Abstract
This study aimed to evaluate the effects of ischemia-reperfusion injury (IRI) on the risk of hepatocellular carcinoma (HCC) recurrence after liver transplantation. Data of 195 patients were retrospectively analysed. Post-reperfusion aspartate (AST), alanine transaminase, and lactate dehydrogenase (LDH) levels were the primary measures of IRI. Tumour recurrence was the primary endpoint. Post-reperfusion AST was a continuous risk factor for tumour recurrence in patients within Milan criteria (p = 0.035), with an optimal cut-off of 1896 U/L. Recurrence-free survival of patients within Milan criteria and post-reperfusion AST of <1896 and ≥1896 U/L was 96.6% and 71.9% at 5 and 3.7 years, respectively (p = 0.006). Additionally, post-reperfusion AST and LDH exceeding the upper quartile significantly increased the risk of HCC recurrence in patients within Milan criteria (p = 0.039, hazard ratio [HR] = 5.99 and p = 0.040, HR = 6.08, respectively) and to a lesser extent, in patients within Up-to-7 criteria (p = 0.028, HR = 3.58 and p = 0.039, HR = 3.33, respectively). No other significant IRI effects were found in patients beyond the Up-to-7 criteria and in analyses stratified for independent risk factors for recurrence: tumour number and differentiation, alpha-fetoprotein, and microvascular invasion. Thus, IRI exerts major negative effects on the risk of HCC recurrence after liver transplantation in patients within standard and extended criteria.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) remains one of the most common indications for liver transplantation1. The Milan criteria defined transplant eligibility for HCC patients for more than two decades; however, the limits are now being expanded according to morphological and biological tumour features2,3,4,5,6. Nevertheless, discussion on widening the pool of potential candidates is controversial owing to a major and relatively constant shortage of deceased donors. Further expansion of the selection criteria will inevitably lead to increased waiting times for both HCC and non-HCC populations. In HCC patients, markedly prolonged times on the waiting list are characterised by more common dropouts, possibly leading to the development of more aggressive tumours7. Owing to increased rates of listing and privileged positions of HCC patients under the current allocation policies, a higher number of HCC transplant candidates may have even more detrimental effects on non-HCC patients’ waiting times and pre-transplant mortality8,9. Because widening the donor pool with living donors and high-risk or extended criteria deceased donors is a common strategy, it appears to have major relevance, particularly for HCC patients.
Experimental studies demonstrate the increased risk of cancer recurrence associated with ischemia-reperfusion injury (IRI)10,11. Changes in hepatic microenvironment caused by IRI promote seeding and the development of metastases, whereas IRI-induced proinflammatory response, release of growth factors, mobilization of progenitor cells, and transformation of cancer cells to more aggressive phenotypes may potentiate the formation and growth of metastases at both local and remote sites12,13,14,15,16. Because grafts procured from high-risk deceased donors and to a lesser extent, partial grafts procured from living donors may be more susceptible to IRI, the use of these grafts may increase the risk of post-transplant HCC recurrence. This hypothesis was subject to numerous studies with inconsistent results. Although transplantations of grafts procured from living donors or high-risk grafts procured from deceased donors after cardiac death or those who were older and had hepatic steatosis or other risk factors were reported to have adverse effects on outcomes after liver transplantations for HCC in several studies, the results of available studies are not completely consistent17,18,19,20,21,22,23. However, recent reports found that prolonged ischemic times, directly related to the magnitude of IRI, increased the risk of post-transplant HCC recurrence24,25. Nevertheless, data on the direct effect of the magnitude of IRI on the risk of HCC recurrence after deceased donor liver transplantation are limited. Therefore, this study aimed to evaluate the association between the degree of graft IRI as indicated by post-reperfusion transaminase and lactate dehydrogenase (LDH) levels and the risk of post-transplant HCC recurrence after deceased donor liver transplantation with respect to patients’ initial risk profile.
Methods
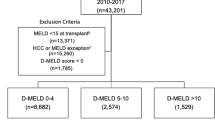
This was a retrospective observational study. In total, 250 liver transplantations were performed for HCC patients between January 2001 and June 2016 at the Department of General, Transplant and Liver Surgery (Medical University of Warsaw). Patients with fibrolamellar HCCs and those with combined HCC/cholangiocarcinoma were not included. After exclusion of 55 patients with missing measurements of transaminase levels 2 h after reperfusion, the final study cohort comprised 195 liver transplant recipients. The study protocol was approved by the institutional review board of the Medical University of Warsaw. Informed consents were not obtained from the patients due to the retrospective nature of the study, which is in line with institutional review board and national regulations. All methods were performed in accordance with the relevant guidelines and regulations. No organs were procured from prisoners.
The degree of IRI was represented by three variables, namely, serum alanine transaminase (ALT), serum aspartate transaminase (AST), and serum LDH levels; each was assessed from a blood sample obtained 2 h after portal reperfusion. These variables were the primary factors of interest. Peak serum bilirubin concentration, international normalised ratio (INR), and gamma-glutamyl transpeptidase (GGTP) activity over the first 7 post-transplant days were additionally analysed as variables associated with IRI. The duration of cold and warm ischemia was defined as the time from clamping of the donor aorta until the removal of the graft from the preservation solution and that from the removal of the graft from the cold preservation solution until portal reperfusion, respectively. The sum of cold and warm ischemic times formed the total ischemic time. All grafts were procured from donors after brain death. Tumour recurrence over the 5-year post-transplant observation period was the primary end-point. Recurrence-free survival was calculated from the date of transplantation until tumour recurrence and censored at the date of last available follow-up, death for non-HCC related causes or 5 years post-transplantation (whichever occurred first). Details on the surgical technique, perioperative care, immunosuppression, and follow-up protocol are provided elsewhere26,27.
First, post-reperfusion ALT, AST, and LDH levels were assessed for their potential effect on the risk of post-transplant tumour recurrence in all patients. Other independent predictors of recurrence were also assessed, including peak post-transplant bilirubin concentration, INR, and GGTP activity. Furthermore, the analyses were adjusted for their potential confounding effects in bivariable analyses. Subgroup analyses were subsequently performed to determine the potential differences in associations between IRI degree and the risk of HCC recurrence according to patients’ initial risk profile, based on fulfilment of selection criteria and established independent predictors of recurrence.
Continuous and categorical variables are given as medians (interquartile ranges) and numbers (percentages). The Kaplan-Meier method was used for survival calculations, and log-rank test was used for intergroup comparisons. A Cox proportional hazards regression analysis was performed to evaluate the associations between particular factors and the risk of recurrence. A multivariable model was created using forward stepwise method with p thresholds of 0.05 and 0.150 for inclusion and exclusion of variables, respectively. An additional series of bivariable analyses were performed to adjust the effects of IRI to potential confounding effects of independent risk factors for tumour recurrence. Spearman correlation coefficients were calculated to evaluate the associations between ischemic times and donor age and post-reperfusion laboratory measurements. Post-reperfusion AST, ALT, and LDH levels; peak post-transplant bilirubin concentration; and peak post-transplant GGTP activity were transformed to their natural logarithms prior to their analyses as continuous variables. Additionally, they were assessed as categorical factors using the upper quartile for division. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cut-offs of continuous factors in predicting recurrence. Hazard ratios (HRs) and c-statistics were presented with 95% confidence intervals (95% CIs). Significance threshold was set to two-tailed p values of 0.05. Analyses were computed in STATISTICA version 13 (Dell Inc., Tulsa, USA) software. The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
The characteristics of the 195 patients are shown in Table 1. Median AST, ALT, and LDH levels assessed 2 h post-reperfusion were 850 U/L (interquartile range: 486–1625 U/L; range 153–14375 U/L), 566 U/L (interquartile range: 304–935 U/L; range 102–9912 U/L), and 2240 U/L (interquartile range: 1322–4670 U/L; range 385–38207 U/L), respectively. Post-reperfusion AST and ALT levels were significantly, yet poorly correlated, with total (both p = 0.001) and cold ischemic times (both p < 0.001), whereas post-reperfusion LDH levels were poorly correlated with cold ischemic time (p = 0.031), intraoperative fresh frozen plasma transfusions (p = 0.002), and intraoperative packed red blood cell transfusions (p = 0.018, Table 2). Donor age and warm ischemic times were not correlated with post-reperfusion AST, ALT, and LDH levels.
The median follow-up period was 37.5 months. A total of 27 patients developed HCC recurrence with recurrence-free survival rates of 90.8%, 83.4%, and 81.0% at 1, 3, and 5 years, respectively. Univariable analyses revealed that post-reperfusion AST (p = 0.521), ALT (p = 0.773), and LDH (p = 0.575) levels and peak 7-day post-transplant bilirubin concentration (p = 0.592), INR (p = 0.553), and GGTP activity (p = 0.534) were not significantly associated with recurrence in all patients (Table 3). There were also no significant differences in recurrence-free survival depending on the quartile of AST (p = 0.725), ALT (p = 0.819), and LDH (p = 0.656) levels (Fig. 1). Similarly, no differences with respect to recurrence-free survival were observed depending on the quartile of peak 7-day postoperative bilirubin concentration (p = 0.849), INR (p = 0.309), and GGTP activity (p = 0.866; Fig. 2). In multivariable analysis, the independent risk factors comprised tumour number (p = 0.004), pre-transplant alpha-fetoprotein concentration (p < 0.001), presence of microvascular invasion (p = 0.014), and poor tumour differentiation (p = 0.007). No significant effects of post-reperfusion AST (all p > 0.250), ALT (all p > 0.403), and LDH (all p > 0.176) levels and peak 7-day postoperative bilirubin concentration (all p > 0.167), INR (all p > 0.230), and GGTP activity (all p > 0.123) on the risk of recurrence were found in analyses adjusted for the effects of these independent predictors. The corresponding series of bivariable analyses are presented in Tables 4 and 5. Additionally, fulfilment of the Milan (p < 0.001; HR 0.17, 95% CI 0.07–0.43); University of California, San Francisco (UCSF, p = 0.009; HR 0.37, 95% CI 0.17–0.78); and Up-to-7 (p < 0.001; HR 0.24, 95% CI 0.11–0.51) criteria significantly reduced the risk of recurrence.
For further analyses, the patients were divided into subgroups based on the fulfilment of selection criteria and independent predictors of recurrence. Cut-offs for tumour number of ≥3 and alpha-fetoprotein concentration of ≥48.3 ng/ml were derived from the corresponding ROC curves. As a continuous variable, post-reperfusion AST significantly influenced the risk of HCC recurrence only in patients within the Milan criteria (p = 0.035, Table 6) with the optimal cut-off of ≥1896 U/L. Additionally, post-reperfusion AST and LDH levels exceeding the upper quartiles were significantly associated with increased risk of recurrence in patients either within the Milan (p = 0.039 and p = 0.040, respectively) or Up-to-7 (p = 0.028 and p = 0.039, respectively) criteria. The degree of IRI, as reflected by post-reperfusion AST, ALT, and LDH levels, did not significantly influence the HCC recurrence risk in patients within the UCSF criteria or in those beyond the Milan, UCSF, or Up-to-7 criteria. No other significant associations between post-reperfusion AST, ALT, and LDH levels and the risk of post-transplant tumour recurrence were observed in subgroups derived from divisions based on tumour number, alpha-fetoprotein concentration, presence of microvascular invasion, and degree of tumour differentiation. In contrast to the significant effects of IRI in patients within the Milan or Up-to-7 criteria, no effects were found for the duration of total ischemia (all p > 0.701), cold ischemia (all p > 0.417), warm ischemia (all p > 0.373), and donor age (all p > 0.276) in these subgroups (Table 7). No significant associations between peak 7-day postoperative bilirubin concentration (all p > 0.081), INR (all p > 0.205), and GGTP activity (p > 0.097) and HCC recurrence risk were identified in subgroup analyses (Table 8).
In patients within the Milan criteria, recurrence-free survival at 1, 3, and 5 years was 98.8%, 96.6%, and 96.6%, respectively, when post-reperfusion AST level was <1896 U/L as opposed to 86.2%, 86.2%, and 71.9% at 1, 3, and 3.7 years, respectively, when post-reperfusion AST level was ≥1896 U/L (p = 0.006, Fig. 3A). Similarly, patients within the Milan criteria and with post-reperfusion LDH level <4670 U/L exhibited 5-year recurrence-free survival of 97.4%, which was significantly higher (p = 0.016) than the 1-, 3-, and 5-year rates of 90.2%, 84.2%, and 78.2%, respectively, observed for those within the Milan criteria and with post-reperfusion LDH level ≥4670 U/L (Fig. 3B). Significant differences with respect to 5-year recurrence-free survival depending on post-reperfusion AST (p = 0.027) and LDH (p = 0.031) levels were also observed for patients within the Up-to-7 criteria (Fig. 3C,D).
Discussion
In the era of donor shortage and increasing utilization of high-risk grafts to partly ameliorate its negative effects, the problem of potential association between the degree of IRI and the risk of HCC recurrence after liver transplantation is of utmost importance. According to the available results of experimental studies, hepatic IRI, universally present in the setting of liver transplantation, increases the risk of metastasis formation both within the ischemic and remote sites through changes in the local microenvironment, induction of inflammatory response, induction of metastatic potential of circulating cancer cells, and systemic release of pro-tumourigenic cytokines12,13,14,15,16. Our study results demonstrate a major negative effect of IRI on the risk of post-transplant HCC recurrence, although limited to patients with low tumour burden.
Importantly, initial analyses performed in all patients failed to reveal any significant associations between post-reperfusion AST, ALT, and LDH levels and HCC recurrence risk, irrespective whether the factors were analysed as continuous or categorical variables. However, the study cohort comprised patients with a wide range of tumour burden due to a liberal selection policy utilised in the authors’ department before establishment of precise criteria5. Nevertheless, a major significant negative effect of post-reperfusion AST and LDH levels was observed for patients within the Milan criteria, which still determine the majority of liver transplant recipients28. Similar findings, although of remarkably lesser extent, were found for patients within the Up-to-7 criteria, whereas the magnitude of IRI did not influence the risk of recurrence in patients beyond the extended criteria. This indicates that the clinical relevance of IRI is limited to generally low-risk populations and diminishes with increasing tumour burden. This appears to be particularly importantly because it demonstrates the possibility of using high-risk grafts to expand the donor pool for high-risk HCC candidates in the context of discussion on widening the boundaries of existing selection criteria2,3,4,5,6,29. Notably, the safe use of extended criteria allografts preferentially for patients with advanced tumours was already reported30. Conversely, none of the subgroup analyses performed in high-risk patients, including those beyond particular selection criteria, with ≥3 tumours, alpha-fetoprotein concentration ≥48.3 ng/mL, or with tumours either poorly differentiated or with microvascular invasion, revealed a significant effect of IRI on the risk of HCC recurrence. Therefore, while these findings point toward the possibility of the utilization of grafts more prone to IRI for high-risk HCC patients, they also indicate limited clinical relevance of reducing IRI in these patients.
In contrast to the use of post-reperfusion transaminases and LDH levels as surrogates of IRI degree in the present study, previous studies focused on the negative effects of prolonged graft ischemia or donor characteristics17,18,19,20,21,22,23,24,25,31. However, the degree of IRI is driven by the interplay of several donor risk factors, of which a single component may not necessarily be an adequate measure of IRI32. In the present study, the laboratory measures of graft ischemia were significantly, yet poorly correlated to graft ischemic times, which in fact is consistent with the results presented by other authors25. This may partly explain the inconsistent results of studies on the effect of duration of graft ischemia and particular donor factors on HCC recurrence risk, as these may not always accurately reflect the magnitude of IRI17,18,19,20,21,22,23,24,25,31.
In contrast to the significant effects of IRI limited to low-risk patients found in the present study, two previous analyses specifically aimed at the effect of ischemic times on tumour recurrence revealed the presence of significant associations particularly in high-risk HCC patients24,25. These populations were characterised by 18F-fluorodeoxyglucose tumour avidness on pre-transplant positron emission tomography and vascular invasion, both of which are known surrogates of biological aggressiveness. Although positron emission tomography data were not available, categorization of patients based on pre-transplant alpha-fetoprotein concentration and tumour differentiation, which are important markers of tumour biology, did not reveal any significant effects of IRI and neither did the analyses stratified for microvascular invasion. The reason for this discrepancy is unclear, although it may be related to a wider spectrum of tumour burden in patients included in the present study. Of note, post-operative peak transaminases did not emerge as risk factors for HCC recurrence in these previous reports. However, we chose post-reperfusion AST, ALT, and LDH levels routinely assessed in our department and not peak levels over the postoperative period in order to minimise the effect of events other than IRI on these parameters.
The results of the present study point toward the importance of strategies aimed to decrease IRI particularly for patients within the standard selection criteria. A single retrospective study revealed decreased magnitude of IRI, as illustrated by low transaminase levels and decreased risk of HCC recurrence in patients receiving prostaglandin E1 analog alprostadil in the early period after liver transplantation33. The protective effects of ischemic preconditioning with respect to the development of metastases were also reported in a recent experimental study14. The use of machine perfusion devices has also been shown to decrease the magnitude of IRI and recently even enabled the development of a strategy to practically eliminate its negative consequences34,35,36. Although the present study does not provide any evidence for the effects of these measures in liver transplantation for HCC, it provides a rationale for prospective trials aimed at addressing this issue.
This study had several limitations besides those inherent to its retrospective nature. Donor characteristics other than a baseline variable of age were neither analysed for associations with post-reperfusion transaminase and LDH levels nor as predictors of tumour recurrence. However, such analyses were beyond the scope of this study, specifically aimed at the effect of IRI on post-transplant HCC recurrence rather than on its determinants. Because all recipients received grafts from donors after brain death, this study did not directly address the issue of using grafts from donors after cardiac death for HCC patients, which was recently shown not to increase the risk of post-transplant recurrence23. Although subject to additional warm ischemia and thus potentially increased magnitude of IRI, their use in HCC patients may be confounded by other factors, including but not limited to, non-random allocation and differences in other donor characteristics. Furthermore, the duration of warm ischemia was not identified as a significant predictor of HCC recurrence. Finally, the main findings of our study are based on the results of univariable subgroup analyses. Therefore, the findings may be confounded by the effects of other risk factors for tumour recurrence. Although there was no particular policy at the authors’ department for the allocation of high-risk grafts to higher-risk HCC patients, the results may also be confounded by non-random allocation of grafts more prone to IRI to patients within the Milan or Up-to-7 criteria, yet at higher initial recurrence risk.
In conclusion, the magnitude of IRI is strongly associated with the risk of tumour recurrence in patients within the Milan criteria and to a lesser extent, in patients within the extended criteria. Available measures to decrease IRI should be evaluated as a method to prevent HCC recurrence after liver transplantation, specifically in patients with low tumour burden.
References
Kim, W. R. et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am. J. Transplant. 17, 174–251 (2017).
Mazzaferro, V. et al. Metroticket 2.0 Model for analysis of competing risks of death following liver transplantation for hepatocellular carcinoma. Gastroenterology. 154, 128–139 (2018).
Notarpaolo, A. et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J. Hepatol. 66, 552–559 (2017).
Halazun, K. J. et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann. Surg. 265, 557–564 (2017).
Grąt, M. et al. The Warsaw Proposal for the Use of Extended Selection Criteria in Liver Transplantation for Hepatocellular Cancer. Ann. Surg. Oncol. 24, 526–534 (2017).
Lai, Q. et al. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann. Surg. 264, 787–796 (2016).
Mehta, N. et al. Wait Time of Less Than 6 and Greater Than 18 Months Predicts Hepatocellular Carcinoma Recurrence After Liver Transplantation: Proposing a Wait Time “Sweet Spot”. Transplantation. 101, 2071–2078 (2017).
Goldberg, D. et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clin. Gastroenterol. Hepatol. 14, 1638–1646 (2016).
Patel, M. S. et al. The race to liver transplantation: a comparison of patients with and without hepatocellular carcinoma from listing to post-transplantation. J. Am. Coll. Surg. 220, 1001–1007 (2015).
Orci, L. A. et al. The role of hepatic ischemia-reperfusion injury and liver parenchymal quality on cancer recurrence. Dig. Dis. Sci. 59, 2058–2068 (2014).
Li, C. X., Man, K. & Lo, C. M. The Impact of Liver Graft Injury on Cancer Recurrence Posttransplantation. Transplantation. 101, 2665–2670 (2017).
Lim, C. et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J. Surg. Res. 184, 888–897 (2013).
Oldani, G. et al. Pre-retrieval reperfusion decreases cancer recurrence after rat ischemic liver graft transplantation. J. Hepatol. 61, 278–285 (2014).
Orci, L. A. et al. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br. J. Surg. 103, 417–426 (2016).
Hamaguchi, Y. et al. Longer warm ischemia can accelerate tumor growth through the induction of HIF-1α and the IL-6-JAK-STAT3 signaling pathway in a rat hepatocellular carcinoma model. J. Hepatobiliary. Pancreat. Sci. 23, 771–779 (2016).
Man, K. et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases–activation of cell invasion and migration pathways. Liver Transpl. 13, 1669–1677 (2007).
Croome, K. P. et al. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transpl. 19, 1214–1223 (2013).
Orci, L. A. et al. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br. J. Surg. 102, 1250–1257 (2015).
Ninomiya, M. et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J. Am. Coll. Surg. 220, 297–304 (2015).
Salgia, R. J., Goodrich, N. P., Marrero, J. A. & Volk, M. L. Donor factors similarly impact survival outcome after liver transplantation in hepatocellular carcinoma and non-hepatocellular carcinoma patients. Dig. Dis. Sci. 59, 214–219 (2014).
Azoulay, D. et al. Living or Brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma: A Multicenter, Western, Intent-to-treat Cohort Study. Ann. Surg. 266, 1035–1044 (2017).
Khorsandi, S. E. et al. Does Donation After Cardiac Death Utilization Adversely Affect Hepatocellular Cancer Survival? Transplantation. 100, 1916–1924 (2016).
Croome, K. P. et al. The Use of Donation After Cardiac Death Allografts Does Not Increase Recurrence of Hepatocellular Carcinoma. Am. J. Transplant. 15, 2704–2711 (2015).
Kornberg, A., Witt, U., Kornberg, J., Friess, H. & Thrum, K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 60, 2832–2839 (2015).
Nagai, S. et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 61, 895–904 (2015).
Krawczyk, M. et al. 1000 liver transplantations at the Department of General, Transplant and LiverSurgery, Medical University of Warsaw–analysis of indications and results. Pol. Przegl. Chir. 84, 304–312 (2012).
Grąt, M. et al. The impact of surgical technique on the results of liver transplantation in patients with hepatocellular carcinoma. Ann. Transplant. 18, 448–459 (2013).
Geissler, E. K. et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 100, 116–125 (2016).
Aravinthan, A. D. et al. Liver Transplantation is a Preferable Alternative to Palliative Therapy for Selected Patients with Advanced Hepatocellular Carcinoma. Ann. Surg. Oncol. 24, 1843–1851 (2017).
Facciuto, M. E. et al. Liver transplantation for hepatocellular carcinoma: defining the impact of using extended criteria liver allografts. Transplantation. 92, 446–452 (2011).
Vagefi, P. A., Dodge, J. L., Yao, F. Y. & Roberts, J. P. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 21, 187–194 (2015).
Ali, J. M. et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transpl. 21, 487–499 (2015).
Kornberg, A., Witt, U., Kornberg, J., Friess, H. & Thrum, K. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment. Pharmacol. Ther. 42, 1101–1110 (2015).
van Rijn, R. et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 104, 907–917 (2017).
Dutkowski, P. et al. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 262, 764–770 (2015).
He, X. et al. The First Case of Ischemia-free Organ Transplantation in Humans: A Proof of Concept. Am. J. Transplant. 18, 737–744 (2018).
Acknowledgements
Michał Grąt received a START 2018 stipend from the Foundation for Polish Science. The Authors would like to thank Editage (www.editage.com) for English language revision of the manuscript.
Author information
Authors and Affiliations
Contributions
Study concept: M.G.; Study design: M.G., M.K., Z.L., K.Z.; Acquisition of data: M.G., M.K., K.W., J.S., M.W., P.K., K.G., W.P., K.Z.; Analyses of data: M.G., Z.L.; Preparation of manuscript: M.G.; Critical revision of the manuscript: All remaining authors; Acceptance of the final version: All authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grąt, M., Krawczyk, M., Wronka, K.M. et al. Ischemia-reperfusion injury and the risk of hepatocellular carcinoma recurrence after deceased donor liver transplantation. Sci Rep 8, 8935 (2018). https://doi.org/10.1038/s41598-018-27319-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27319-y
This article is cited by
-
Postoperative Supplemental Oxygen in Liver Transplantation (PSOLT) does not reduce the rate of infections: results of a randomized controlled trial
BMC Medicine (2023)
-
Effect of intermittent Pringle maneuver on perioperative outcomes and long-term survival following liver resection in patients with hepatocellular carcinoma: a meta-analysis and systemic review
World Journal of Surgical Oncology (2023)
-
FOXO1 regulates Th17 cell-mediated hepatocellular carcinoma recurrence after hepatic ischemia-reperfusion injury
Cell Death & Disease (2023)
-
Risk Factors of Positive Resection Margin in Hepatectomy for Resectable Ruptured Hepatocellular Carcinoma: Risk Prediction and Prognosis
Journal of Gastrointestinal Surgery (2023)
-
The effect of the number of hepatic inflow occlusion times on the prognosis of ruptured hepatocellular carcinoma patients after hepatectomy
BMC Surgery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.