Abstract
Knowledge of metabolic risk factors for end-stage kidney disease (ESKD) in the general population is limited when considering the competing event death in risk analysis. The aim of our prospective observational study was to investigate how blood pressure and metabolic factors might influence the risks for ESKD and death before ESKD in a large Austrian population-based cohort with long-term follow-up. 177,255 participants (53.8% women; mean age 42.5 years) were recruited between 1988 and 2005 and linked to the Austrian Dialysis and Transplant Registry and the National Mortality Registry. Over a mean follow-up of 16 years 358 participants reached ESKD and 19,512 participants died. Applying fully adjusted cause-specific Cox proportional hazards models elevated fasting blood glucose, hypertension, hypertrigylceridemia and hypercholesterolemia were associated with a higher relative risk for ESKD than for death before ESKD, whereas elevated γ-glutamyltransferase was associated with an increased relative risk of death but not ESKD. Results were similar using continuous or categorical exposure variable measures in the general cohort but differed in selected high-risk populations. These findings might help improve the design of renal risk factor modification trials and kidney disease awareness and prevention programs in the general population, which may ultimately decrease the burden of ESKD.
Similar content being viewed by others
Introduction
Epidemiological studies indicate that the metabolic syndrome and its components (elevated blood pressure, elevated triglycerides, low HDL cholesterol, impaired fasting blood glucose and central obesity) are associated with increased cardiovascular morbidity and all-cause mortality in the general population1. Furthermore, the metabolic syndrome is linked to the development and progression of chronic kidney disease (CKD)2,3,4. A meta-analysis of 31 studies clearly showed an association between the metabolic syndrome and cardiovascular disease, as well as kidney disease5. CKD itself is associated with a large burden of morbidity and mortality6,7, exceeding the mortality risk of the general population by 10- to 20-fold8.
Approximately one in 40 middle-aged men and one in 60 women will develop end-stage kidney disease (ESKD) during their lifetime9. There is some evidence to suggest that the presence of the metabolic syndrome and its components is associated with an increased risk for ESKD rather than an increased risk for death10. However, estimating the ESKD risk requires a careful approach that considers the competing event death before ESKD. As shown by Turin et al. the lifetime risk for ESKD in the general population is significantly attenuated with the competing risk death before ESKD (relative risk reduction in men by 36%, in women by 23%). In contrast, adjusting for the competing risk of death does not affect ESKD risk in people with impaired kidney function9. Evaluation of the influence of known risk factors on the long-term risk for these two competing events might provide new insights to strengthen risk reduction efforts in the general population to prevent ESKD-associated burden of morbidity and treatment costs.
Such studies applying cause-specific risk models in the general population are limited. Previous studies were performed in populations at high risk for ESKD, with either preexisting CKD10, hypertension11, diabetes mellitus12 or cardiovascular disease13, but not in a large general population-based cohort without a priori predefined increased risk for kidney disease. Moreover, these studies included older patients10,11,14 and used either only clinical10 or administrative data sets11,12,15.
The objectives of the present prospective, longitudinal study were to investigate the possible influence of blood pressure and metabolic factors on the long-term risks for ESKD and death before ESKD in a large general population based cohort with long follow-up. In addition, these associations were explored in three ESKD high-risk subgroups encompassing participants with prevalent obesity, diabetes mellitus and hypertension.
Results
Study cohort characteristics
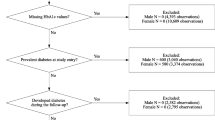
The analyzed cohort included 177,255 participants (53.8% women) with a mean age at baseline of 42.5 (SD 15.4) years and 2,829,500 person-years of follow-up taking part in the Vorarlberg Health Monitoring and Prevention Program (VHM&PP) (Table 1). By merging the VHM&PP data and the OEDTR data, we identified patients with ESKD requiring dialysis or kidney transplantation, who had also taken part in the VHM&PP program. During a mean follow-up time of 16.0 (SD 5.3) years 358 patients reached ESKD (135 women and 223 men, 0.2%, 13.4 per 100,000 person years), and 19,512 participants died (11%, 730 per 100,000 person years). Mean age at the start of renal replacement therapy was 63.7 (SD 12.7) years and mean time from baseline until ESKD was 10.5 (SD 5.5) years. Overall, 11.4% of the participants were obese, 34.2% hypertensive and 30.0% smokers. Of the participants reaching ESKD 23.2% were obese, 76.4% hypertensive and 39.7% smokers.
Association between risk factors and the competing risks ESKD and death before ESKD in the entire cohort
As shown in Table 2 for the entire study population, the fully adjusted Cox regression model (HR, 95% confidence interval) using continuous exposure variable measures revealed stronger associations with ESKD than with death before ESKD for increasing systolic blood pressure [per 5 mmHg HR 1.11 (1.07–1.14) versus 1.03 (1.02–1.03)], fasting blood glucose levels [per mmol/L HR 1.20 (1.16–1.24) versus 1.07 (1.06–1.07)], triglyceride levels [per log mmol/L HR 1.58 (1.29–1.94) versus 1.10 (1.07–1.14)] and total cholesterol levels [per log mmol/L HR 1.16 (1.07–1.26) versus 0.95 (0.94–0.96)]. Increased GGT levels were not associated with ESKD risk, but with increased risk for death [per log U/L HR 1.36 (1.32–1.38)].
Models with exposure variables categorized according to clinically relevant cut-offs showed stronger associations with ESKD than with death before ESKD for diabetes mellitus, hypertension, hypertriglyceridemia and hypercholesterolemia (Fig. 1 and Table 3). The relative risk (HR, 95% confidence interval) for ESKD was highest for participants with diabetes mellitus (HR 4.62, 3.54–6.03), followed by hypertension (HR 2.89, 2.22–3.77), hypertriglyceridemia (HR 2.08, 1.32–3.28) and hypercholesterolemia (HR 1.61, 1.29–2.00).
Cause-specific hazard ratios for ESKD and Death before ESKD Hazard ratios are taken from Table 3. All cause-specific models are fully adjusted. Obesity: BMI ≥ 30 kg/m2; diabetes mellitus: fasting blood glucose ≥6.9 mmol/L; hypertension: systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or being on antihypertensive therapy; hypertriglyceridemia: triglycerides ≥2.3 mmol/L; hypercholesterolemia: total cholesterol ≥6.2 mmol/L; elevated gamma-GT: for men ≥61 U/L, for women ≥36 U/L.
Association between risk factors and the competing risks ESKD and death before ESKD in selected high-risk populations
Table 4 summarizes cause-specific relative risks in the three selected high-risk populations of obese, hypertensive and diabetic participants using cause-specific Cox regression risk models. Among obese participants, stronger associations with ESKD than with death before ESKD were found for fasting blood glucose (per mmol/L HR 1.27, 1.20–1.34), diastolic blood pressure (per 5 mmHg HR 1.18, 1.05–1.32) and total cholesterol levels (per mmol/L HR 1.33, 1.16–1.51). In hypertensive participants, a higher relative risk for ESKD was found in participants with increasing blood levels of fasting glucose (HR 1.19, 1.14–1.23), triglycerides (HR 1.63, 1.30–2.05) and total cholesterol (HR 1.18, 1.08–1.29), increasing systolic (HR 1.11, 1.07–1.15) and diastolic (HR 1.06, 1.00–1.13) blood pressure and smokers (HR 1.44, 1.11–1.87). Among diabetic participants a higher relative risk for ESKD than for death was detected for increasing fasting blood glucose levels (HR 1.16, 1.10–1.23), systolic blood pressure (HR 1.09, 1.03–1.17) and total cholesterol levels (HR 1.19, 1.05–1.36). In all three high-risk groups, increasing GGT levels revealed a stronger association with death before ESKD than with ESKD (obesity HR 1.36, 1.29–1.43; hypertension HR 1.33, 1.30–1.36; and diabetes mellitus HR 1.25, 1.18–1.32).
Discussion
The main findings of our large population-based cohort study using cause-specific competing risk analyses are that various metabolic risk factors and blood pressure are associated with a higher relative risk for ESKD than for death before ESKD. When investigating the effects of the individual risk factors, fasting blood glucose, blood pressure, triglycerides, and total cholesterol had a stronger influence on the relative risk for ESKD than on death. Only for GGT was the relative risk for death before ESKD higher than the risk for ESKD. Likewise, when predefined definitions of obesity, diabetes mellitus, hypertension, hypertriglyceridemia and hypercholesterinemia were used as categorical variables, all these clinical indicators had a greater impact on ESKD risk than on risk for death before ESKD.
Our findings, namely that elevated levels of fasting blood glucose, blood pressure and triglycerides were associated with a higher relative risk for ESKD, are in line with previously described results in patients with known CKD Stages 3 and 410. In this study, low HDL cholesterol levels were associated with increased mortality, whereas we found an inverse association for total cholesterol. Contrary to our study, obesity and hypertension were associated with reduced all-cause mortality, which could be explained by the fact that these patients were older (mean age 72.3 years) and already had prevalent CKD.
The association between obesity, ESKD and mortality seems to be complex. The effects of obesity are modified by the absence (so-called metabolic healthy obesity) or presence of other components of the metabolic syndrome. Panwar et al. found a decreased risk for ESKD in obese individuals without the metabolic syndrome, but an increased incidence rate in those with the metabolic syndrome16. They also showed that high blood pressure, triglycerides and fasting blood glucose increase the risk for ESKD. We confirm and extend these findings in a more than 8-fold larger population-based cohort encompassing younger participants, longer follow-up and additional statistical methods considering the important competing event death before ESKD. In the high-risk group of obese participants in our cohort, fasting blood glucose, diastolic blood pressure and total cholesterol were associated with an increased relative risk for ESKD in cause-specific models. This is consistent with the literature showing that excess weight affects ESKD risk via diabetes mellitus and hypertension10,17,18. In line with our observation, although in a population of older age and higher co-morbidity burden, Lohr et al.14 using a competing risk model found that higher systolic blood pressure was associated with an increased incidence of CKD, but not with increased mortality.
Although no causal inference can be made from an observational study like ours due to its design, one might hypothesize that the higher relative risk for ESKD than for death before ESKD could be explained by the potential pathophysiological mechanisms linking the components of the metabolic syndrome to CKD and ESKD. Insulin resistance leads to inflammation and increased oxidative stress19, obesity induces the secretion of pro-inflammatory cytokines such as leptin, interleukin-6 and tumor necrosis factor-alpha by adipose tissue20, resulting in glomerulosclerosis due to enhanced expression of intra-renal transforming growth factor-beta21. Additionally, obesity leads to glomerular and podocyte hypertrophy and mesangial matrix expansion22. Trigylcerides may cause renal damage by promoting pro-inflammatory cytokine production23. As our population-based study included a middle-aged cohort, therapeutic interventions may have reduced the cardiovascular mortality associated with these risk factors and allowed survival during the observation period but may have been less successful in preventing progression from CKD to ESKD. In contrast, a variety of other causes, which are not influenced by the risk factors analyzed in our study, for example malignancy, contribute to mortality, therefore increasing absolute risk for death.
In our study, elevated GGT was found to be associated with an increased risk for death from all causes, but not for ESKD. GGT is considered neither a classical cardiovascular metabolic risk factor nor a component of metabolic syndrome3. However, increased levels of GGT may be a marker of non-alcoholic fatty liver disease24 and be associated with the risk for the metabolic syndrome independently of alcohol intake25. In the Framingham Heart Study (mean age 44 years, 52% women, and therefore comparable with our study population) higher GGT levels were prospectively associated with cardiovascular disease and all-cause mortality, even after adjustment for established cardiovascular risk factors and hepatic enzymes26. Earlier work in our cohort identified baseline GGT levels as well as a longitudinal increase in GGT as being independently associated with cardiovascular death27,28. Our finding of an increased risk for death but not for ESKD with higher GGT levels during the same follow-up might reflect the development of a risk factor cluster that constitutes not only vascular morbidity but also other conditions increasing the risk for death.
Additional subdistribution analyses using the Fine and Gray model29 revealed similar results compared to the cause-specific Cox models, supporting a higher relative risk for ESKD than for death before ESKD for fasting blood glucose, blood pressure, triglycerides, and total cholesterol in the whole cohort and also in the three selected high-risk populations of obese, hypertensive and diabetic participants. In this paper, we have chosen to report cause-specific relative risks obtained from Cox models of the cause-specific hazards. Just like a standard Cox model, these relative risks express the effect on the intensity of the event under study, either ESKD or death before ESKD, and it is this relative risk concept that is amenable for our discussion of the results above. Some authors (e.g., Schmoor et al.30) suggest that one may additionally consider Fine and Gray results as a summary of the cause-specific relative risks, but as stated above, results were numerically comparable and we have therefore chosen not to reproduce them. We also note that numerically comparable results are not uncommon comparing these two models31. Another issue is that the interpretation of the results obtained from a Fine and Gray model is currently a subject of debate in the biostatistical literature32,33. Also for this reason, we have preferred to report cause-specific hazard ratios.
This is one of the largest population-based studies with long-term follow-up to assess risk for ESKD and death before ESKD and investigate the effects of metabolic factors on that risk. In addition, we applied competing risk regression analyses calculating cause-specific models considering the important competing event death that precludes ESKD as the end-point of interest. Furthermore, we analyzed these risk factors in pre-specified high-risk groups, defined by the presence of diabetes mellitus, hypertension or obesity. The results of these group-specific analyses further support our findings in the general population cohort using clinically relevant cut-points.
Notwithstanding these advantages, several limitations of our study have to be kept in mind. Despite multivariable adjustments for established risk factors, potential residual confounding due to other unmeasured lifestyle or medical factors including physical activity and medical treatment could have influenced the observed associations. In particular, information on estimated or measured glomerular filtration rate und albuminuria as markers of renal function at baseline was lacking, because these variables were not included in the VHM&PP (starting in 1988). The ESKD incidence rates during the study period were 14.5 per 100,000 persons per year for the inhabitants of Vorarlberg in general and 13.4 per 100,000 persons per year for the VHM&PP cohort. This slightly lower incidence rate in the VHM&PP cohort is likely due to the fact that healthier persons tend to participate in prevention programs. This should be considered when generalizing the results. The VHM&PP program started enrollment nearly 30 years ago. Metabolic risk management has improved during this time, affecting the relative risk for ESKD and death. However, precisely due to the remote start of the program this study stands out for its long follow-up of 16 years.
The influence of blood pressure and the metabolic factors diabetes mellitus, hypertriglyceridemia and hypercholesterolemia on the relative risk for ESKD found in our study may contribute to stimulate kidney function screening and awareness programs in individuals at risk. Targeting these factors might allow better dialysis-free survival. As diabetes mellitus, hypertension, hypertriglyceridemia and hypercholesterolemia are associated with a higher relative risk for ESKD, future therapeutic trials aiming at improving these conditions should include ESKD as an outcome of interest.
In summary, components of the metabolic syndrome were associated with a higher relative risk for end-stage kidney disease than for its competing event death in a large general population-based cohort of middle-aged adults. In contrast, elevated GGT levels indicated a higher relative risk for death before ESKD. These risk factors vary in their significance depending on the prevalence of one of the three preexisting cardiovascular high-risk phenotypes diabetes, hypertension or obesity. Such information might improve the design of renal risk factor modification trials and enable risk-adapted chronic kidney disease and end-stage kidney disease awareness and prevention programs in the general population, which may ultimately decrease the burden of chronic kidney disease.
Methods
Study Population
Between January 1, 1988 and June 30, 2005 177,255 individuals participated in the Vorarlberg Health Monitoring and Prevention Program (VHM&PP), a general population-based risk factor surveillance program in Vorarlberg, Austria’s westernmost state with approx. 360,000 inhabitants in 201534. Every adult in Vorarlberg above the age of 20 years was invited to participate in the program. The screening examinations were performed by the local general practitioner according to a standard protocol, which was described in detail earlier35. The program included a physical examination with measurement of height, body weight, systolic and diastolic blood pressure, total cholesterol, triglycerides, γ-glutamyltransferase (GGT), and fasting blood glucose. Participants’ smoking status was inquired, and participants were categorized into “never smokers” and “ever smokers”. The data collection protocol remained unchanged throughout the years of recruitment, therefore the same factors were available for all 177,255 individuals participating in this study. The choice of risk factors included in this study is based on earlier work describing associations between these factors and ESKD in renal risk populations10,17 or between these factors and cardiovascular morbidity and mortality in the general population27,28,36,37. All participants gave written informed consent to use their data for scientific purposes.
The VHM&PP cohort was linked with the Austrian Dialysis and Transplant Registry (OEDTR) and the National Mortality Registry. The OEDTR collects data on all patients receiving chronic renal replacement therapy (hemodialysis, peritoneal dialysis, kidney transplantation) in Austria since 1964 and has almost complete follow-up38. Ethics approval for this study was obtained from the Ethics Committee of the State of Vorarlberg. All study procedures were performed in accordance with the Declaration of Helsinki and relevant guidelines. Written informed consent was obtained from all VHM&PP participants, and all patients registered in the OEDTR signed a declaration of consent to permit their data to be transferred to the registry.
Statistical analyses
A prospective cohort study was conducted to describe differences between the risk for ESKD and death before ESKD. Outcomes were defined as ESKD requiring hemo-/peritoneal dialysis or kidney transplantation, and death before ESKD (=death from all causes without prior ESKD). Predictors (exposure variables) were analyzed quantitatively, and were also categorized on the basis of clinically relevant definitions. Thus, BMI was calculated as body weight in kilograms divided by squared height in meters. Obesity was defined according to the WHO definition with BMI ≥30 kg/m2 39. Fasting blood glucose levels >6.9 mmol/L were defined as diabetes40. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or being on antihypertensive therapy41. Triglycerides ≥2.3 mmol/L defined hypertriglyceridemia and total cholesterol ≥6.2 mmol/L hypercholesterolemia42. For GGT, gender-specific cut-offs according to the local central laboratory were used. For men elevated GGT values were defined as ≥61 U/L, and for women elevated GGT values were defined as ≥36 U/L. Each participant´s first examination was used for baseline exposure data. The follow-up by the Austrian Dialysis and Transplant Registry and the National Mortality Registry is population-based and virtually complete. In the exposure variables only few missing variables occurred, which in relation to the size of the dataset are unlikely to have influenced the results.
Cause-specific risk models were calculated to analyze the association between different risk factors and ESKD and death before ESKD30. Participants free of either endpoint on December 31, 2009 (end of follow-up) were administratively censored. Survival time until the initiation of renal replacement therapy or until death served as outcome parameter for the Cox regression models. Due to skewness, triglyceride and GGT values were logarithmized. Separate analyses were done for continuous and categorical exposure variables (with categorization based on clinical definitions as described above). Smoking status (ever vs never smoker) was included in both analyses. All models were adjusted for sex, age, smoking status and additionally for all included risk factors. Furthermore, we investigated the association in clinically relevant selected high-risk groups, encompassing participants with prevalent arterial hypertension, diabetes mellitus and obesity. Interaction was investigated by including a product term in the Cox regression equation. All tests were two-sided, and statistical significance was defined as a P value ≤ 0.05. All calculations were performed using the statistical analysis software SAS, release 9.3 (SAS Institute, Cary, NC, USA).
Data availability
The datasets generated during and analyzed during the current study are not publicly available due to the Austrian law which prohibits public availability of health-related personal data but are available from hans.concin@aks.or.at on reasonable request.
References
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645, https://doi.org/10.1161/CIRCULATIONAHA.109.192644 (2009).
Prasad, G. V. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J Nephrol 3, 210–219, https://doi.org/10.5527/wjn.v3.i4.210 (2014).
Tanner, R. M., Brown, T. M. & Muntner, P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep 14, 152–159, https://doi.org/10.1007/s11906-012-0254-y (2012).
Thomas, G. et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clinical journal of the American Society of Nephrology: CJASN 6, 2364–2373, https://doi.org/10.2215/cjn.02180311 (2011).
Galassi, A., Reynolds, K. & He, J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 119, 812–819, https://doi.org/10.1016/j.amjmed.2006.02.031 (2006).
Couser, W. G., Remuzzi, G., Mendis, S. & Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80, 1258–1270, https://doi.org/10.1038/ki.2011.368 (2011).
Eckardt, K. U. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382, 158–169, https://doi.org/10.1016/S0140-6736(13)60439-0 (2013).
Jardine, A. G., Gaston, R. S., Fellstrom, B. C. & Holdaas, H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 378, 1419–1427, https://doi.org/10.1016/S0140-6736(11)61334-2 (2011).
Turin, T. C. et al. Lifetime risk of ESRD. J Am Soc Nephrol 23, 1569–1578, https://doi.org/10.1681/ASN.2012020164 (2012).
Navaneethan, S. D. et al. Metabolic syndrome, ESRD, and death in CKD. Clinical journal of the American Society of Nephrology: CJASN 8, 945–952, https://doi.org/10.2215/CJN.09870912 (2013).
Sim, J. J. et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int 88, 622–632, https://doi.org/10.1038/ki.2015.142 (2015).
Jiang, Y., Osgood, N., Lim, H. J., Stang, M. R. & Dyck, R. Differential mortality and the excess burden of end-stage renal disease among First Nations people with diabetes mellitus: a competing-risks analysis. CMAJ 186, 103–109, https://doi.org/10.1503/cmaj.130721 (2014).
Lea, J. P., Crenshaw, D. O., Onufrak, S. J., Newsome, B. B. & McClellan, W. M. Obesity, end-stage renal disease, and survival in an elderly cohort with cardiovascular disease. Obesity (Silver Spring) 17, 2216–2222, https://doi.org/10.1038/oby.2009.70 (2009).
Lohr, J. W., Golzy, M., Carter, R. L. & Arora, P. Elevated systolic blood pressure is associated with increased incidence of chronic kidney disease but not mortality in elderly veterans. J Am Soc Hypertens 9, 29–37, https://doi.org/10.1016/j.jash.2014.10.008 (2015).
Derose, S. F. et al. Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int 76, 629–637, https://doi.org/10.1038/ki.2009.209 (2009).
Panwar, B. et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int 87, 1216–1222, https://doi.org/10.1038/ki.2014.384 (2015).
Nagel, G. et al. Body mass index and metabolic factors predict glomerular filtration rate and albuminuria over 20 years in a high-risk population. BMC Nephrol 14, 177, https://doi.org/10.1186/1471-2369-14-177 (2013).
Redon, J. & Lurbe, E. The kidney in obesity. Curr Hypertens Rep 17, 555, https://doi.org/10.1007/s11906-015-0555-z (2015).
Locatelli, F., Pozzoni, P. & Del Vecchio, L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol 17, S81–85, https://doi.org/10.1681/ASN.2005121332 (2006).
Wisse, B. E. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15, 2792–2800, https://doi.org/10.1097/01.ASN.0000141966.69934.21 (2004).
Wolf, G. et al. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int 56, 860–872, https://doi.org/10.1046/j.1523-1755.1999.00626.x (1999).
Serra, A. et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int 73, 947–955, https://doi.org/10.1038/sj.ki.5002796 (2008).
Wahba, I. M. & Mak, R. H. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN 2, 550–562, https://doi.org/10.2215/CJN.04071206 (2007).
Franzini, M. et al. Accuracy of b-GGT fraction for the diagnosis of non-alcoholic fatty liver disease. Liver Int 32, 629–634, https://doi.org/10.1111/j.1478-3231.2011.02673.x (2012).
Liu, C. F., Zhou, W. N. & Fang, N. Y. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract 66, 692–698, https://doi.org/10.1111/j.1742-1241.2012.02959.x (2012).
Lee, D. S. et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 27, 127–133, https://doi.org/10.1161/01.ATV.0000251993.20372.40 (2007).
Ruttmann, E. et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 112, 2130–2137, https://doi.org/10.1161/CIRCULATIONAHA.105.552547 (2005).
Strasak, A. M. et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol 28, 1857–1865, https://doi.org/10.1161/ATVBAHA.108.170597 (2008).
Fine, J. P. & Gray, R. J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94, 496–509, https://doi.org/10.2307/2670170 (1999).
Schmoor, C., Schumacher, M., Finke, J. & Beyersmann, J. Competing risks and multistate models. Clin Cancer Res 19, 12–21, https://doi.org/10.1158/1078-0432.CCR-12-1619 (2013).
Grambauer, N., Schumacher, M. & Beyersmann, J. Proportional subdistribution hazards modeling offers a summary analysis, even if misspecified. Stat Med 29, 875–884, https://doi.org/10.1002/sim.3786 (2010).
Andersen, P. K. & Keiding, N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med 31, 1074–1088, https://doi.org/10.1002/sim.4385 (2012).
Beyersmann, J. a. S., T. in Handbook of Survival Analysis (ed J. Klein) (Chapman & Hall(CRC, 2014).
Ulmer, H., Kelleher, C., Diem, G. & Concin, H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: the Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J 24, 1004–1013 (2003).
Klenk, J. et al. Body mass index and mortality: results of a cohort of 184,697 adults in Austria. Eur J Epidemiol 24, 83–91, https://doi.org/10.1007/s10654-009-9312-4 (2009).
Fritz, J. et al. Mediation analysis of the relationship between sex, cardiovascular risk factors and mortality from coronary heart disease: findings from the population-based VHM&PP cohort. Atherosclerosis 243, 86–92 (2015).
Zitt, E. et al. Anthropometric and Metabolic Risk Factors for ESRD Are Disease-Specific: Results from a Large Population-Based Cohort Study in Austria. PloS one 11, e0161376 (2016).
Wimmer, F., Oberaigner, W., Kramar, R. & Mayer, G. Regional variability in the incidence of end-stage renal disease: an epidemiological approach. Nephrol Dial Transplant 18, 1562–1567 (2003).
WHO. BMI classification, http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
American Diabetes Association. Diagnosing Diabetes and Learning about Prediabetes, http://www.diabetes.org/diabetes-basics/diagnosis/.
Chobanian, A. V. et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252, https://doi.org/10.1161/01.HYP.0000107251.49515.c2 (2003).
Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Acknowledgements
We would like to thank Elmar Stimpfl for excellent technical support and Georg Posch from the Agency for Preventive and Social Medicine (aks) as well as Markus Wallner, Christian Bernhard and Gabriela Dür from the Vorarlberg State Government. We are indebted to all general practitioners who collected the data for the VHM&PP health examinations, and the staff of all dialysis units in Vorarlberg who provided the data for the OEDTR.
Author information
Authors and Affiliations
Contributions
E.Z., K.L. and G.N. wrote the main manuscript text; E.Z. and K.L. planned the study and interpreted the data; C.P., R.S.P., R.K. and G.N. did the statistical analyses and prepared the figure; H.C. and J.B. critically reviewed the manuscript. All authors reviewed the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zitt, E., Pscheidt, C., Concin, H. et al. Long-term risk for end-stage kidney disease and death in a large population-based cohort. Sci Rep 8, 7729 (2018). https://doi.org/10.1038/s41598-018-26087-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26087-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.