Abstract
Although influenza may cause death in the geriatric population, the best method for predicting mortality in this population is still unclear. We retrospectively recruited older people (≥65 yr) with influenza visiting the emergency department (ED) of a medical center between January 1, 2010, and December 31, 2015. We performed univariate and multivariate logistic regression to identify independent mortality predictors and then developed a prediction score. Four hundred nine older ED patients with a nearly equal sex ratio were recruited. Five independent mortality predictors were identified: severe coma (Glasgow Coma Scale score ≤8), past histories of cancer and coronary artery disease, elevated C-reactive protein levels (>10 mg/dl), and bandemia (>10% band cells). We divided the patients into three mortality risk and disposition groups: (1) low risk (1.1%; 95% confidence interval [CI], 0.5–3.0%); (2) moderate risk (16.7%; 95% CI, 9.3–28.0%); and (3) high risk (40%; 95% CI, 19.8–64.2%). The area under the receiver operating characteristic curve and the Hosmer-Lemeshow goodness of fit of the GID score were 0.86 and 0.578, respectively. The GID score is an efficient and simple tool for predicting mortality in older ED patients with influenza. Further studies are warranted to validate its use.
Similar content being viewed by others
Introduction
The rapidly ageing population is a global issue. In the United States, the older population (aged ≥65 yr) is estimated to reach 89 million people by 2050, potentially constituting around 28% of the total U.S. population1. Taiwan has one of the most rapidly aging populations in the world2,3. The geriatric population in Taiwan in 2017 represents 13.3% of the country’s total population, and it is estimated that it will grow to 20% by 20252,4,5. This aging population has a significant impact on the health care system. In 2015, 38.5% of Taiwan National Health Insurance expenditures were contributed by the geriatric population6. A recent study in Taiwan demonstrated that older people also account for 35.6% of emergency medical services calls7.
Influenza is a common cause of death in older people8. The morbidity and mortality caused by influenza are often attributed to secondary bacterial infections and complications8. Although influenza seasons may vary in severity, the older population is most vulnerable to severe influenza9. A 2004 study in the U.S. reported that the older population accounted for about 186,000 excess hospitalizations10. Another study in the U.S. in 2003 reported that older persons accounted for 44,000 excess deaths resulting from all causes11. During influenza seasons, deciding whether to admit older person with influenza to the hospital is very difficult because of limited medical resources; therefore, predicting mortality in older people with influenza and their subsequent disposition become very important issues. Although researchers in several studies have reported mortality predictions for, and the critical care resource use of patients with influenza, most of these studies were focused on mortality prediction for adults in general, the prediction of specific influenza subtypes, and prediction of hospitalization12,13,14. Mortality prediction for older people with all subtypes of influenza is still unclear. Therefore, we conducted this study with the aim of delineating this issue.
Results
In total, we recruited 409 patients for this study. The mean age (±SD) was 79.5 (±8.3) yr, and the sex ratio was nearly equal (Table 1). The most common past histories were hypertension (64.3%), diabetes (39.8%), chronic obstructive pulmonary disease (27.1%), and coronary artery disease (25.1%). In the analysis of subtypes, influenza A was dominant (68%), whereas coinfection with influenza A and B comprised 2.7% of subtypes. The most common complications of influenza were pneumonia (67.5%), urinary tract infection (18.1%), and sepsis (8.3%). Admission and 30-day mortality rates were 83.9% and 4.9%, respectively. All patients were treated with either oseltamivir (94%) or zanamivir (6%) immediately when influenza was diagnosed in the emergency department (ED). Because the patients who had been treated in other hospitals were excluded, the recruited patients were treated with the antivirals for the first time during this influenza episode.
Univariate logistic regression analysis yielded ten univariate mortality predictors, each with a significance of p < 0.1: tachypnea, severe coma, hypertension, coronary artery disease, cancer, bedridden, leukocytosis, bandemia, anemia, and elevated C-reactive protein levels (CRP) (Table 2). Multivariate logistic regression analysis identified five independent mortality predictors: severe coma, elevated CRP, cancer, coronary artery disease, and bandemia. Specific points for each predictor were assigned to develop the geriatric influenza death (GID) score (Table 3).
According to GID score, the patients were divided into the following three risk subgroups: (1) low risk, defined as mortality risk of 1.1% (4/334; 95% confidence interval [CI], 0.5−3.0%); (2) moderate risk, defined as mortality risk of 16.7% (10/60; 95% CI, 9.3−28.0%); and (3) high risk, defined as mortality risk of 40.0% (6/15; 95% CI, 19.8−64.2%) (Fig. 1). The individual mortality risk for GID score is as follows: (1) score of 0, 0.6% (1/174); (2) score of 1, 1.9% (3/160); (3) score of 2, 16.7% (10/60); (4) score of 3, 42.9% (6/14); and (5) score of 4, 0% (0/1). Owing to the similar mortality risk of scores 0 and 1 and the small sample size of score 4, we combined scores 0 and 1 into the low-risk subgroup and scores 3 and 4 into the high-risk subgroup. The area under the receiver operating characteristic curve (AUC) of the GID score was 0.86 (95% CI, 0.77−0.94), which suggests excellent discrimination (Fig. 2). The Hosmer-Lemeshow goodness of fit was 0.578. The causes of death were pneumonia (15 [75%] of 20), bacteremia (2 [10%] of 20), and combined pneumonia and urinary tract infection (3 [15%] of 20). The classifications of influenza among the deaths were type A, 60% (12 of 20); type B, 25% (5 of 20); and types A and B, 15% (3 of 20).
Discussion
We developed the GID score, consisting of five independent mortality predictors, to help predict 30-day mortality and suggested disposition of older people with influenza in the emergency department. The GID score had excellent discrimination and a good fit. After calculating the GID score, we found that high-risk patients with scores ≥3 are considered critically ill, and admission to the intensive care unit is suggested; moderate-risk patients with a score of 2 are recommended to be admitted to the general ward; and low-risk patients with scores ≤1 may be admitted to the general ward or discharged with outpatient department follow-up after ED treatment. This tool may help in determining how to effectively allocate medical resources and thus preserve them for patients with greater need. In addition, because the GID score was developed in the geriatric population with influenza by univariate and multivariate logistic regression analyses, it is more important and specific than the tools used for the overall geriatric population.
Severe coma is the strongest predictor of mortality in older ED patients with influenza. This finding is compatible with the Third International Consensus Definitions for Sepsis and Septic Shock, published in 2016, which suggest that altered mentation be one of the variables used to predict mortality among patients with infection (quick Sepsis-related Organ Failure Assessment [qSOFA] criteria)15. However, the state of altered mentation is often difficult to define, and should be compared with the patient’s baseline mental status. Using severe coma for the variable of mentation is more realistic and more feasible to quantify16. CRP is believed to be correlated with sepsis-related mortality. The researchers in a study that included 2785 adult patients in Denmark reported that CRP independently predicted 30-day mortality if the level was >10 mg/dl17. Researchers in another study also reported that risk of sepsis-related mortality appeared to have increased when the third-day CRP value was >10 mg/dl18. Researchers in a large nationwide study in the United States reported that patients with a history of cancer are at increased risk for acquiring sepsis and subsequently dying as a result19. Patients with cancer often are in an immunocompromised state due to the use of chemotherapy, radiation, or other immune-modulating therapy to combat the underlying malignancy19. They may also have impaired leukocyte function due to the malignancy itself, which makes them vulnerable to infection19. Influenza may lead to the exacerbation of preexisting cardiac disease and direct cardiac involvement, resulting in myocarditis and deterioration of heart failure20. The influenza virus is also assumed to play an important role in atherogenesis or atherothrombosis by destabilizing present vulnerable plaques, which may trigger acute myocardial infarctions and eventually result in an increased chance of mortality21. Bandemia is found to be a predictor of mortality in the diseases associated with infection21,22, and is one of the criteria for systemic inflammatory response syndrome (SIRS)15. Although the qSOFA has been emphasized as a better tool for identifying sepsis in recent studies, the SIRS criteria may remain useful for the identification of infection15.
We could not conclude that influenza vaccination was not effective despite this study showing a non-significant association between influenza vaccination and mortality. The sample is limited to older person presenting to the ED with influenza, thus providing a limited and potentially biased population with which to measure any association between influenza vaccine and outcome. In addition, the effectiveness of the influenza vaccine may varies from season to season11,23. The influenza vaccination plays an important role in attempts to reduce the mortality burden of influenza11. A previous study showed the benefits of inactivated influenza vaccine in preventing deaths resulting from complications of influenza infection11.
The prevalence of chronic obstructive pulmonary disease in this study was higher than in the overall geriatric population. A nationwide study in Taiwan reported that the prevalence of chronic obstructive pulmonary disease was around 8.8% in the population of ≥70 years in 200224. The probable reason is that older persons with chronic obstructive pulmonary disease are more susceptible to influenza infections due to impaired pulmonary function24.
Despite this study’s strength of proposing a novel tool for predicting mortality in older ED patients, it has some limitations. First, some of the data may not have been collected completely, owing to the nature of a retrospective study design. Second, the results of this study may not be generalizable to other nations or other regions in Taiwan, owing to its being a single-center study. Further studies are warranted to validate the use of the GID score. Third, the influenza pharyngeal or nasal swabs used in this study—although the most practical choice for most medical facilities—are not a gold standard method with confirmed false-positive and false-negative rates. Further advanced examinations, such as reverse transcriptase–polymerase chain reaction, immunofluorescence assay, or viral culture, are needed to confirm the diagnosis of influenza. Fourth, some other laboratory markers, such as procalcitonin, lactic acid, and albumin, which are associated with mortality in patients with severe sepsis, were not included in this study. The reason why we did not include these measurements in our study is that it is not practical to perform these tests or to obtain these data for every older ED patient with suspected influenza. Fifth, there may be substantial differences in patients presenting within the influenza season and at during the off-season, because influenza is a seasonal illness. The variation between seasons is important, as different strains can have different severities for specific populations, such as older people. Therefore, subsequent studies about this issue are also warranted. Sixth, the susceptibility to antivirals was not included, which may affect the prognosis of influenza.
In conclusion, the GID score is an easy, simple, and efficient tool for predicting 30-day mortality and facilitating decision making for the disposition of older ED patients with influenza. The GID score also may potentially be applicable in other medical settings. Furthermore, using the ratio of the actual to expected number of deaths based on the GID score may help in the evaluation of the quality of care in older persons with influenza. Despite the advantages of the GID score, it cannot completely replace the clinical judgment of the treating physician.
Methods
Study design, setting, and participants
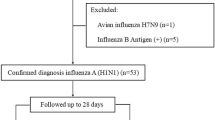
We conducted this study in an 825-bed, university-affiliated medical center in northern Taiwan. The center has a 40-bed ED providing care for approximately 55,000 patients per year16,25. Older people comprised about 33% of all ED patients. All older persons who visited the ED between January 1, 2010, and December 31, 2015, were recruited if they met both of the following criteria: (1) having a fever, defined as tympanic temperature ≥37.2 °C or a baseline temperature elevated ≥1.3 °C16,25; and (2) influenza infection, defined as a positive influenza pharyngeal or nasal swab result (either type A or type B)26. Overall, 479 older ED patients met the criteria for influenza. 409 patients were recruited after we excluded 70 patients who had insufficient data or had been treated in other hospitals. The recruited patients were divided into two groups on the basis of their 30-day outcome: survival vs. mortality. The study flowchart is shown in Fig. 3.
Data sources, measurement, and definitions of the categorical variables
We retrospectively collected data, including demographic characteristics, vital signs, past history, laboratory data, complications, and outcomes, from medical records by an emergency physician. Information on the variables of interest for each patient was recorded (Tables 1 and 2). Any variable not recorded in the patient’s medical record was considered negative. We defined categorical variables as the following, in accordance with previous studies in emergency or geriatric medicine:
- 1.
-
2.
Bedridden: Eastern Cooperative Oncology Group performance status 4 (completely disabled, cannot carry on any self-care, and totally confined to bed or chair)25,30.
- 3.
-
4.
Tachypnea: Respiratory rate >20/min25.
-
5.
Leukocytosis: White blood cell count >12,000 cells/mm3 16,25,31.
-
6.
Bandemia: Immature band form count >10%15.
-
7.
Anemia: Hemoglobin <12 mg/dl32.
-
8.
Thrombocytopenia: Platelet count <150 × 103/mm3 33.
-
9.
Renal impairment: Serum creatinine >2 mg/dL27.
-
10.
Elevated CRP: >10 mg/dL34.
Complications of influenza include pneumonia, urinary tract infection, sepsis, meningitis, hepatitis, and others. The diagnosis of complications was based on the treating physician’s documentation in the medical record. The treatment of influenza in the study hospital was based on the clinical practice guidelines of the Infectious Diseases Society of America35.
Definition of endpoint
The primary endpoint was 30-day mortality16,25,26,28,29,36. Older persons who survived ≥30 days were considered “survivors” for this analysis. Telephone follow-up was used to ascertain 30-day survival if the patient was discharged before 30 days.
Ethics statement
This study was conducted according to the Declaration of Helsinki. Because this was an observational study, the Cathay General Hospital Institutional Review Board approved the study protocol and waived the need for informed consent (written and oral) from the participants.
Statistical methods
IBM SPSS Statistics version 23.0 for Mac (IBM, Armonk, NY, USA) was used to conduct all statistical analyses. The power was 0.82 using G-power 3.0 for analysis. Continuous data are presented as mean ± SD. An independent samples t test, or the Mann-Whitney-Wilcoxon test was used for continuous variables. Pearson’s chi-square test or Fisher’s exact test was used for categorical variables. Univariate variables with p < 0.1 were included in the multivariate stepwise (forward) logistic regression analysis for investigating independent mortality predictors37. The level of significance was set at 0.05 (two-tailed). Weights were assigned to each independent mortality predictor according to the predicted b values of the multivariate logistic regression analysis divided by 2 and rounded to the nearest integer27. Then, we developed a GID score and calculated the GID score for each patient. The stability of the GID score was evaluated by bootstrapping methods, for which we conducted random sampling from actual study patients, and 1000 hypothetical study populations were generated. We also estimated the bootstrapped effect size and 95% CI for each coefficient. The AUC was used to evaluate the discrimination of the score by bootstrap methods. We also used the Hosmer-Lemeshow test to evaluate goodness of fit.
References
Ortman, J. M., Velkoff, V. A. & Hogan, H. An aging nation: the older population in the United States. Washington, DC: US Census Bureau; 2014, http://www.census.gov/prod/2014pubs/p25-1140.pdf (2014).
National development council, department of executive, Taiwan. Population Projections for R.O.C. (Taiwan): 2016~2060, http://www.ndc.gov.tw/en/cp.aspx?n=2E5DCB04C64512CC&s=002ABF0E676F4DB5 (2017).
Ke, Y. T. et al. Emergency geriatric assessment: a novel comprehensive screen tool for geriatric patients in the emergency department. Am J Emerg Med. 36, 143–146 (2018).
Huang, C. C. et al. Chronic osteomyelitis increases long-term mortality risk in the elderly: a nationwide population-based cohort study. BMC Geriatr. 16, 72 (2016).
Wu, C. J. et al. Septic arthritis significantly increased the long-term mortality in geriatric patients. BMC Geriatr. 17, 178 (2017).
National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. The National Health Insurance Statistics, 2015, http://www.nhi.gov.tw/english/Content_List.aspx?n=70805F6752EE7B9E&topn=616B97F8DF2C3614 (2015).
Huang, C. C. et al. Elderly and Nonelderly Use of a Dedicated Ambulance Corps’ Emergency Medical Services in Taiwan. Biomed Res Int. 2016, 1506436 (2016).
Wong, C. M., Chan, K. P., Hedley, A. J. & Peiris, J. S. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 39, 1611–1617 (2004).
Nichol, K. L., Nordin, J. D., Nelson, D. B., Mullooly, J. P. & Hak, E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 357, 1373–1381 (2007).
Thompson, W. W. et al. Influenza-associated hospitalizations in the United States. JAMA. 292, 1333–1340 (2004).
Thompson, W. W. et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 289, 179–186 (2003).
Oh, W. S. et al. A prediction rule to identify severe cases among adult patients hospitalized with pandemic influenza A (H1N1) 2009. J Korean Med Sci. 26, 499–506 (2011).
Adeniji, K. A. & Cusack, R. The Simple Triage Scoring System (STSS) successfully predicts mortality and critical care resource utilization in H1N1 pandemic flu: a retrospective analysis. Crit Care. 15, R39 (2011).
Hak, E., Wei, F., Nordin, J., Mullooly, J. & Poblete, S. Development and validation of a clinical prediction rule for hospitalization due to pneumonia or influenza or death during influenza epidemics among community-dwelling elderly persons. J Infect Dis. 189, 450–458 (2004).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 315, 801–810 (2016).
Chung, M. H. et al. Geriatric Fever Score: A New Decision Rule for Geriatric Care. Plos One. 9, e110927 (2014).
Gradel, K. O. et al. Does C-reactive protein independently predict mortality in adult community-acquired bacteremia patients with known sepsis severity? APMIS. 121, 835–842 (2013).
Devran, O. et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip Respir Med. 7, 47 (2012).
Danai, P. A., Moss, M., Mannino, D. M. & Martin, G. S. The epidemiology of sepsis in patients with malignancy. Chest. 129, 1432–1440 (2006).
Mamas, M. A., Fraser, D. & Neyses, L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 130, 304–309 (2008).
Madjid, M., Naghavi, M., Litovsky, S. & Casscells, S. W. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 108, 2730–2736 (2003).
Tsai, C. L. et al. Impact of diabetes on mortality among patients with community-acquired bacteremia. J Infect. 55, 27–33 (2007).
Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Effectiveness, 2005–2018, https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm (2018).
Wang, Y. C. et al. Prevalence and risks of chronic airway obstruction: a population cohort study in taiwan. Chest. 131, 705–710 (2007).
Chung, M. H. et al. Hypotension, bedridden, leukocytosis, thrombocytopenia and elevated serum creatinine predict mortality in geriatric patients with fever. Geriatr Gerontol Int. 15, 834–839 (2015).
Boivin, G., Hardy, I., Tellier, G. & Maziade, J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 31, 1166–1169 (2000).
Huang, C. C. et al. Predicting the Hyperglycemic Crisis Death (PHD) Score: A New Decision Rule for Emergency and Critical Care. Am J Emerg Med. 31, 830–834 (2013).
Huang, C. C. et al. Infection, Absent Tachycardia, Cancer History, and Severe Coma Are Independent Mortality Predictors in Geriatric Patients with Hyperglycemic Crises. Diabetes Care. 36, e151–152 (2013).
Huang, H. S. et al. Predicting the mortality in geriatric patients with dengue fever. Medicine (Baltimore). 96, e7878 (2017).
Oken, M. M. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5, 649–655 (1982).
Dellinger, R. P. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39, 165–228 (2013).
Mukhopadhyay, D. & Mohanaruban, K. Iron deficiency anaemia in older people: investigation, management and treatment. Age Ageing. 31, 87–91 (2002).
Marik, P. E. Don’t miss the diagnosis of sepsis! Crit Care. 18, 529 (2014).
Luzzani, A. et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 31, 1737–1741 (2003).
Harper, S. A. et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 48, 1003–1032 (2009).
Huang, C. C. et al. Cancer History, Bandemia, and Serum Creatinine Are Independent Mortality Predictors in Patients with Infection-Precipitated Hyperglycemic Crises. BMC Endocr Disord. 13, 23 (2013).
Laupacis., A., Sekar, N. & Stiell, I. G. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 277, 488–494 (1997).
Acknowledgements
This work was supported by Chi-Mei Medical Center, [Grant number CMNCKU10620]. We thank Miss Ti Hsu for the English revision.
Author information
Authors and Affiliations
Contributions
J.Y.C., C.C. Hsu, C.C. Huang, and H.R.G. designed and conceived this study and wrote the manuscript. J.Y.C. performed the statistical analysis. J.H.C., W.L.C. and H.J.L. provided professional suggestions and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, JY., Hsu, CC., Chen, JH. et al. Geriatric influenza death (GID) score: a new tool for predicting mortality in older people with influenza in the emergency department. Sci Rep 8, 9312 (2018). https://doi.org/10.1038/s41598-018-27694-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27694-6
This article is cited by
-
Using artificial intelligence to predict adverse outcomes in emergency department patients with hyperglycemic crises in real time
BMC Endocrine Disorders (2023)
-
Projections of heat-related excess mortality in China due to climate change, population and aging
Frontiers of Environmental Science & Engineering (2023)
-
Two-stage prediction model for in-hospital mortality of patients with influenza infection
BMC Infectious Diseases (2021)
-
Predicting outcomes in older ED patients with influenza in real time using a big data-driven and machine learning approach to the hospital information system
BMC Geriatrics (2021)
-
Gender differences and influenza-associated mortality in hospitalized influenza A patients during the 2018/19 season
Infection (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.