D., Palencia, R. & Khan, S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab. Syndr. Obes. 6, 327–338 (2013).
Mooradian, A. D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 5, 150–159 (2009).
Alam, F., Asiful I.M., Sasongko, T.H. & Gan, S.H. Type 2 diabetes mellitus and Alzheimer’s disease: bridging the pathophysiology and management. 22, 4430–42 (2016).
Janson, J. et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53, 474–481 (2004).
Lima, M. M. et al. Does Parkinson’s disease and type-2 diabetes mellitus present common pathophysiological mechanisms and treatments? CNS Neurol. Disord. Drug Targets 13, 418–428 (2014).
Hu, G., Jousilahti, P., Bidel, S., Antikainen, R. & Tuomilehto, J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 30, 842–847 (2007).
Peppa, M. & Vlassara, H. Advanced glycation end products and diabetic complications: a general overview. Hormones 4, 28–37 (2005).
Negre-Salvayre, A., Salvayre, R., Augé, N., Pamplona, R. & Portero-Otín, M. Hyperglycemia and glycation in diabetic complications. Antioxid. Redox Signal 11, 3071–3109 (2009).
Luthra, M. & Balasubramanian, D. Nonenzymatic glycation alters protein structure and stability. A study of two eye lens crystallins. J. Biol. Chem. 268, 18119–18127 (1993).
Liu, J., Ru, Q. & Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 49, 170–183 (2012).
Adrover, M. et al. Mechanistic insights in glycation-induced protein aggregation. Biomacromolecules 15, 3449–3462 (2014).
Mariño, L. et al. Glycation of lysozyme by glycolaldehyde provides new mechanistic insights in diabetes-related protein aggregation. ACS Chem. Biol. 12, 1152–1162 (2017).
Oliveira, L. M. et al. Insulin glycation by methylglyoxal results in native-like aggregation and inhibition of fibril formation. BMC Biochem. 12, 41 (2011).
Oliveira, L. M. et al. Insights into the molecular mechanism of protein native-like aggregation upon glycation. Biochim. Biophys. Acta 1834, 1010–1022 (2013).
Ledru, F. et al. New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J. Am. Coll. Cardiol. 37, 1543–1550 (2001).
Ulrich, P. & Cerami, A. Protein glycation, diabetes, and aging. Recent. Prog. Horm. Res. 56, 1–22 (2001).
Vistoli, G. et al. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 47(Suppl 1), 3–27 (2013).
Ahmed, U., Anwar, A., Savage, R. S., Thornalley, P. J. & Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 18, 250 (2016).
Aronson, D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J. Hypertens. 21, 3–12 (2003).
Stern, D. M., Yan, S. D., Yan, S. F. & Schmidt, A. M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 1, 1–15 (2002).
Gomes, R. A. et al. Protein glycation in vivo: functional and structural effects on yeast enolase. Biochem. J. 416, 317–326 (2008).
Shinohara, M. et al. Overexpression of glyoxalase- I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Invest. 101, 1142–1147 (1998).
Peyroux, J. & Sternberg, M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. 54, 405–416 (2006).
Engelen, L., Stehouwer, C. D. & Schalkwijk, C. G. Current therapeutic interventions in the glycation pathway: evidence from clinical studies. Diabetes Obes. Metab. 15, 677–689 (2013).
Voziyan, P. A. & Hudson, B. G. Pyridoxamine: the many virtues of a maillard reaction inhibitor. Ann. N.Y. Acad. Sci. 1043, 807–816 (2005).
Adrover, M., Vilanova, B., Frau, J., Muñoz, F. & Donoso, J. The pyridoxamine action on Amadori compounds: A reexamination of its scavenging capacity and chelating effect. Bioorg. Med. Chem. 16, 5557–5569 (2008).
Voziyan, P. A. et al. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J. Biol. Chem. 278, 46616–46624 (2003).
Sajithlal, G. B., Chithra, P. & Chandrakasan, G. The role of metal-catalyzed oxidation in the formation of advanced glycation end products: an in vitro study on collagen. Free Radic. Biol. Med. 25, 265–269 (1998).
Sajithlal, G. B., Chithra, P. & Chandrakasan, G. An in vitro study on the role of metal catalyzed oxidation in glycation and crosslinking of collagen. Mol. Cell. Biochem. 194, 257–263 (1999).
Xiao, H., Cai, G. & Liu, M. Fe2+-catalyzed non-enzymatic glycosylation alters collagen conformation during AGE-collagen formation in vitro. Arch. Biochem. Biophys. 468, 183–192 (2007).
Hayase, F., Shibuya, T., Sato, J. & Yamamoto, M. Effects of oxygen and transition metals on the advanced Maillard reaction of proteins with glucose. Biosci. Biotechnol. Biochem. 60, 1820–1825 (1996).
Reddy, N.R. Occurrence, distribution, content and dietary intake of phytate. In: Foods Phytates (Reddy, N. R. & Sathe, S. K., eds). CRC Press, Boca Raton, FL, pp. 36–63 2002.
Vasca, E. et al. Complex formation between phytic acid and divalent metal ions: a solution equilibria and solid state investigation. Anal. Bioanal. Chem. 374, 173–178 (2002).
Rodrigues-Filho, U. P. et al. Heterometallic manganese/zinc-phytate complex as a model compound for metal storage in wheat grains. J. Inorg. Biochem. 99, 1973–1982 (2005).
Hawkins, P. T. et al. Inhibition of iron-catalysed hydroxyl radical formation by inositol polyphosphates: a possible physiological function for myo-inositol hexakisphosphate. Biochem. J. 294, 929–934 (1993).
Gupta, R. K., Gangoliya, S. S. & Singh, N. K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 52, 676–684 (2015).
Reddy, M. B., Hurrell, R. F., Juillerat, M. A. & Cook, J. D. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am. J. Clin. Nutr. 63, 203–207 (1996).
Grases, F., Simonet, B. M., Prieto, R. M. & March, J. G. Dietary phytate and mineral bioavailability. J. Trace. Elem. Med. Biol. 15, 221–228 (2001).
Grases, F., Simonet, B. M., Perello, J., Costa-Bauzá, A. & Prieto, R. M. Effect of phytate on element bioavailability in the second generation of rats. J Trace Elem. Med. Biol. 17, 229–234 (2004).
Lau, E. M. & Woo, J. Nutrition and osteoporosis. Curr. Opin. Rheumatol. 10, 368–372 (1998).
Cullumbine, H., Basnayake, V., Lemottee, J. & Wickramanayake, T. W. Mineral metabolism on rice diets. Br. J. Nutr. 4, 101–111 (1950).
Walker, A. R., Fox, F. W. & Irving, J. T. Studies in human mineral metabolism: 1. The effect of bread rich in phytate phosphorus on the metabolism of certain mineral salts with special reference to calcium. Biochem. J. 42, 452–462 (1948).
Grases, F. & Costa-Bauza, A. Phytate (IP6) is a powerful agent for preventing calcifications in biological fluids: usefulness in renal lithiasis treatment. Anticancer Res. 19, 3717–3722 (1999).
Grases, F. et al. Phytate acts as an inhibitor in formation of renal calculi. Front. Biosci. 12, 2580–2587 (2007).
Grases, F. et al. Anticalculus effect of a triclosan mouthwash containing phytate: a doubleblind, randomized, three-period crossover trial. J. Periodontal Res. 44, 616–621 (2009).
Grases, F. et al. Phytate reduces age-related cardiovascular calcification. Front. Biosci. 13, 7115–7122 (2008).
Sanchis, P. et al. Protective Effect of Myo-Inositol Hexaphosphate (Phytate) on Abdominal Aortic Calcification in Patients With Chronic Kidney Disease. J. Ren. Nutr. 26, 226–36 (2016).
Fernández-Palomeque, C. et al. Relationship between Urinary Level of Phytate and Valvular Calcification in an Elderly Population: A Cross-Sectional Study. PLoS One 10, 0136560 (2015).
López-González, A. A. et al. Protective effect of myo-inositol hexaphosphate (phytate) on bone mass loss in postmenopausal women. Eur. J. Nutr. 52, 717–26 (2013).
Vucenik, I. & Shamsuddin, A. M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J. Nutr. 133, 3778–3784 (2003).
Xu, Q., Kanthasamya, A. G. & Reddy, M. B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology 245, 101–108 (2008).
Sakurai, T., Kimura, S., Nakano, M. & Kimura, H. Oxidative modification of glycated low density lipoprotein in the presence of iron. Biochem. Biophys. Res. Commun. 177, 433–439 (1991).
Loske, C. et al. Transition metal-mediated glycoxidation accelerates cross-linking of beta-amyloid peptide. Eur. J. Biochem. 267, 4171–4178 (2000).
Kang, J. H. Oxidative damage of DNA by the reaction of amino acid with methylglyoxal in the presence of Fe(III). Int. J. Biol. Macromol. 33, 43–48 (2003).
Prieto, R. M. et al. Effects of Mediterranean diets with low and high proportions of phytate-rich foods on the urinary phytate excretion. Eur. J. Nutr. 49, 321–6 (2010).
Wei, Y. et al. D-ribose in glycation and protein aggregation. Biochim. Biophys. Acta. 1820, 488–494 (2012).
Sun, T. et al. The abnormally high level of uric d-ribose for type-2 diabetics. Prog. Biochem. Biophys. 40, 816–825 (2013).
Rimbach, G. & Pallauf, J. Phytic acid inhibits free radical formation in vitro but does not affect liver oxidant or antioxidant status in growing rats. J. Nutr. 128, 1950–1955 (1999).
Nagai, R., Murray, D. B., Metz, T. O. & Baynes, J. W. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 61, 549–59 (2012).
Cooper, G. J. Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes mellitus and Alzheimer’s disease. Drugs. 71, 1281–320 (2011).
Baynes, J. W. & Murray, D. B. The metal chelators, trientine and citrate, inhibit the development of cardiac pathology in the Zucker diabetic rat. Exp Diabetes Res. 1, 1–6 (2009).
Dammann, P., Sell, D. R., Begall, S., Strauch, C. & Monnier, V. M. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell’s mole-rat (Fukomys anselli). J. Gerontol. A Biol. Sci. Med. Sci. 67, 573–583 (2012).
Semba, R. D. et al. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am. J. Kidney Dis. 53, 51–58 (2009).
Hegab, Z., Gibbons, S., Neyses, L. & Mamas, M. A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 4, 90–102 (2012).
Meerwaldt, R. et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J. Am. Soc. Nephrol. 16, 3687–3693 (2005).
Machado, J. T. et al. N-acetylcysteine prevents endoplasmic reticulum stress elicited in macrophages by serum albumin drawn from chronic kidney disease rats and selectively affects lipid transporters, ABCA-1 and ABCG-1. Atherosclerosis 237, 343–352 (2014).
Tan, K. C. et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep 29, 329–333 (2006).
Wang, A. Y. et al. Skin autofluorescence associates with vascular calcification in chronic kidney disease. Arterioscler. Thromb. Vasc. Biol 34, 1784–1790 (2014).
Jiang, J. et al. Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis 224, 187–194 (2012).
Enomoto, M. et al. Positive association of serum levels of advanced glycation end products with thrombogenic markers in humans. Metabolism 55, 912–917 (2006).
Guosheng, L. et al. The relationship of serum AGE levels in diabetic mothers with adverse fetal outcome. J. Perinatol. 29, 483–488 (2009).
Vos, F. E., Schollum, J. B. & Walker, R. J. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 4, 368–375 (2011).
Florkowski, C. HbA1c as a diagnostic test for diabetes mellitus – reviewing the evidence. Clin. Biochem. Rev. 34, 75–83 (2013).
Prabhavathi, K., Kirthana, K. U. & Goturu, J. Glycosylated haemoglobin (HbA1c) - a marker of circulating lipids in type 2 diabetic patients. J. Clin. Diagn. Res. 8, 20–23 (2014).
Cho, N. H. et al. Optimal HbA1c cutoff for detecting diabetic retinopathy. Acta Diabetol. 50, 837–842 (2013).
Lin, C. C. et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am. J. Med. 126, 1017–1017 (2013).
Lee, W. J., Jang, S., Lee, S. H. & Lee, H. S. Correlation between the severity of diabetic peripheral polyneuropathy and glycosylated hemoglobin levels: a quantitative study. Ann. Rehabil. Med. 40, 263–270 (2016).
Hong, L. F. et al. Glycosylated hemoglobin A1c as a marker predicting the severity of coronary artery disease and early outcome in patients with stable angina. Lipids Health Dis. 13, 89–89 (2014).
Nybo, M., Hodgson, L. A., Kawasaki, R., Wong, T. Y. & Grauslund, J. Hemoglobin is associated with retinal vascular fractals in type 1 diabetes patients. Ophthalmic Epidemiol. 21, 327–32 (2014).
Sherwani, S. I., Khan, H. A., Ekhzaimy, A., Masood, A. & Sakharkar, M. K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights. 11, 95–104 (2016).
Omoruyi, F. O. et al. The Potential Benefits and Adverse Effects of Phytic Acid Supplement in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Sci. 2013, 172494 (2013).
Byun, K. et al. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 177, 44–55 (2017).
Foster, S. R., Omoruyi, F. O., Bustamante, J., Lindo, R. L. & Dilworth, L. L. The effect of combined inositol hexakisphosphate and inositol supplement in streptozotocin-induced type 2 diabetic rats. Int J Exp Pathol. 97, 397–407 (2016).
Lee, S. H. et al. Effects of dietary phytic acid on serum and hepatic lipid levels in diabetic KK mice. Nutr. Res. 25, 869–876 (2005).
Liu, N., Ru, Y., Wang, J. & Xu, T. Effect of dietary sodium phytate and microbial phytase on the lipase activity and lipid metabolism of broiler chickens. Br. J. Nutr. 103, 862–868 (2010).
Yuangklang, C., Wensing, T., Lemmens, A. G., Jittakhot, S. & Beynen, A. C. Effect of sodium phytate supplementation on fat digestion and cholesterol metabolism in female rats. J. Anim. Physiol. Anim. Nutr. (Berl). 89, 373–8 (2005).
Venn, B. J. & Mann, J. I. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 58, 1443–61 (2004).
Chandalia, M. et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 342, 1392–1398 (2000).
Anderson, J. W. et al. Metabolic effects of high-carbohydrate, high-fiber diets for insulin-dependent diabetic individuals. Am. J. Clin. Nutr. 54, 936–943 (1991).
Heighton, L., Schmidt, W. F. & Siefert, R. L. Kinetic and equilibrium constants of phytic acid and ferric and ferrous phytate derived from nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 56, 9543–9547 (2008).
Chobanian, A. V. et al. Seventh report of theJoint National Committee on Prevention, Detection, Evaluation, andTreatment of High Blood Pressure. Hypertension 42, 1206–52 (2003).
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106, 3143–421 (2002).
Grases, F. et al. Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. Biofactors 15, 53–61 (2001).
Grases, F., Simonet, B. M., March, J. G. & Prieto, R. M. Inositol hexakisphosphate in urine: the relationship between oral intake and urinary excretion. BJU Int. 85, 138–42 (2000).
Berga, F., Rodriguez, A., Costa-Bauzá, A. & Grases, F. Novel Colorimetric Determination of Phytate in Urine. Analytical Letters 49, 307–318 (2016).
Acknowledgements
P.S. expresses her appreciation for a postdoctoral fellowship (PD/009/2013) to the Conselleria de Educació, Cultura i Universitats del Govern de les Illes Balears and the European Social Fund, through the ESF Operational Programme of the Balearic Islands 2013–2017.
Author information
Authors and Affiliations
Contributions
All authors make substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data. P.S. and R.R. shares the first authorship. F.G. and L.M. are the senior’s researchers of the group. P.S., F.G. and L.M. conceived and designed the study. F.B., R.F. and A.C. collected the samples and performed laboratory analysis. M.A. and P.S. conceived and developed the “in vitro” studies. R.R. and L.M. performed the recruitment and the visits of the patients. R.R. collected the data. P.S. analysed and interpreted the data. P.S., M.A., L.M. and F.G. wrote the manuscript. All authors revised the manuscripts for important intellectual content.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanchis, P., Rivera, R., Berga, F. et al. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci Rep 8, 9619 (2018). https://doi.org/10.1038/s41598-018-27853-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27853-9
This article is cited by
-
Daily phytate intake increases adiponectin levels among patients with diabetes type 2: a randomized crossover trial
Nutrition & Diabetes (2023)
-
Phytic acid: a reservoir of phosphorus in seeds plays a dynamic role in plant and animal metabolism
Phytochemistry Reviews (2023)
-
Improving the nutritional quality of yam flour by substitution with Curculigo pilosa and in vitro digestibility and sensory analysis of its pasta
Journal of Food Measurement and Characterization (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.