Abstract
Cigarette smoke exposure is a major risk factor in chronic obstructive pulmonary disease (COPD) and its interactions with genetic variants could affect lung function. However, few gene-smoking interactions have been reported. In this report, we evaluated the effects of gene-smoking interactions on lung function using Korea Associated Resource (KARE) data with the spirometric variables—forced expiratory volume in 1 s (FEV1). We found that variations in FEV1 were different among smoking status. Thus, we considered a linear mixed model for association analysis under heteroscedasticity according to smoking status. We found a previously identified locus near SOX9 on chromosome 17 to be the most significant based on a joint test of the main and interaction effects of smoking. Smoking interactions were replicated with Gene-Environment of Interaction and phenotype (GENIE), Multi-Ethnic Study of Atherosclerosis-Lung (MESA-Lung), and COPDGene studies. We found that individuals with minor alleles, rs17765644, rs17178251, rs11870732, and rs4793541, tended to have lower FEV1 values, and lung function decreased much faster with age for smokers. There have been very few reports to replicate a common variant gene-smoking interaction, and our results revealed that statistical models for gene-smoking interaction analyses should be carefully selected.
Similar content being viewed by others
Introduction
The spirometric measurement forced expiratory volume in the first second (FEV1) reflects the physiological and functional state of the lungs; this measure has been used as the gold standard for diagnosing a lung disease, classifying its severity, assessing its progression over time, and monitoring the treatment response1. Furthermore, this parameter is a predictor of other morbidities and mortality in the general population, even independent of smoking history2,3. Reduced FEV1 is a characteristic of chronic obstructive pulmonary disease (COPD), a leading cause of mortality and morbidity worldwide4,5. The prevalence and burden of COPD are expected to increase in the coming decades owing to continued exposure to COPD risk factors and the aging population5.
Multiple risk factors for COPD have been identified, and smoking has been recognized as the major risk factor for a rapid decline in lung function and consequent development of COPD. However, only a minority of smokers develop COPD6, and there are substantial differences in the sensitivity to smoking among individuals. These differences are partly attributable to genes and/or their interactions with smoking. Heritability estimates of lung function range from 39% to 66%7,8. Moreover, a hereditary severe deficiency in alpha-1 antitrypsin, encoded by SERPINA1 on chromosome 149, is the best known genetic risk factor for the development of COPD. However, severe alpha-1 antitrypsin deficiency accounts for only about 1% of the patients with COPD10. Thus, improving our understanding about disease pathogenesis and progression would require studies on genetic susceptibility loci and their interactions with smoking.
Recently, many genome-wide association studies (GWASs) have been conducted to identify the genetic loci associated with lung function levels, and many genome-wide significant loci have been identified. The necessity of replication across populations with diverse ethnic or environmental characteristics has been reported11. However, loci identified from GWASs have often failed to be replicated in different populations12. There are many reasons for this inconsistency, and several studies have shown a partial relation with gene-environment interactions11,13. Smoking has a strong effect on lung function, and the effects of gene-smoking interactions on lung function have been repeatedly highlighted14,15,16. However, the effects of gene-smoking interactions on variability in lung function in different ethnicities are not clear. Furthermore, because COPD is expected to be the fifth most common disease worldwide, with the third highest mortality rate5 in 2020, and because its burden, including financial cost, is predicted to increase, early prediction of lung function may be important for developing individualized therapeutic strategies, and further studies are required to identify genetic factors that predict the risk for a subsequent rapid decline in lung function across ethnicities.
In this study, we aimed to identify the genetic variants interacting with smoking on FEV1 using genome-wide interaction studies (GWISs). We considered various models in terms of smoking-related variables and variance-covariance structure, and the best model was chosen by Akaike information criterion (AIC) for each dataset. GWISs were conducted using the Korea Associated Resource (KARE) data. We detected that SNPs with the smallest P-values are located near SOX9. The SOX9 has been reported to be involved in lung branching morphogenesis17 and recovery of lung function after lung injury18. It should be noted that genetic association of SOX9 was firstly detected by Hankcock et al., but their interaction P-values were larger than 0.0519. We replicated interactions in the Gene-Environment of Interaction and phenotype (GENIE), Multi-Ethnic Study of Atherosclerosis-Lung (MESA-Lung), and COPDGene studies. Our findings provide important insights into our understanding about lung disease prevention and control.
Methods
Data description
Our analyses consisted of two phases—discovery and replication. For the discovery phase, we conducted GWISs on FEV1 using KARE data. For the replication phase, we considered GENIE, MESA-Lung, and COPDGene data, and replicated the significant results identified from the filtering step. Detailed procedure for genotyping, quality controls (QC), and imputations for each data are described in the Supplementary Text 1.
KARE
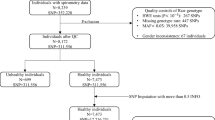
Data collected by the KARE project were used for GWASs. Participants were recruited from the rural Ansung and urban Ansan cohorts. Initiated in 2001 as part of the Korean Genome Epidemiology Study (KoGES), the initial samples included 5,018 and 5,020 participants aged 40–69 years from Ansung and Ansan areas, respectively. After QC of genotypes, there were 8,534 participants between the age of 40 and 69 years with at least one spirometry test and genotype data (see Supplementary Text 1 for detailed procedures about QC). Among these participants, 4,001 were men and 4,533 were women. The values of FEV1 were observed up to three times every two years, and 19,557 measurements were used for the analyses. Smoking history was obtained through a questionnaire, and smoking status and pack years were used for association analyses as covariates. Smoking status was categorized as never smokers, former smokers, and current smokers. Never smokers were defined as individuals who had never smoked, and former smokers were participants who had smoked previously, but stopped smoking prior to the survey. Current smokers were individuals who stated that they currently smoked during the investigation, or who had a record of smoking and did not belong to the other two categories. According to our categorization, there were 4,926 never smokers, 1,742 former smokers, and 1,866 current smokers in our cohort.
GENIE
GENIE data were used to replicate SNPs identified from GWISs using KARE data. The GENIE cohort consisted of 7,999 participants, who had visited Seoul National University Gangnam Center during 201420. They agreed to participate in genetic studies and donated blood samples, and after QC, there were 5,971 participants (3,404 men and 2,567 women) with spirometry and smoking-related variables (see Supplementary Text 1 for detailed procedures about QC). Spirometry and smoking-related variables were repeatedly measured up to 11 times. Smoking-related variables were obtained by questionnaire. Based on their responses, smoking status was categorized into three groups, similar to the smoking status categories used in the KARE data. The numbers of never smokers, former smokers, and current smokers were 3,396, 1,804, and 771, respectively.
MESA-Lung non-Hispanic whites
MESA-Lung data were used to replicate SNPs identified from GWISs using KARE data. MESA was a prospective cohort initiated to investigate cardiovascular diseases. Participants consisted of 6,814 men and women aged 45–84 years. As a subgroup of the MESA cohort, the MESA-Lung study enrolled 3,965 participants who were sampled from the MESA cohort, and agreed to participate in the genetic analysis and to measure their lung functions21. The MESA-Lung study was composed of four populations—non-Hispanic whites (NHWs; 35%), African-Americans (AAs; 26%), Hispanics (23%), and Chinese-Americans (16%)21—and we considered only NHWs. After QC of genotypes, 1,033 participants had both spirometry and smoking-related variables with genotype data, including 459 never smokers, 468 former smokers, and 106 current smokers (see Supplementary Text 1 for detailed procedures about QC).
COPDGene
COPDGene was a multi-center study on smokers with and without COPD, and included AAs and NHWs. All participants had at least 10 pack years of smoking, and their ages were between 45 and 80 years. Pre- and post-bronchodilator spirometric data were obtained for all participants with standardized spirometry (EasyOne Spirometer; Zurich, Switzerland); to be consistent with other studies, we focused on pre-bronchodilator spirometry. After QC of genotypes, 7,760 NHWs and 3,300 AAs were enrolled for replication studies22 (see Supplementary Text 1 for detailed procedures about QC). AAs consisted of 2,643 current smokers and 657 former smokers, and NHWs consist of 2,616 current smokers and 4,054 former smokers.
Statistical analysis
Smoking has a significant effect on respiratory function, and it was found that variances of FEV1 can differ by smoking status. Linear mixed models which allow heteroscedasticity according to smoking status were computationally very intensive and we consider a two step approach; (i) filtering step and (ii) testing step. In the filtering step, participants were stratified according to the smoking status and a likelihood ratio test with 3 degrees of freedom which allow heteroscedasticity according to smoking status were applied to select the most significantly associated SNPs. Filtering step was considered only for discovery data, and the likelihood ratio test will be called 3 DF test in the remainder of this article. For testing step, we consider several variance-covariance structures for the linear mixed models, and the best model was chosen with AIC. Then former, current, and never smokers were pooled, and linear mixed models with the smallest AIC were applied to the SNPs selected from the filtering step.
Filtering step: genome- wide interaction studies (GWISs) with KARE data
We considered FEV1 (mL) as spirometric measures, which were used to identify the genetic variants interacting with smoking. GWISs were conducted using KARE data. We found that there were no substantial differences in spirometric measures between current smokers and former smokers for KARE data with AIC, and both groups were combined into a single group; ever smokers. To handle heteroscedasticity, we conducted stratified analyses which applied linear mixed models to never smokers and ever smokers separately for GWISs. For both groups, sex, age at baseline measurement, height, body mass index (BMI), elapsed time from the baseline measurement, and interaction of age and sex were included as covariates. It was reported that lung function decline accelerates after he or she becomes 35 years old23, and all participants in KARE data were around 40–69 years old at the baseline. Thus, effect of ages on the baseline pulmonary function measurements and that of elapsed time on their decrement were expected to be substantially different, and it is a main reason why the baseline age and elapsed time from the baseline measurement were considered as different covariates. To adjust for population substructure strictly, principal component (PC) analyses were applied to the genetic relationship matrix, and the first 10 PC scores were included as covariates24. FEV1 of each participant was measured up to 3 times, and FEV1 at each time point was included as response variables. The similarities among repeated spirometric measures for each participant were handled with a random intercept. We let y ij be FEV1 values for participant i at time point j, and they were assumed to follow multivariate normal (MVN) distribution. We denote elapsed time from baseline measurement and PC scores for participant i and component k by time ij and pc i k. Then the linear mixed model for ever smokers becomes
For never smokers, we consider
It should be noted that pack years are 0 for never smokers and were included as covariates only for ever smokers. We compared several structures for Σ and Σ′, and selected an unstructured covariance structure. The proposed models were applied to detect gene-smoking interaction of FEV1 average levels. To identify SNPs interacting with smoking on spirometric measures, we considered H0: \({\beta ^{\prime} }_{7}={\beta }_{8}={\beta }_{9}=0.\) This could be tested by summing a likelihood ratio test with 2 degrees of freedom (DF) for ever smokers and a likelihood ratio test with 1 DF for never smokers. The summed likelihood ratio test followed the chi-square test with 3 DF under the null hypothesis, and this statistic would be denoted as the 3 DF test. The most significant SNPs were selected for further analyses of gene-smoking interaction effects.
We compare the results from 3 DF test with the homoscedasticity model. For homoscedasticity model, ever and never smokers were pooled and linear mixed model was fitted. We assume the same variances between never smokers and ever smokers, and the same coefficients of covariates as follows:
Then P-values for homoscedasticity model were obtained by likelihood-ratio tests with 3 degrees of freedom for H0: \({\beta }_{10}={\beta }_{11}={\beta }_{12}=0.\) This will be called homoscedasticity test in the remainder of this article.
Testing step: estimating the effects of SNPs and SNP-smoking interaction effects with KARE data
The 3 DF test provides P-values for overall effects about the main and interaction effects. However, it could not identify interaction effects of SNPs with smoking on lung function because never and ever smokers were separately analyzed. Furthermore, stratified analyses are usually less powerful compared to analyses using pooled data. Thus, former, current, and never smokers in KARE data were pooled and analyzed by a linear mixed model.
While building the linear mixed model, we first assessed variance-covariance structures by smoking status. If heteroscedasticity is not correctly taken into account, the false positive rates cannot be controlled with P-values25. Furthermore, modeling the relationship between smoking-related variables and FEV1 is not clear. If lung function of the participants worsens, they tend to quit smoking, and such indication biases make the relationships complicated. Thus, the appropriate choice of smoking-related variables may depend on sampling strategies. We considered various models with the choices of smoking-related variables and variance-covariance structure by smoking status for each dataset, and the best model was selected with the AICs. Notably, SNPs were not included for the model selection, and this step did not violate any statistical inference.
Supplementary Table S1 presents AICs for various models fitted to FEV1 for KARE data. Supplementary Table S1 shows that KARE data has the smallest AIC when smoking status had two levels (never versus ever smokers) instead of three levels, and different variances between never smokers and ever smokers were assumed. All covariates used for GWISs of KARE data were also included as covariates in our linear mixed model. We found that coefficients of some covariates differed according to smoking status, and interactions of some covariates and smoking-related variables were considered. For example, interactions between smoking status and time effects were significant at the 0.05 level and included as covariate. Furthermore, to control the effects of confounders on interaction effects, the interaction between confounders and environmental factors of interest should be considered for gene-environmental analyses26. Therefore, we considered interactions between PC scores for adjusting the population substructures and smoking status regardless of significance at 0.05 level. We considered g = 1 and 2 indicate ever smokers and never smokers, respectively, and n i is the number of repeatedly observed measurements for participant i. The best model selected with AIC was as follows:
This model includes two smoking-related variables—smoking status, and pack years. Therefore, both were used to define the interactions between SNP and smoking. To test gene and environment interactions, we considered
Replication studies
SNPs selected from GWISs using KARE data were replicated with GENIE, MESA-Lung, and COPDGene data. For each dataset, we considered various variance-covariance structures, and the best model was selected with AICs. Supplementary Tables S2–S4 show AICs for GENIE, MESA-Lung, and COPDGene data, respectively, which were used as replication studies. The selected models with AICs were used to replicate the effects of SNPs and their interactions with smoking. Notably, SNPs and their interactions were not considered for model selection. A final model for replication data is described in Supplementary Text 2.
Data availability
All data analyzed in this article were utilized in previously published articles (KARE: Cho, Go et al.27; GENIE: Choe, Lee et al.20; MESA-Lung: Hankinson, Kawt et al.21; COPDGene: Castaldi, Cho et al.22).
Results
Descriptive statistics
Table 1 shows baseline characteristics of participants in the KARE, GENIE, MESA-Lung, and COPDGene data. KARE and GENIE data were from the Korean population and included both baseline and longitudinal data. MESA-Lung and COPDGene were cross-sectional data, and participants in MESA-Lung were NHWs, whereas COPDGene data consisted of AAs and NHWs. In the KARE data, there were 8,534 participants, of which 47% were men. In the KARE data, participants were 40–69 years old, and the percentage of never smokers, former smokers, and current smokers were 58%, 20%, and 22%, respectively. The GENIE cohort composed of participants who were regularly screened for health, and their average lung function values were expected to be better than those of the general population. There were 5,971 participants, of which 57% were men. Participants were repeatedly measured an average of 3.13 times. In the GENIE data, participants were 30–84 years old, which explained the largest range of FEV1. In the GENIE data, the percentages of never smokers, former smokers, and current smokers were 56.9%, 30.2%, and 12.9%, respectively. MESA-Lung data consisted of 1,033 participants, of which 50.5% were men. These participants were 45–84 years old, and 44.4%, 45.3%, and 10.3% were never, former, and current smokers, respectively. Lastly, COPDGene data consisted of AAs and NHWs. The number of AAs was 3,300, of which 56% were men; these participants were 45–80 years old, and the percentages of former and current smokers were 20% and 80%, respectively. The number of NHWs was 6,670, of which 52% were men. Participants were 45–80 years old, and 61% and 39% were former and current smokers, respectively. Unlike KARE, GENIE, and MESA-Lung data, there were no never smokers in the COPDGene data, and participants in the COPDGene data had the lowest mean FEV1 and the highest pack years.
Heterogeneity of FEV1 decline along ages
Figure 1 shows the estimated FEV1 according to age and their 95% confidence intervals. The generalized additive models were applied for MESA-Lung and COPD gene data. KARE and GENIE data have the repeated measures of FEV1 levels, and the generalized additive mixed models were used. According to Figure 1, there were substantial differences among FEV1 according to the smoking status for each dataset. Current smokers in KARE and MESA-Lung data tended to have the lowest FEV1 values, followed by former smokers. For GENIE data, there were no differences in FEV1 values among never, former, and current smokers. Interestingly, for NHWs and AAs in COPDGene data, average FEV1 values of former smokers were smaller than those of current smokers, even though the differences were quite small for NHWs. This difference likely relates to selection bias, because participants with the worse lung function tended to quit smoking28,29, and only heavy smokers were considered for COPDGene. These results suggest that the same model could not be applied to different data to identify SNPs interacting with smoking, and the best model for the choice of smoking-related covariates and variance-covariance structures were selected with AICs.
GWISs of FEV1 with KARE data
In the discovery phase, associations of 310,515 SNPs were tested by applying the 3 DF test to KARE data. For the GWISs of FEV1, we included ten PC scores to adjust population substructure, and they explain 0.41% of genetic variances. Scatter plot for the first two PC scores and scree plot are provided in Supplementary Figure S1. Figure 2A presents the QQ-plot for 3 DF tests, and it shows that the proposed 3 DF tests generally preserve nominal significance levels. Variance-inflation factors (VIF) were estimated test was also applied30 and Figure 2B shows its QQ plot. Figure 2B reveals evidence of some inflation, and VIF of homoscedasticity model, 1.22, is substantially larger than 1. Supplementary Figure S2 is a Manhattan plot of results from the 3 DF test. We further checked the effects of heteroscedasticity according to smoking status on FEV1/FVC by applying the same methods, and there were no significant results (Supplementary Table S5). Supplementary Figure S3A,B are based on their results from 3 DF test and homoscedasticity test, respectively. VIFs for both models were 1.01 and 1.12. Therefore, we can conclude that heteroscedasticity according to smoking status should be carefully addressed for identifying the interaction effects between SNPs and smoking on lung function.
Table 2 shows the most significant SNPs from results of 3 DF tests for FEV1. These selected SNPs were located in the upstream region of SOX9 on chromosome 17 and had similar minor allele frequencies (MAFs). Furthermore the linkage distribution plot in Figure 3A reveals that these factors were highly correlated, which indicates that four significant SNPs actually indicate same association signal. Figure 3B shows the regional plot for this region. Those 4 SNPs are located within topologically associated domains (TAD) of SOX9 region (68.67~70.45 Mb of chromosome 17 in hg19)31,32,33. DNA sequences within a TAD physically interact with each other more frequently than with sequences outside the TAD, and thus our most significant SNPs may affect the expression of SOX9. Therefore, our results indicate that SOX9 may be functionally related with FEV1.
Effects of SNPs and SNP-smoking interactions on spirometric measures in KARE data
3 DF tests cannot separately estimate the main effects of SNPs and their interaction effects with smoking. In addition, ever and never smokers were separately analyzed, and such stratified analyses were less powerful than analyses with pooled data. Thus, all participants were pooled and analyzed with linear mixed models. Supplementary Table S1 shows AICs for the various models, and the selected model assumed different variances between never and ever smokers, corresponding to the linear mixed model eq. (5). This method was computationally very intensive and was applied to the most significant SNPs from GWISs. The selected models are summarized in Supplementary Table S6, and the Wald test of selected models for rs17765644, which has the smallest P-value in filtering step, are provided in Table 3. These results were obtained with PROC MIXED in SAS(version 9.4). The results for the other three SNPs are provided in Supplementary Tables 7–9 The results also showed that P-value for testing H0: \({\beta }_{{\rm{SNP}}}={\beta }_{\mathrm{SNP}-\mathrm{smoking}}={\beta }_{\mathrm{SNP}-\mathrm{PY}}=0\) was close to the Bonferroni-adjusted 0.05 significance level (1.61 × 10−7), and their SNP-smoking group interactions were significant at the 0.05 significance level, even though there were no significant interactions between SNPs and pack years. Coefficients for both the main association of rs17765644 and its interaction with smoking status were −0.025 and −0.029, respectively. This implies that the FEV1 of never smokers tend to be lower around (number of minor alleles) × 0.025 and ever-smokers are further lower around (number of minor alleles) × 0.029. Figure 4 shows the interacting effect of SNP with smoking status.
Replication studies with GENIE, MESA-Lung, and COPDGene data
To validate the potential association of lung function with smoking, the four SNPs selected with KARE data were replicated in GENIE, MESA-Lung, and COPDGene data. According to the results from KARE data, the main effects of those four SNPs were negative, and their effects were more profound for ever smokers. Based on these factors, we conducted one-tailed tests for the main and interaction effects at the 0.05 significance level for replication studies.
Table 3 shows the results of replication studies for rs17765644. The best model for GENIE data was selected with AICs, and the selected model is shown in the eq. (1) of Supplementary Text 2 and Supplementary Table S6. According to Table 3, the main effect of rs17765644 was not significant for GENIE data. Smoking status had three levels, and two dummy variables were defined for GENIE data. Never smokers were used as the reference level, and P-value for overall test about the main effect of SNP, and interaction effects for SNP-smoking status, and SNP-pack years was significant at the 0.1 significance level. Interaction P-values between rs17765644 and dummy variables for former and current smokers were 0.052 and 0.049, respectively. The interaction effect between rs17765644 and dummy variables for current smokers was −0.024, which was much smaller than that between the SNP and dummy variable for former smokers. If former and current smokers were combined into ever smokers, and two levels were defined for smoking status, the estimated interaction effects between smoking status and rs17765644 and its P-values were −0.019 and 0.04, respectively.
The best model for MESA-Lung data was also selected with AICs, and the selected model is shown in the eq. (2) of Supplementary Text 2 and Supplementary Table S6. The smoking status for the best model has two levels, never and ever smokers. The dummy variable for smoking status is coded as 1 for ever smokers and as 0 for never smokers. As shown in Table 3, the P-value for overall effects was 0.0037. P-value for the main effect of rs17765644 was 0.008. The interaction effect between rs17765644 and smoking status was not significant, but its interaction with pack years was significant (P-value = 0.014). Thus, we concluded that the FEV1 values of ever and never smokers were not proportional to the coded genotypes, but the amount of decrease according to the pack years was proportional. Results for the other SNPs had a pattern similar to that of rs17765644 because they were highly correlated (Figure 3). These results are shown in Supplementary Tables 7–9
Lastly, AAs and NHWs in COPDGene data for rs17765644 were utilized to replicate the main effect and its interaction with smoking. For COPDGene data, there were no never smokers, and smoking status had two levels, former and current smokers. We found that former and current smokers had different FEV1 values, and the best model was selected with AIC. The selected models for AAs and NHWs are shown in the eqs (3) and (4) of Supplementary Text 2, respectively, and summarized in Supplementary Table S6. Former smokers were used as the reference level for smoking status. However, because there were no never smokers, and the directions of regression coefficients from the KARE data could not be considered, we used two-tailed tests. For AAs, the interaction effects of rs17765644 were significant at the 0.1 significance level (P-value = 0.082). Interestingly, rs11870732 (Supplementary Table S8) had significant interactions with smoking status (P-value = 0.037). For NHWs, the main effect of rs17765644 was significant at the 0.05 significance level, and the regression coefficient was −0.066, which was consistent with the data from other studies. However, its interaction effect with smoking status had the opposite direction (P-value = 0.054). This result may be attributable to the selection bias, and former smokers may have smoked more than the current smokers. The results for the other SNPs showed patterns similar to those of rs17765644.
Discussion
We conducted GWISs of lung function (FEV1) to identify genetic variants interacting with cigarette smoking. We identified such an interaction using a joint test near the previously described SOX9 locus on chromosome 17 in KARE. These findings were further explored in GENIE, MESA-Lung, and COPDGene. In this report, firstly, we replicated the main effects of SOX9 on FEV1 values in Koreans, as shown in NHWs. Secondly, we found some evidence for SOX9 gene-smoking interaction effects on FEV1, and former/current smokers with minor alleles of the selected SNPs near SOX9 tended to have lower FEV1 values, even though the interaction effects were not strong. According to our results, the coefficient of gene-smoking interaction showed the same direction as the main SNP effect and its amount was almost the same. There have been very few studies showing a significant gene-smoking interaction effect, which could be replicated in other populations, though there were inconsistencies in the interaction effects for different ethnicities. Finally, we highlighted that the statistical model for the analysis of gene-smoking interactions should be carefully selected. The effects of smoking on FEV1 were very strong, and the means and variances could differ according to smoking status. Notably, when the mis-specified variance-covariance matrices were taken into account, QQ plots were inflated, and type-1 errors were not controlled.
SOX9 has been extensively studied and shown to have pivotal roles in the lung epithelium during branching morphogenesis17. The epithelial-mesenchymal transition (EMT) is the process through which epithelial cells become mesenchymal-like, playing crucial roles in tissue repair and several pathological processes, including tissue fibrosis, tumor invasiveness, and metastasis. EMT is involved in specific steps in embryogenesis and organ development; however, this concept has been challenged by recent evidence showing that terminally differentiated epithelium can be changed to mesenchymal cells, even in adulthood34. This process can be activated by tissue injury or pathological stresses, and inappropriately controlled processes may induce tissue fibrosis and cancer. SOX9 induces migratory fibroblasts responsible for extracellular matrix (ECM) deposition and tissue destruction by EMT35. SOX9 induces lung fibrosis mediated by transforming growth factor (TGF)-β1 repair signaling, characterized by inappropriate ECM deposition; this can result in the destruction of tissue architecture and function34,35. Recent studies have suggested that SOX9 activation is essential for the recovery of lung function after acute lung injury, and SOX9 inhibition induces impaired recovery18. Smoking, which includes exposure to several oxidants and free radicals, causes numerous pulmonary diseases through inflammatory processes, leading to cell recruitment to the lung, activation of signaling pathways, and upregulation of proteins, consequently contributing to disruption of the lung ECM. This process varies from person to person; therefore, genetic susceptibility and gene-smoking interactions have been suggested to contribute to disease progression. Previous genome-wide joint meta-analyses of SNPs by smoking interactions on FEV1 and FEV1/FVC across 19 studies (total N = 50,047) demonstrated that SOX9 was associated with FEV1 and was expressed at higher levels in the airway epithelium in smokers than in non-smoking adults of the European ancestry19. Our findings were consistent with the results of a previous study, and the SOX9 gene-smoking interaction effects were verified in several ethnicities (Korean, NHWs, and AAs).
However, despite our interesting findings, some inconsistencies were observed in our replication results. For example, an interaction between smoking status and SNPs was found for KARE and GENIE data; however, for MESA-Lung data, the interaction between pack years and SNPs was significant. For COPDGene data, there were no never smokers, and former/current smokers were compared. Coefficients of smoking status and SNP interactions were significant at the 0.1 significance level, but had different directions for NHWs and AAs. There were multiple explanations for these phenomena, including ethnic differences36,37. Genetic ancestry itself is not assumed to be a cause for this difference, but could account for differences in lung function and susceptibility to smoking. For example, the structures of smoking experience vary by population38 implying complicated relationships between genetics, ethnicity, smoking, and lung function39. Therefore, the effects of gene-smoking interactions can be heterogeneous among different populations. In our replication study, we considered diverse ethnicities. According to our results, the replication with GENIE data was quite consistent with that of KARE data, and both were based on the Korean population. However, MESA-Lung and COPDGene data consisted of NHWs and AAs. Small differences between GENIE and KARE data could be explained by the characteristics of the participants. For example, KARE data were based on rural and urban community populations, and GENIE data were composed of participants who underwent regular health screening and received routine medical care. Medical care and routine health check-ups are often positively related to socioeconomic status40.
Furthermore, the effects of smoking on FEV1 are substantial; however, modeling its relationship with FEV1 is not clear. For example, we found that the effects of gene-smoking status were significant in the KARE and GENIE data, and in the MESA-Lung data, the effects of gene-pack years were significant. Smoking does not have a linear relationship with FEV1, and non-linear relationships41,42 have been reported frequently. The importance of various factors, such as smoking cessation time, smoking behavior, duration, and total dose, has been shown43,44 to explain the effects of smoking. In this report, we utilized the smoking status and pack years based on a self-reported smoking history. The prevalence of smoking from self-reporting surveys is usually underestimated, and the degree of underestimation varies among different countries45,46,47. To minimize this heterogeneity among different studies, we selected the best model with AIC. However, the appropriate definition for smoking is still unclear, and further studies are still necessary.
COPD is expected to be the third leading cause of mortality worldwide within a few years, and identifying genetic variants interacting with smoking would be beneficial in terms of social burden, aging, and the growing importance of personalized medicine48. However, statistical models that correctly model the effects of gene-smoking interactions based on lung function are complicated, and successful gene-smoking interaction analyses have been very limited. The proposed method illustrates the complexity of gene-smoking interaction analyses, and to identify consistent gene-smoking interactions, a statistical model should be developed that considers the non-linear relationships between smoking history and lung function.
References
Chandra, D. et al. Airflow limitation and endothelial dysfunction. unrelated and independent predictors of atherosclerosis. American journal of respiratory and critical care medicine 194, 38–47 (2016).
Kurl, S., Jae, S. Y., Kauhanen, J., Ronkainen, K. & Laukkanen, J. A. Impaired pulmonary function is a risk predictor for sudden cardiac death in men. Ann Med 47, 381–385, https://doi.org/10.3109/07853890.2015.1036111 (2015).
Mannino, D. M., Buist, A. S., Petty, T. L., Enright, P. L. & Redd, S. C. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 58, 388–393 (2003).
Murphy, S. L., Kochanek, K. D., Xu, J. & Arias, E. Mortality in the United States, 2014. NCHS Data Brief, 1–8 (2015).
Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187, 347–365, https://doi.org/10.1164/rccm.201204-0596PP (2013).
Terzikhan, N. et al. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol 31, 785–792, https://doi.org/10.1007/s10654-016-0132-z (2016).
Klimentidis, Y. C. et al. Heritability of pulmonary function estimated from pedigree and whole-genome markers. Frontiers in genetics 4, 174 (2013).
Palmer, L. et al. Familial aggregation and heritability of adult lung function: results from the Busselton Health Study. European Respiratory Journal 17, 696–702 (2001).
Hersh, C. P., DeMeo, D. L. & Silverman, E. K. National Emphysema Treatment Trial state of the art: genetics of emphysema. Proceedings of the American Thoracic Society 5, 486–493 (2008).
Lieberman, J., Winter, B. & Sastre, A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest 89, 370–373 (1986).
Imboden, M. et al. Modification of the association between PM10 and lung function decline by cadherin 13 polymorphisms in the SAPALDIA cohort: a genome-wide interaction analysis. Environ Health Perspect 123, 72–79, https://doi.org/10.1289/ehp.1307398 (2015).
Smolonska, J., Wijmenga, C., Postma, D. S. & Boezen, H. M. Meta-analyses on suspected chronic obstructive pulmonary disease genes: a summary of 20 years’ research. Am J Respir Crit Care Med 180, 618–631, https://doi.org/10.1164/rccm.200905-0722OC (2009).
Kim, H. J. et al. CDH13 gene-by-PM10 interaction effect on lung function decline in Korean men. Chemosphere 168, 583–589, https://doi.org/10.1016/j.chemosphere.2016.11.022 (2017).
Curjuric, I. et al. Different genes interact with particulate matter and tobacco smoke exposure in affecting lung function decline in the general population. PloS one 7, e40175 (2012).
Hallberg, J. et al. Genetic and environmental influence on lung function impairment in Swedish twins. Respiratory research 11, 92 (2010).
He, J.-Q., Connett, J. E., Anthonisen, N. R., Paré, P. D. & Sandford, A. J. Glutathione S-transferase variants and their interaction with smoking on lung function. American journal of respiratory and critical care medicine 170, 388–394 (2004).
Rockich, B. E. et al. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proceedings of the National Academy of Sciences 110, E4456–E4464 (2013).
Li, L. et al. Sox9 activation is essential for the recovery of lung function after acute lung injury. Cellular Physiology and Biochemistry 37, 1113–1122 (2015).
Hancock, D. B. et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet 8, e1003098 (2012).
Choe, E. K. et al. Search for genetic factor association with cancer-free prostate-specific antigen level elevation on the basis of a genome-wide association study in the Korean population. European Journal of Cancer Prevention (2017).
Hankinson, J. L. et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. CHEST Journal 137, 138–145 (2010).
Castaldi, P. J. et al. Genome-wide association identifies regulatory Loci associated with distinct local histogram emphysema patterns. American journal of respiratory and critical care medicine 190, 399–409 (2014).
Sharma, G. & Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clinical interventions in aging 1, 253 (2006).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics 38, 904–909 (2006).
Long, J. S. & Ervin, L. H. Using heteroscedasticity consistent standard errors in the linear regression model. The American Statistician 54, 217–224 (2000).
Keller, M. C. Gene× environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological psychiatry 75, 18–24 (2014).
Cho, Y. S. et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nature genetics 41, 527–534 (2009).
Willemse, B., Postma, D., Timens, W. & Ten Hacken, N. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. European Respiratory Journal 23, 464–476 (2004).
Burr, M. L., Phillips, K. M. & Hurst, D. N. Lung function in the elderly. Thorax 40, 54–59 (1985).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Smyk, M., Akdemir, K. C. & Stankiewicz, P. SOX9 chromatin folding domains correlate with its real and putative distant cis-regulatory elements. Nucleus 8, 182–187 (2017).
Franke, M. et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature (2016).
Pervjakova, N. & Prokopenko, I. The TAD-pathway for GWAS signals. European Journal of Human Genetics 25, 1179–1180 (2017).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation 119, 1420–1428 (2009).
Pritchett, J., Athwal, V., Roberts, N., Hanley, N. A. & Hanley, K. P. Understanding the role of SOX9 in acquired diseases: lessons from development. Trends in molecular medicine 17, 166–174 (2011).
Whitrow, M. J. & Harding, S. Ethnic differences in adolescent lung function: anthropometric, socioeconomic, and psychosocial factors. American journal of respiratory and critical care medicine 177, 1262–1267 (2008).
Korotzer, B., Ong, S. & Hansen, J. E. Ethnic differences in pulmonary function in healthy nonsmoking Asian-Americans and European-Americans. American journal of respiratory and critical care medicine 161, 1101–1108 (2000).
Aschard, H. et al. Challenges and opportunities in genome-wide environmental interaction (GWEI) studies. Human genetics 131, 1591–1613 (2012).
Aldrich, M. C. et al. Genetic ancestry-smoking interactions and lung function in African Americans: a cohort study. PloS one 7, e39541 (2012).
Mackenbach, J. P., Stronks, K. & Kunst, A. E. The contribution of medical care to inequalities in health: differences between socio-economic groups in decline of mortality from conditions amenable to medical intervention. Social Science & Medicine 29, 369–376 (1989).
Castaldi, P. J. et al. Impact of non-linear smoking effects on the identification of gene-by-smoking interactions in COPD genetics studies. Thorax 66, 903–909 (2011).
Ambrose, J. A. & Barua, R. S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American college of cardiology 43, 1731–1737 (2004).
Leffondré, K., Abrahamowicz, M., Siemiatycki, J. & Rachet, B. Modeling smoking history: a comparison of different approaches. American journal of epidemiology 156, 813–823 (2002).
Pandeya, N. et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. American journal of epidemiology 168, 105–114 (2008).
West, R., Zatonski, W., Przewozniak, K. & Jarvis, M. J. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiology and Prevention Biomarkers 16, 820–822 (2007).
Gorber, S. C., Schofield-Hurwitz, S., Hardt, J., Levasseur, G. & Tremblay, M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine & Tobacco Research 11, 12–24 (2009).
Wong, S. L., Shields, M., Leatherdale, S., Malaison, E. & Hammond, D. Assessment of validity of self-reported smoking status. Health reports 23, D1 (2012).
MacDonald-Wilson, K. L., Deegan, P. E., Hutchison, S. L., Parrotta, N. & Schuster, J. M. Integrating personal medicine into service delivery: empowering people in recovery. Psychiatric rehabilitation journal 36, 258 (2013).
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1302) and the National Research Foundation of Korea (2017M3A9F3046543). MHC and EK were supported by the R01HL113264 (MHC and EKS). The COPDGene project was supported by Award Number R01 HL089897 and Award Number R01 HL089856 from the National Heart, Lung, and Blood Institute. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
Author information
Authors and Affiliations
Contributions
Boram Park, So-My Koo, Hyeon-Jong Yang and Sungho Won wrote the main manuscript text, and Boram Park, Jaehoon An and MoonGye Lee analyzed and interpreted the results. Hae Yeon Kang, Dandi Qiao, Michael H. Cho, Joohon Sung and Edwin K. Silverman provided the data. Michael H. Cho, Joonhon Sung, Edwin K. Silverman, Hyeon-Jong Yang and Sungho Won revised this paper critically for important intellectual content. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, B., Koo, SM., An, J. et al. Genome-wide assessment of gene-by-smoking interactions in COPD. Sci Rep 8, 9319 (2018). https://doi.org/10.1038/s41598-018-27463-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27463-5
This article is cited by
-
Genetics of chronic respiratory disease
Nature Reviews Genetics (2024)
-
SRY-related high-mobility group box 9 (SOX9) alleviates cigarette smoke extract (CSE)-induced inflammatory injury in human bronchial epithelial cells by suppressing stromal interaction molecule 1 (STIM1) expression
Inflammation Research (2022)
-
Effect of 6p21 region on lung function is modified by smoking: a genome-wide interaction study
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.