Abstract
In this article, room temperature ethanol sensing behavior of p-type Ce doped SnO2 nanostructures are investigated successfully. Interestingly, it is examined that the abnormal n to p-type transition behavior is caused by Ce doping in SnO2 lattice. In p-type Ce doped SnO2, Ce ion substituting the Sn is in favor of generating excess holes as oxygen vacancies, which is associated with the improved sensing performance. Although, p-type SnO2 is one of the important materials for practical applications, it is less studied as compared to n-type SnO2. Pure and Ce doped SnO2 nanostructures were successfully synthesized by chemical co-precipitation method. The structure, surface morphology, unpaired electrons (such as free radicals), and chemical composition of obtained nanoparticles were studied by various kinds of characterization techniques. The 9% Ce doped SnO2 sensors exhibit maximum sensor response of ~382 for 400 ppm of ethanol exposure with fast response time of ~5 to 25 sec respectively. Moreover, it is quite interesting that such enhancement of ethanol sensing is unveiled at room temperature, which plays a key role in the quest for better ethanol sensors. These remarkably improved sensing results are attributed to uniformly distributed nanoparticles, lattice strain, complex defect chemistry and presence of large number of unpaired electrons on the surface.
Similar content being viewed by others
Introduction
In past six decades, metal oxide semiconductors (MOS) have been one of the most prevailing gas sensing materials among large miscellany of materials on the grounds of its low production cost, tranquility during use and prominent selectivity for various gases in addition to high detection1,2,3,4,5. Over the past few decades, an intense requirement of gas sensors has become progressively greater by virtue of elevated atmospheric pollution leading towards the emission of hazardous gases6,7,8,9,10. One of the vital requirements of such gas sensors can be ethanol sensors. Those working on ethanol synthesis, experience hardship from the huge probability of being victims of digestive track cancer and increased risk of respiratory symptoms of a threshold limit (1000 ppm). Thus, an increasing demands and emerging challenges for detection of ethanol gas at ppm/ppb level have enforced the researchers to fabricate low cost, high performance and relatively stable ethanol sensors. With the growing interest in progression of sensing devices, more and more exhilarating results of MOS have been reported in literature10,11,12,13,14. The gas sensors developed by various types of MOS exhibited good sensitivity to ethanol at different operating temperatures (OT)15,16,17,18,19 suggesting that most of the ethanol sensors work at higher temperature. Various MOS such as TiO2, GeO2, Cr2O3, Mn2O3, NiO, CuO, CdO, CeO2, MgO, BaO, In2O3, WO3, V2O3, Fe2O3, Nb2O5, MoO3, Co3O4, Ta2O5, La2O3, Nd2O3 etc. are well-known and widely used for metal oxide based sensors for the detection of different chemical inputs20,21.
Among them, pure SnO2 is one of the most prominent n-type, wide band gap (3.6 eV) MOS with a carrier concentration of 5.7 × 1020 cm−3 making it an outstanding material for chemical gas sensors22,23,24. Although pure SnO2 has an excellent sensitivity but the major drawbacks are its poor selectivity which hinders a lot to meet up all practical key parameters such as high sensitivity, faster response-recovery time, stability and low power consumption for realistic sensor applications20,25,26,27,28. Thus, despite of substantial research work carried out on sensing materials, still there is a scope of exploration to achieve higher sensitivity and better selectivity for hazardous gases at room temperature (RT). For that, metal ion doping in SnO2 lattice may be one of the influencing factors for the furtherance in sensing properties of SnO229. In case of doped SnO2, substitutional and interstitial point defects may exist depending on the rearrangement of the internal defects in SnO2 lattice30. In addition, the semiconducting behavior can be predicted depending on the type of doping (donor or acceptor) introduced to the host material in order to tailor the band bending and band alignments on which the amount of gas sensing enhancement relies. It is noteworthy that gas sensing has been explored using SnO2 exhibiting n-type behavior but their equivalent p-type counterpart was unexpectedly absent for a long time. In past a decade, the exploration of novel p-type SnO2 for gas sensors has been started31,32,33 and the researchers and technologists around the globe are making an effort to discover an effective method to attain the p-type conduction in the n-type SnO2. In literature, p-type conductivity in SnO2 has been achieved by doping MSn metal ions (where, M = Li, Al, Ga, In and Cd) acting as a substitutional dopant in SnO2 playing an important role to enhance the gas sensing characteristics30,34,35. Hence, the understanding of synthesis and fabrication of p-type SnO2 gas sensors would be a worthy research area to open up several new possibilities in device applications.
In this study, authors have used the state of art to explore p-type conduction mechanism in Ce-doped SnO2 to characterize RT ethanol sensing. Although Ce-doped n-type SnO2 based sensors have already been used for the detection of ethanol but reported operating temperatures of these sensors are as high as 300 to 500 °C21,27,36 and no reports have been shown on p-type conduction in Ce-doped SnO2 ethanol sensors at RT. Therefore, the fabrication of stable and highly selective gas sensing materials working at RT is still highly desirable. Present work demonstrated the RT ethanol sensors based on p-type Ce-doped SnO2 nanostructured devices to understand their mechanism under consideration of several aspects that influence the sensor performance including defect chemistry, surface morphology, several diffusion processes, and associated unpaired electrons in the form of free radicals. However, from the literature and present study, authors realized that the level of understanding of these mechanisms is still lacking and in-depth research in this area is required. To the best of our knowledge, it is the first systematic report on p-type Ce doped SnO2 nanostructure based RT ethanol sensor. The results described in this article indicates that 9 wt % Ce doped SnO2 sensor is highly sensitive and selective towards ethanol detection at RT.
Results
XRD analysis
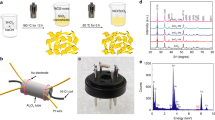
The XRD patterns of pure and Ce doped SnO2 nanostructured samples are elucidated in Fig. 1. As it can be seen that, pure SnO2 have tetragonal rutile structure which is indexed with JCPDS file 00-041-144537. It has been observed from Fig. 1(b–d) that Ce doped SnO2 nanostructured samples have all the peaks of SnO2 with a few peaks of CeO2. These peaks were indexed with the JCPDS files of SnO2 and CeO2 (JCPDS file no.: 01-075-0120)38. The XRD pattern of pure and Ce doped SnO2 nanomaterials have been refined by Rietveld refinement using FULLPROF Suite programme. The background for all samples was refined by 6th degree Polynomial function. The pseudo-voigt profile function (linear combination of Gaussian and Lorentzian) was used to describe the peak shape of all the samples. The Rietveld refinement data of pure and Ce doped SnO2 nanomaterial samples are summarized in Table 1. It can be seen from Fig. 1 that the diffraction peaks of all the samples were well fitted. The lattice parameters, atomic position and phase fraction are listed in Table 1. The increase in magnitude of lattice parameter and cell volume of Ce doped SnO2 are shown in Table 1. The lattice parameter ‘a’ and ‘c’ of the Ce doped SnO2 unit cell is found to increase linearly with the Ce concentration following Vegards law39,40 which is shown in figure (see Supporting Information, Fig. S1). It is also observed that the full width at half maxima (FWHM) increases with an increase in Ce content in the SnO2. Hence, this indicates that Ce doping in the SnO2 lattice leads to decrease in crystallite size. From the increased lattice constant with the addition of Ce content, it is confirmed that Ce ions have been effectively doped in SnO2 lattice and some part is present in the form of CeO236,41. The substitution of Sn by Ce creates vacancies and disturb local arrangements of the crystal structure which is further confirmed from ESR analysis. Similar observations have been reported in the literature by Yang et al.42. The slight deviation in atomic position has been observed that may induce lattice strain which is further confirmed from the Williamson-Hall plot43 (see Supporting Information, Fig. S2). The value of average micro-strain for pure SnO2 is 1.28 × 10−3 and found to be increased in case of 9% Ce doped SnO2 nanomaterial which is shown in Table 1. It shows that with the increased Ce content in SnO2, the little deviation in atomic position is found and lattice constant also increases with an increase in micro strain values from 1.28 × 10−3 to 3.4 × 10−3. Due to incorporation of Ce ions into the SnO2 lattice, this causes increase in lattice parameter and micro strain. The influences of increased lattice parameter and micro strain on sensing characteristics have been discussed at the end of this section. The surface morphology and elemental composition of the sensor materials have been studied using FESEM and EDS measurement (see Supporting Information, Figs S3 and S4).
TEM and HRTEM analysis
Figure 2(a–c) shows the TEM images of pure and Ce doped SnO2 nanomaterials. From TEM images, it is clear that the particle size is decreasing with the addition of Ce content. The nanoparticles are random/polygon in shape with the average particle size of ~16 nm, ~12 nm and ~6 nm for Pure SnO2, and Ce doped SnO2 (0, 6 and 9%) respectively. 9% Ce doped SnO2 nanomaterial results in formation of uniformly distributed nanoparticles of approximately ~6 nm particle size, which is twice of the Debye length for SnO2. The Fig. 3(a,b) shows the HRTEM images of pure and Ce doped SnO2 nanomaterials where we can clearly see the lattice resolution of individual nanoparticles. The distance between the lattice planes for pure SnO2 is found to be 0.33 nm which is associated to the (110) plane of tetragonal SnO2. The marked lattice fringes of 9% Ce doped SnO2 sample is found to be 0.35 nm and 0.25 nm corresponding to (110) and (101) planes of tetragonal SnO2 respectively. The selected area diffraction (SAD) patterns recorded for pure and Ce doped SnO2 nanomaterials are shown in Fig. 3(c,d) which clearly reveal the polycrystalline nature of pure and Ce doped SnO2. The diffraction rings of pure SnO2 are indexed as (110), (101), (200), (211) and (301) confirming the tetragonal rutile structure. SAD pattern acquired for 9% Ce doped SnO2 also confirms the formation of tetragonal rutile structure. These results are consistent with the above mentioned XRD results.
ESR analysis
The presence of unpaired electrons in the form of free radicals and charge carriers has been studied using ESR spectroscopy. The Fig. 4 shows the ESR dispersion derivative (dp/dH) as a function of magnetic field. The ESR measurements were performed at microwave frequency of ~9.45 GHz (X-band). When the external magnetic field is applied, the spin interactions of the unpaired electrons can be studied from ESR spectra recorded for pure and Ce doped SnO2 at RT. As it is known that the spin quantum number (s) of ½ with the magnetic moments consisting of two magnetic components ms = +½ and −½ in parallel and antiparallel respectively. These magnetic components are aligned to the applied field with the strength of Bo having a specific energy. There is a possibility of either absorption or emission of electromagnetic radiation by unpaired electrons and they also consist of two energy levels between which the unpaired electrons can move. The change in the two energy levels of an electron can be obtained by ΔE = gμ B B0, where, g and μ B are electron’s g factor and Bohr magnetron (μ B = 9.274 × 10−24 JT−1) respectively. ESR spectra show a resonance at 336.07, 336.1, 336.2 and 336.4 mT for pure and Ce doped SnO2 (0, 3, 6 and 9%) samples respectively. Although resonance field is not affected much, Fig. 4 shows that peak to peak line width and power absorption during resonance increases remarkably upon Ce addition. Small ∆Hpp and high intensity observed in 9% Ce doped SnO2 may be attributed to the existence of large number of intrinsic oxygen vacancies. The g-value for all samples were calculated from resonance magnetic field as shown in Table 2, which is nearly equal to the g–value of (2.008) of free electron44. The g-factor was calculated as \(g=h\vartheta /{\mu }_{B}{B}_{0}\) similar to the reported in literature45. The width of the ESR signal was found to be the lowest (~0.92) in 9% Ce doped SnO2 sample. The narrowing of ESR line is attributed to strong exchange of unpaired electrons and delocalization of unpaired electrons over a large system of conjugated chemical bonds46. Thus, the defect chemistry of Ce-doped samples were characterized by studying the presence of free electrons using ESR spectroscopy and the analysis suggested that Ce doping in SnO2 create a huge amount of free electrons which is certainly responsible for improved sensitivity.
XPS analysis
The core level x-ray photoelectron spectra’s of 9 wt.% Ce doped SnO2 sample has been investigated in vacuum of the order of 2.4 × 10−9 torr. All obtained peaks were fitted by the coalition of Lorentzian and Gaussian function to reduce the least square error of the fit. A linear and tougaard background function was used to account the inelastic background in the XPS spectrum. All the XPS spectra shown in Fig. 5, are referenced to C 1 s peak (284.6 eV). The deconvoluted XPS core level Sn 3d spectra’s of 9 wt.% Ce doped SnO2 sample indicates the presence of Sn with two oxidation state i.e Sn2+ and Sn4+ respectively shown in Fig. 5(a)47,48,49,50. Figure 5(b) shows the deconvoluted XPS core level Ce 3d spectra of 9 wt.% Ce doped SnO2 sample. The valence state of Ce 3d was determined by deconvolation of Ce 3d spectra. The Ce 3d spectra is fitted with the five subpeaks. These subpeaks corresponding to Ce3+ and Ce4+ valance state respectively. In Fig. 5(b), binding energy at 882.2 eV, 900.8 eV and 916.7 eV sub peaks were assigned to Ce4+ valance state51,52,53, whereas 885.8 eV and 903.9 eV sub peaks were attributed to Ce3+ valence state54,55. Above results confirmed that 9 wt.% Ce doped SnO2 sample displayed the presence of mixed valence state of Ce3+ and Ce4+ with the relative percentage of 70% and 30% respectively. This suggest that the content of Ce3+ is higher than Ce4+ valence state, which indicates that Ce3+ ion were substituted into SnO2 lattice. Hence Ce3+ substitution at Sn4+ site is a firm reason for n to p-type transition by Ce doping in SnO2 lattice which is further confirmed from Hall measurement results (see Supporting Information, Table S1). The substitution at Sn4+ by Ce3+ triggered the creation of oxygen vacancy defects in SnO2 lattice, which is well matched with ESR results. The O 1 s spectrum for 9 wt.% Ce doped SnO2 presented in the Fig. 5(c). It has been observed from the Fig. 5(c), that two components are present at 530.5 eV and 531.7 eV. This first peak (530.5 eV) is associated to lattice oxygen bound to Sn56,57 and second peak at 531.7 eV corresponds to oxygen deficient region in SnO2 matrix58,59.
Sensing measurements
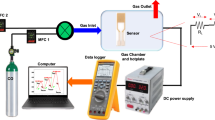
The gas sensing measurements of pure and Ce doped SnO2 sensors were performed at RT for ethanol detection. Before starting the measurements, each sensor was initially purged with synthetic air for 10 minutes to stabilise the base line resistance. The exposure time to the chemical inputs was maintained to be 5 sec and for recovery, the exposure to reference synthetic air was maintained to 10 sec respectively. The resistance of sensing materials increased or decreased to the exposure and removal of chemical inputs. The gas sensitivity is defined as follows;
where, Rg is the electrical resistance in presence of gas and Ra is the electrical resistance in clean synthetic air atmosphere only. Figure 6(a,b) shows the repeatable operation cycles of 9 and 6% Ce doped SnO2 sensors in the ethanol exposure at RT. The response signal of 9, 6 and 3% Ce doped SnO2 sensors for various amount of concentration at RT is shown in Fig. 6(a–c). Figure 6(d) shows the response signal of 9% Ce doped SnO2 sensors for NO2 and ethanol exposure. It has also been observed that sensor response (recovery) times are of the order of ~5–30 secs (30–60 secs) as seen from Fig. 6(a–d). The sensor response of pure and Ce doped SnO2 as a function of ethanol concentration is shown in Fig. 7(a). It is evident from the Fig. that 9% Ce doped SnO2 sensor exhibits higher sensitivity for various amount of ethanol concentration at RT. The 9% Ce doped SnO2 sensor shows a striking improvement of RT ethanol sensing behaviour as compared to pure SnO2. Selectivity behaviour of the pure and Ce doped SnO2 sensor was tested with 400 ppm of C2H5OH (ethanol), NH3 (ammonia) and NO2 gas.
(a) Reproducibility of 9% Ce doped SnO2 sensor response for 200, 300 & 400 ppm of ethanol (b) Changes in sensing response exhibited for 200, 300 and 400 ppm of ethanol by 6% Ce doped SnO2 sensor and (c) Response of 3% Ce doped SnO2 sensor for 100, 200, 300 & 400 ppm of ethanol (d) Response of 9% Ce doped SnO2 sensor for NO2 and ethanol.
The sensitivity values for all the above-mentioned chemical inputs are shown in Fig. 7(b). We can clearly observe from the Fig. 7(b) that 9% Ce doped SnO2 sensor is highly selective to ethanol gas at RT. The use of the sensing device for longer duration with accuracy depends upon reproducibility, reliability and repeatability. The sensor performance should not vary with time and operation cycles. The stability of 9% Ce doped SnO2 sensor towards ethanol at RT has been studied for 180 days. It has been confirmed that 9% Ce doped SnO2 sensor displays better stability and repeatability even at the end of 6th month which is ~97–98% as compared to initial measurements (see Supporting Information, Fig. S5). Such stability and repeatability analysis suggest that the 9% Ce doped SnO2 sensor can be used for long duration, which is one of the major advantages for practical applications.
Discussion
As it is clear that the undoped SnO2 will never exhibit p-type semiconducting behaviour. In order to overcome the barriers regarding the enhancement of the gas sensing, different amount of Ce has been doped into pure n-type SnO2 and sensing measurements of p-type Ce doped SnO2 has been studied systematically. As shown in Fig. 7(a), a linear sensor response has been observed for C2H5O5 concentration (ppm) varying from 100 to 400 ppm indicating enhanced sensing for 9 wt% Ce doped SnO2 material at RT. As discussed from XRD measurements, it is evident that Ce has been incorporated in SnO2 lattice which causes increase in lattice parameter and micro strain. From the above analysis, it is confirmed that defect chemistry has been modified with the incorporation of Ce into SnO2. The assumptions regarding defect states can be made in order to identify the type of defects induced in the structure with doping: (i) substitution of Ce in place of Sn (CeSn) (ii) interstitial oxygen (Oi) formation because of the occupation of Sn position by Ce atom and (iii) a complex defect (CeSn + 2Oi) may be present at the Sn site. Figure 8 shows the schematic illustration of defect chemistry caused by Ce doping in SnO2 lattice. In order to justify the drastically enhanced sensing mechanism in Ce doped SnO2, one needs to identify the dominance of the defects as mentioned above and an in-depth analysis is required. As discussed earlier from the ESR measurements, it is clear that there is a strong exchange of unpaired electrons and delocalized unpaired electrons in the form of free radicals. Small ∆Hpp and high intensity is observed in 9% Ce-doped SnO2 may be attributed to the existence of large number of intrinsic oxygen vacancies. This suggested that the increase in the Ce doping concentrations leads to the increase in the interstitial oxygen atoms present in the structure/system. This is because there is a reduction in the Sn content via substitution of Ce (CeSn) which is also evident from EDS analysis (see Supporting Information, Fig. S4). Hence, it is revealed that the doping conditions have been resulted in Sn-poor/O-rich condition which should be the reason for p-type semiconductor34,60. From the literature, it is confirmed that the CeSn acts as a donor because of the lower formation energy. In addition, the complex defect (CeSn + 2Oi) present at the Sn site consists of relatively high formation energy and behaves as an acceptor. It is here to be understood that there is a chance of existence of self-compensation between lower formations energized donors and acceptors respectively. Such self-compensation will suppress CeSn by (CeSn + 2Oi) complex defect and can be predicted to behave like an acceptor concluding that the Ce doped SnO2 behaves as a p-type semiconductor. It has been confirmed that, when C2H5OH gas exposed to all the sensors, the 9% Ce-doped SnO2 sensor shows the highest response (SR = 382) for 100 to 400 ppm concentrations of C2H5OH at RT. The improved sensors response of the p-type Ce-doped SnO2 nanostructures to ethanol at RT can be studied in more detail in order to clarify sensing mechanism. The defect of the p-type Ce-doped SnO2 and their sensing mechanism can be explained using the following reactions 3, 4 and 5 (in Kröger-Vink notation)30,61.
However, CeSn is the Ce substitution in Sn sites and \({O}_{0}^{X}\) is the lattice oxygen and \({V}_{0}^{\cdot \cdot }\,\) denotes the two positively charged vacancies on an oxygen sites. Thus, in p-type Ce-doped SnO2, the adsorption of negatively charged oxygen ion can produce the holes for conduction which is shown in the reaction-462. The interactions between ethanol and surface adsorbed oxygen in p-type Ce-doped SnO2 can be described by reaction 5. The resulting gas-sensing equation may be considered according to the charges of the adsorbed oxygen ions under the hypothesis of complete oxidation of C2H5OH.
Thus, complete oxidation of C2H5OH in the above reactions increased the resistance and concurrently decreases the conductance of the surface region of p-type Ce-doped SnO2 upon exposure to ethanol. Such changes in resistance while exposure to ethanol in Ce-doped sensors shown in Fig. 6.
In order to summarize the improved gas sensing behaviour of 9 wt % Ce doped SnO2 sensor towards ethanol at RT, four major factors are responsible (i) more structural defects present in the sample, (ii) particle size becomes twice of the Debye length and (iii) high surface to volume ratio and (iv) presence of a large number of unpaired electrons on the sensor surface. It can therefore, be inferred from XRD analysis that Ce doping in SnO2 leads to an increase in lattice strain as well as deviation from atomic position of the host material observed. This deviation from atomic position of the host material and increased lattice strain will result in the formation of more structural defects within the system. When Sn is substituted by isovalent Ce atoms, Ce4+ could be easily reduced to Ce3+ which leads to the formation of an acceptor-like defects resulting the Ce doped SnO2 behaviour as a p-type semiconductor30. On the other hand, it was essential to mention that above 3% Ce, the semiconducting behaviour transforms into p-type which has been one of the reason for the drastic increase in ethanol sensing. Analogous behaviour has been reported by Chattopahhyay and co-workers in case of Al doped SnO263. It has been reported that below 12.05% Al substitution in SnO2, the sample exhibited n-type semiconducting behaviour, and it transforms to p-type above it. Aforementioned XPS results indicated that Ce3+ substitution at Sn4+ site leads to the formation of an acceptor-like defects in Ce doped SnO2 sample and introduced the excess amount of holes which in turn increases oxygen vacancies in the lattice64,65,66,67. Such increase in the defects can contribute well in the direction of achieving improved sensing to ethanol at RT. The highest sensor response noticed in 9 wt.% Ce-doped SnO2 is associated to increased oxygen vacancies in SnO2 lattice that provides active sites for adsorption of ethanol gas molecules at RT. Comprehending the statement that enhanced RT sensor response to ethanol in 9 wt.% Ce-doped sensors may result in formation of oxygen vacancies in the host materials. This conclusive statement is in agreement with ESR studies. From ESR studies, it is well-defined that oxygen vacancies are higher in case of 9 wt.% Ce-doped sensors. In addition, power absorption and peak to peak line width during resonance increases remarkably with Ce doping. Small ∆Hpp and highest intensity is noticed in 9% Ce doped SnO2 and resulted into the existence of large number of free electrons in the form of oxygen vacancies. Increase in sensitivity due to higher oxygen vacancies is previously reported in literature68,69. FESEM studies also confirmed that 9 wt % Ce doped SnO2 sensor exhibited the lowest grain size ~8 (2) nm and the number of grain boundaries increases by decreasing the gain size (see Supporting Information, Fig. S3). Therefore, surface areas increase steadily with the increased Ce concentration. Hence 9 wt % Ce doped SnO2 sensor shows a higher sensor response to ethanol at RT due to their larger surface areas. More surface areas provide more active sites for the adsorption of the chemical inputs which are responsible for the improvement of the gas sensor response. As discussed earlier from the TEM analysis, the variation in the particle size from 16 nm to 6 nm has been observed for the doping wt% ranging from 0 to 9% respectively. To understand the effect of particle size on the sensing mechanism, one needs to establish the relationship of particle size with Debye length depending on the type of metal oxide, the analyzed gas, and detection mechanism to be taken place28. The main fundamental parameter to evaluate the gas response is related to the Debye length (LD). There may be three cases to be assumed: (i) d ≫ 2Ls (ii) d ~ 2Ls (iii) d < 2Ls. From the literature, it is considered that LD of SnO2 is about ~3 nm. In case of 0%, 3% and 6% Ce-SnO2, assumption 1 has been satisfied stating that the gas sensitivity may be practically independent of d because of the conduction mechanism taking place across the grain boundary is limited by Schottky barriers. But in case of 9% Ce-SnO2, the particle size becomes twice of the Debye length i.e. d ~ 2Ls. This shows that the conductive channels between grains are overlapped attributed to the complete depletion of space charge region while interacting with the oxygen molecules. Hence, whole particle takes part in the gas sensing and as a result higher sensitivity is observed. So, the major factor for the higher sensing are attributed to the increased concentration of adsorbed oxygen species on the fully depleted junctions across the grains resulting into the higher sensing for ethanol at RT. In Table 3, the ethanol sensing response of the 9 wt% Ce doped SnO2 sensors are compared with those of the earlier published reports70,71,72,73,74,75,76,77,78,79,80,81,82. Table 3 displays marterials used, deposition technique, operating temperatures, sensor response, and recovery times of some ethanol sensors. However, sensor response of SnO2 based sensors are good, but operating temperatures are very high. On the other hand, it must also be observed from the present study that the 9 wt% Ce doped SnO2 sensor remarkably enhanced the ethanol sensor response at RT with faster response (recovery) time ~5–20 sec (~30–60 sec). Hence, the strikingly improved sensor response of 9% Ce-doped SnO2 sensor upon exposure of C2H5OH is associated to the uniformly distributed nanoparticles with size approximately twice of the Debye length, high surface area, defect chemistry and presence of a large number of unpaired electrons of the sensor surface.
Conclusions
In conclusion, systematic studies have been performed on the synthesis, characterization and RT gas sensing behaviour of p-type Ce doped SnO2 sensor. Pure and Ce doped SnO2 sensor have been successfully synthesized by chemical co-precipitation method and used to detect ethanol at RT. To the best of our knowledge, this is the first time a p-type Ce-doped SnO2 has been studied and demonstrated to meet the requirements for highly sensitive, selective and long-time stable low cost sensing device. The maximum sensitivity is ~382 for 400 ppm with fast response time of ~5 to 25 sec have been obtained in 9% Ce doped SnO2 towards ethanol at RT. The high sensitivity of Ce doped SnO2 sensor towards C2H5OH is attributed to the uniformly distributed nanoparticles with size approximately twice of the Debye length, defect chemistry, presence of a large number of unpaired electrons, and creating the excess of holes, as oxygen vacancies. This study suggests a practical approach to the fabrication and design of high performance ethanol sensors working at RT.
Experimental
Materials and Reagents
Tin(IV) chloride pentahydrate (SnCl4.5H2O), cerium(III) Chloride heptahydrate (CeCl3·7H2O), and stearic acid were purchased from Sigma-Aldrich. Ethanol (C2H5OH) and ammonia were purchased from Merck limited. All reagents were used without further purification.
Synthesis
In present work, pure and Ce doped SnO2 samples are synthesized with three different Ce concentrations (the mole ratio of Ce/Sn = 3, 6 and 9 wt%) through a chemical co-precipitation method. Ethanol is used as a solvent for the synthesis of metal oxide nanoparticles. In a typical synthesis of Ce doped SnO2 sensor, SnCl4.5H2O and CeCl3.7H2O in stoichiometric proportions were dissolved in ethanol (C2H5OH). For the precipitation of the solution, ammonia and stearic acid was added drop wise to the precursor solution with continuous stirring and the final pH was maintained at 10. Obtained precipitates were filtered and washed several times with absolute alcohol and distilled water, then dried in a vacuum oven at 95 °C for 4 hours. These obtained powder samples (undoped and Ce doped SnO2) were annealed at 800 °C for six hours using a muffle furnace and were cool down at the rate of 100 °C per hour. The obtained powder samples have been used to fabricate the sensor device. The gold interdigital six finger electrodes patterns were deposited on the alumina substrates by thermal evaporation. The width of each finger is 500 µm and the interspacing between the fingers is 700 µm respectively. Then, the gas sensor was fabricated by drop casting technique. The pure and Ce-doped SnO2 dispersed in appropriate amount of ethanol followed by ultrsonication for 30 minutes. Thereafter, the solution was drop casted on the Au interdigital electrodes patterned substrate. The deposited sensor films were sintered at 95 °C for 30 minutes in a vacuum oven.
Characterizations
The samples were characterized using X-ray diffraction (XRD, Bruker D8 advanced diffractometer) in the scanning range of 20–80° (2θ) with the step size of 0.0195° using Cu-Kα radiations at 1.54 Ǻ wavelength. The Rietveld refinement of XRD data has been done by FULLPROF program. Surface morphology and chemical composition of sensor materials were determined using field emission scanning electron microscope (FESEM, Model Σigma, Carl Ziess). The morphological and structural information were carried out using high-resolution transmission electron microscope (TEM, FEI Technai G230, Hillsboro, U.S.A.). To study the presence of unpaired electrons, electron spin resonance (ESR) spectra was recorded at RT by using ESR X-band spectrometer operating at 9.45 GHz frequency, 2.99 mW power with the sweeping time of 2 mins. X-ray photoelectron spectroscopy XPS (Perkin Elmer-1257 with hemispherical section analyzer with 25 meV resolution) has been used. XPS set up consist of dual anode X-ray source, which is capable of generating Al Kα (1486.6 eV) or Mg Kα (1253.6 eV) X-ray radiations. The sensing measurements were performed in custom designed gas sensing set up attached with mass flow controllers for precise measurement of gas flow at ppm level39,83.
References
Rout, C. S., Hegde, M., Govindaraj, A. & Rao, C. Ammonia sensors based on metal oxide nanostructures. Nanotechnology 18, 205504 (2007).
Quang, N. H., Van Trinh, M., Lee, B.-H. & Huh, J.-S. Effect of NH 3 gas on the electrical properties of single-walled carbon nanotube bundles. Sensors and Actuators B: Chemical 113, 341–346 (2006).
Rao, A. et al. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Applied Materials & Interfaces (2017).
Franke, M. E., Koplin, T. J. & Simon, U. Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter? Small 2, 36–50 (2006).
Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Materials Science and Engineering: B 139, 1–23 (2007).
Yamazoe, N. & Miura, N. Environmental gas sensing. Sensors and Actuators B: Chemical 20, 95–102 (1994).
Qu, F., Yuan, Y. & Yang, M. Designed Synthesis of Sn3N4 Nanoparticles through Soft Urea Route with Excellent Gas Sensing Properties. Chemistry of Materials (2017).
Watson, J. The tin oxide gas sensor and its applications. Sensors and Actuators 5, 29–42 (1984).
Wang, S., Liu, X. & Zhang, M. Reduction of Ammineruthenium (III) by Sulfide Enables In Vivo Electrochemical Monitoring of Free Endogenous Hydrogen Sulfide. Analytical Chemistry (2017).
Wetchakun, K. et al. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sensors and Actuators B: Chemical 160, 580–591 (2011).
Zhang, J., Qin, Z., Zeng, D. & Xie, C. Metal-oxide-semiconductor based gas sensors: screening, preparation, and integration. Physical Chemistry Chemical Physics 19, 6313–6329 (2017).
Korotcenkov, G., Brinzari, V. & Cho, B. K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: a review. Microchimica Acta 183, 1033–1054 (2016).
Koo, W.-T. et al. Heterogeneous Sensitization of Metal–Organic Framework Driven Metal@ Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. Journal of the American Chemical Society 138, 13431–13437 (2016).
Mirzaei, A., Leonardi, S. & Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceramics International 42, 15119–15141 (2016).
Xu, X., Fan, H., Liu, Y., Wang, L. & Zhang, T. Au-loaded In2O3 nanofibers-based ethanol micro gas sensor with low power consumption. Sensors and Actuators B: Chemical 160, 713–719, https://doi.org/10.1016/j.snb.2011.08.053 (2011).
Hwang, I.-S. et al. Synthesis and gas sensing characteristics of highly crystalline ZnO–SnO2 core–shell nanowires. Sensors and Actuators B: Chemical 148, 595–600, https://doi.org/10.1016/j.snb.2010.05.052 (2010).
Khoang, N. D., Trung, D. D., Van Duy, N., Hoa, N. D. & Van Hieu, N. Design of SnO2/ZnO hierarchical nanostructures for enhanced ethanol gas-sensing performance. Sensors and Actuators B: Chemical 174, 594–601, https://doi.org/10.1016/j.snb.2012.07.118 (2012).
Moon, C. S., Kim, H.-R., Auchterlonie, G., Drennan, J. & Lee, J.-H. Highly sensitive and fast responding CO sensor using SnO2 nanosheets. Sensors and Actuators B: Chemical 131, 556–564, https://doi.org/10.1016/j.snb.2007.12.040 (2008).
Wang, Y., Wu, X., Li, Y. & Zhou, Z. Mesostructured SnO2 as sensing material for gas sensors. Solid-State Electronics 48, 627–632, https://doi.org/10.1016/j.sse.2003.09.015 (2004).
Batzill, M. & Diebold, U. The surface and materials science of tin oxide. Progress in Surface Science 79, 47–154, https://doi.org/10.1016/j.progsurf.2005.09.002 (2005).
Pourfayaz, F., Mortazavi, Y., Khodadadi, A. & Ajami, S. Ceria-doped SnO2 sensor highly selective to ethanol in humid air. Sensors and Actuators B: Chemical 130, 625–629, https://doi.org/10.1016/j.snb.2007.10.018 (2008).
Alcántara, R. et al. Preparation, sintering, and electrochemical properties of tin dioxide and Al-doped tin dioxides obtained from citrate precursors. Chemistry of materials 12, 3044–3051 (2000).
Kılıç, Ç. & Zunger, A. Origins of coexistence of conductivity and transparency in SnO2. Physical Review Letters 88, 095501 (2002).
Aswal, D. K. & Gupta, S. K. Science and technology of chemiresistor gas sensors. (Nova Publishers, 2007).
Das, S. & Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Progress in Materials Science 66, 112–255, https://doi.org/10.1016/j.pmatsci.2014.06.003 (2014).
Akira, F., Taneo, N. & Yoshihiro, H. Hall-Effect Measurement on Polycrystalline SnO 2 Thin Films. Japanese Journal of Applied Physics 27, 552 (1988).
Pourfayaz, F., Khodadadi, A., Mortazavi, Y. & Mohajerzadeh, S. S. CeO2 doped SnO2 sensor selective to ethanol in presence of CO, LPG and CH4. Sensors and Actuators B: Chemical 108, 172–176, https://doi.org/10.1016/j.snb.2004.12.107 (2005).
Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Materials Science and Engineering: R: Reports 61, 1–39, https://doi.org/10.1016/j.mser.2008.02.001 (2008).
Wang, Z., Zhou, H., Han, D. & Gu, F. Electron compensation in p-type 3DOM NiO by Sn doping for enhanced formaldehyde sensing performance. Journal of Materials Chemistry C 5, 3254–3263 (2017).
Zhang, G., Xie, C., Zhang, S., Zhang, S. & Xiong, Y. Defect chemistry of the metal cation defects in the p-and n-doped SnO2 nanocrystalline films. The Journal of Physical Chemistry C 118, 18097–18109 (2014).
Tran, Q.-P., Fang, J.-S. & Chin, T.-S. Optical Properties and Boron Doping-Induced Conduction-Type Change in SnO2 Thin Films. Journal of Electronic Materials 45, 349 (2016).
Varley, J. et al. High-throughput design of non-oxide p-type transparent conducting materials: data mining, search strategy and identification of boron phosphide. Chemistry of Materials (2017).
Wahila, M. J. et al. Lone-pair stabilization in transparent amorphous tin oxides: a potential route to p-type conduction pathways. Chemistry of Materials 28, 4706–4713 (2016).
Scanlon, D. O. & Watson, G. W. On the possibility of p-type SnO2. Journal of Materials Chemistry 22, 25236–25245, https://doi.org/10.1039/C2JM34352E (2012).
Rastomjee, C. S. et al. An investigation of doping of SnO2 by ion implantation and application of ion-implanted films as gas sensors. Thin Solid Films 279, 98–105, https://doi.org/10.1016/0040-6090(95)08156-9 (1996).
Qin, W., Xu, L., Song, J., Xing, R. & Song, H. Highly enhanced gas sensing properties of porous SnO2-CeO2 composite nanofibers prepared by electrospinning. Sensors and Actuators B: Chemical 185, 231–237, https://doi.org/10.1016/j.snb.2013.05.001 (2013).
McCarthy, G. J. & Welton, J. M. X-ray diffraction data for SnO2. An illustration of the new powder data evaluation methods. Powder Diffraction 4, 156–159 (1989).
Rüdorff, W. & Valet, G. Über das Ceruranblau und Mischkristalle im System CeO2 · UO2 · U3O8. Zeitschrift für anorganische und allgemeine Chemie 271, 257–272 (1953).
Kumar, M., Kumar, A. & Abhyankar, A. Influence of texture coefficient on surface morphology and sensing properties of w-doped nanocrystalline tin oxide thin films. ACS applied materials & interfaces 7, 3571–3580 (2015).
Lou, X. et al. Epitaxial Growth of Mg × Ca1×x O on GaN by Atomic Layer Deposition. Nano letters 16, 7650–7654 (2016).
Jiang, Z. et al. Highly sensitive and selective butanone sensors based on cerium-doped SnO2 thin films. Sensors and Actuators B: Chemical 145, 667–673 (2010).
Yang, L. et al. Efficient hydrogen evolution over Sb doped SnO2 photocatalyst sensitized by Eosin Y under visible light irradiation. Nano Energy (2017).
Cullity, B. Element of X-ray Diffraction, Addison-Wesley Reading. MA Google Scholar (1978).
Shi, S., Gao, D., Xu, Q., Yang, Z. & Xue, D. Singly-charged oxygen vacancy-induced ferromagnetism in mechanically milled SnO2 powders. RSC Advances 4, 45467–45472, https://doi.org/10.1039/C4RA05475J (2014).
Irzh, A., Genish, I., Klein, L., Solovyov, L. A. & Gedanken, A. Synthesis of ZnO and Zn nanoparticles in microwave plasma and their deposition on glass slides. Langmuir 26, 5976–5984 (2010).
Volodin, A. & Cherkashin, A. ESR spectra of O2− on SnO2. effect of adsorbed CO on the conditions of stabilization of O2−. Reaction Kinetics and Catalysis Letters 17, 323–327 (1981).
Grutsch, P., Zeller, M. & Fehlner, T. Electronic energies of tin compound. Inorganic Chemistry 12, 1432 (1973).
Stranick, M. A. & Moskwa, A. SnO by XPS. Surface Science Spectra 2, 45–49 (1993).
Ansari, S., Fouad, H., Shin, H.-S. & Ansari, Z. Electrochemical enzyme-less urea sensor based on nano-tin oxide synthesized by hydrothermal technique. Chemico-biological interactions 242, 45–49 (2015).
Zhang, S., Kang, P. & Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. Journal of the American Chemical Society 136, 1734–1737 (2014).
Nefedov, V., Gati, D., Dzhurinskii, B., Sergushin, N. & Salyn, Y. V. X-ray electron study of oxides of elements. Zhurnal Neorganicheskoi Khimii 20, 2307–2314 (1975).
Paparazzo, E., Ingo, G. & Zacchetti, N. X‐ray induced reduction effects at CeO2 surfaces: An x‐ray photoelectron spectroscopy study. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 9, 1416–1420 (1991).
Dauscher, A. et al. Characterization by XPS and XAS of supported Pt/TiO2 · CeO2 catalysts. Surface and Interface Analysis 16, 341–346 (1990).
Praline, G., Koel, B., Hance, R., Lee, H.-I. & White, J. X-ray photoelectron study of the reaction of oxygen with cerium. Journal of Electron Spectroscopy and Related Phenomena 21, 17–30 (1980).
Ingo, G., Paparazzo, E., Bagnarelli, O. & Zacchetti, N. XPS studies on cerium, zirconium and yttrium valence states in plasma‐sprayed coatings. Surface and Interface Analysis 16, 515–519 (1990).
Supothina, S. & De Guire, M. R. Characterization of SnO2 thin films grown from aqueous solutions. Thin Solid Films 371, 1–9 (2000).
Kumar, V. et al. The role of surface and deep-level defects on the emission of tin oxide quantum dots. Nanotechnology 25, 135701 (2014).
Chen, M. et al. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Applied Surface Science 158, 134–140 (2000).
Lee, S. et al. Oxygen partial pressure dependent electrical conductivity type conversion of phosphorus-doped ZnO thin films. Journal of Physics D: Applied Physics 47, 065306 (2014).
Godinho, K. G., Walsh, A. & Watson, G. W. Energetic and Electronic Structure Analysis of Intrinsic Defects in SnO2. The Journal of Physical Chemistry C 113, 439–448, https://doi.org/10.1021/jp807753t (2009).
Jyh Ming, W. A room temperature ethanol sensor made from p-type Sb-doped SnO2 nanowires. Nanotechnology 21, 235501 (2010).
Choi, K.-I. et al. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sensors and Actuators B: Chemical 146, 183–189 (2010).
Ahmed, S. F., Khan, S., Ghosh, P. K., Mitra, M. K. & Chattopadhyay, K. K. Effect of Al doping on the conductivity type inversion and electro-optical properties of SnO2 thin films synthesized by sol-gel technique. Journal of Sol-Gel Science and Technology 39, 241–247, https://doi.org/10.1007/s10971-006-7808-x (2006).
Kar, A. & Patra, A. Optical and Electrical Properties of Eu3+-Doped SnO2 Nanocrystals. The Journal of Physical Chemistry C 113, 4375–4380, https://doi.org/10.1021/jp810777f (2009).
Wei, W., Dai, Y. & Huang, B. Role of Cu Doping in SnO2 Sensing Properties Toward H2S. The Journal of Physical Chemistry C 115, 18597–18602, https://doi.org/10.1021/jp204170j (2011).
Liu, X., Iqbal, J., Wu, Z., He, B. & Yu, R. Structure and Room-Temperature Ferromagnetism of Zn-Doped SnO2 Nanorods Prepared by Solvothermal Method. The Journal of Physical Chemistry C 114, 4790–4796, https://doi.org/10.1021/jp909178x (2010).
Tianshu, Z., Hing, P., Li, Y. & Jiancheng, Z. Selective detection of ethanol vapor and hydrogen using Cd-doped SnO2-based sensors. Sensors and Actuators B: Chemical 60, 208–215, https://doi.org/10.1016/S0925-4005(99)00272-5 (1999).
Ge, Y. et al. Highly sensitive and rapid chemiresistive sensor towards trace nitro-explosive vapors based on oxygen vacancy-rich and defective crystallized In-doped ZnO. Sensors and Actuators B: Chemical 244, 983–991 (2017).
Šutka, A. & Gross, K. A. Spinel ferrite oxide semiconductor gas sensors. Sensors and Actuators B: Chemical 222, 95–105 (2016).
Kim, K.-W. et al. The selective detection of C2H5OH using SnO2–ZnO thin film gas sensors prepared by combinatorial solution deposition. Sensors and Actuators B: Chemical 123, 318–324 (2007).
Khorami, H. A., Keyanpour-Rad, M. & Vaezi, M. R. Synthesis of SnO2/ZnO composite nanofibers by electrospinning method and study of its ethanol sensing properties. Applied Surface Science 257, 7988–7992 (2011).
Jinkawa, T., Sakai, G., Tamaki, J., Miura, N. & Yamazoe, N. Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides. Journal of Molecular Catalysis A: Chemical 155, 193–200 (2000).
Yi, Z., Bing, Y.-f, Chang, L., Zheng, W.-t & Zou, G.-t Self-assembly of hierarchical ZnSnO3-SnO2 nanoflakes and their gas sensing properties. Transactions of Nonferrous Metals Society of China 22, 2451–2458 (2012).
Rumyantseva, M. et al. Nanocomposites SnO2/Fe2O3: sensor and catalytic properties. Sensors and Actuators B: Chemical 118, 208–214 (2006).
Chen, X. et al. Self-assembly of ZnO nanoparticles into hollow microspheres via a facile solvothermal route and their application as gas sensor. CrystEngComm 15, 7243–7249 (2013).
Ge, L. et al. Trisodium citrate assisted synthesis of ZnO hollow spheres via a facile precipitation route and their application as gas sensor. Journal of Materials Chemistry 21, 10750–10754 (2011).
Nikan, E., Khodadadi, A. A. & Mortazavi, Y. Highly enhanced response and selectivity of electrospun ZnO-doped SnO2 sensors to ethanol and CO in presence of CH4. Sensors and Actuators B: Chemical 184, 196–204 (2013).
Ji, F. et al. 2D-MoO3 nanosheets for superior gas sensors. Nanoscale 8, 8696–8703 (2016).
Zhang, P.-P., Zhang, H. & Sun, X.-H. A uniform porous multilayer-junction thin film for enhanced gas-sensing performance. Nanoscale 8, 1430–1436 (2016).
Li, W. et al. Enhanced ethanol sensing performance of hollow ZnO–SnO2 core–shell nanofibers. Sensors and Actuators B: Chemical 211, 392–402 (2015).
Liu, T. et al. Porous tungsten trioxide nanolamellae with uniform structures for high-performance ethanol sensing. CrystEngComm 18, 8411–8418 (2016).
Ma, X., Song, H. & Guan, C. Enhanced ethanol sensing properties of ZnO-doped porous SnO2 hollow nanospheres. Sensors and Actuators B: Chemical 188, 193–199 (2013).
Kumar, M., Kumar, A. & Abhyankar, A. SnO2 based sensors with improved sensitivity and response‐recovery time. Ceramics International 40, 8411–8418 (2014).
Acknowledgements
This research was supported by the Post-Doctoral Research Program through Incheon National University (INU), Incheon, Republic of Korea and Basic Science Research Program through the National Research Foundation (NRF) of Korea by the Ministry of Education (NRF-2016R1C1B1012088).
Author information
Authors and Affiliations
Contributions
M.K. performed the experiments and wrote the manuscript. V.B. interpreted the data. J.H.Y. and A.C.A. designed the experiments and supervised the project. S.H.P. helped in XPS measurements of the samples. A.K. and J.K. polished the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, M., Bhatt, V., Abhyankar, A.C. et al. New insights towards strikingly improved room temperature ethanol sensing properties of p-type Ce-doped SnO2 sensors. Sci Rep 8, 8079 (2018). https://doi.org/10.1038/s41598-018-26504-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26504-3
This article is cited by
-
Fabrication of samarium doped SnO2 thin films using facile spray pyrolysis technique for photocatalysis application
Ionics (2024)
-
Reliable liquified petroleum gas sensing at room temperature by nanocrystalline SnO2 thin film deposited by Langmuir–Blodgett method
Applied Physics A (2023)
-
Structural, magnetic, and optical properties of ZnFe2O4/RO (RO = CdO, NiO, Ga2O3, SnO2, and TiO2) nanocomposites
Journal of Materials Science (2023)
-
Facile Synthesis and Characterization of Microstructure and Optical Properties of Pure and Zn Doped SnO2 Nanorods
Journal of Cluster Science (2022)
-
Plasma treatment effect on SnO2–GO nano-heterojunction: fabrication, characterization and optoelectronic applications
Applied Physics A (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.