Abstract
While high-risk human papillomavirus (HPV) infection is a well-established risk factor for cervical cancer, there are likely other factors within the local microenvironment that contribute to cervical carcinogenesis. Here we investigated relationships between HPV, vaginal pH, vaginal microbiota (VMB) composition, level of genital immune mediators and severity of cervical neoplasm. We enrolled women with low- and high-grade cervical dysplasia (LGD, HGD), invasive cervical carcinoma (ICC), and healthy controls. HPV16, HPV45, HPV58, and HPV31 were the most prevalent in our cohort with HPV16 and HPV31 genotypes more prevalent in Hispanics. Vaginal pH was associated with ethnicity and severity of cervical neoplasm. Lactobacillus dominance decreased with the severity of cervical neoplasm, which correlated with elevated vaginal pH. Hispanic ethnicity was also associated with decreased Lactobacillus dominance. Furthermore, Sneathia was enriched in all precancerous groups, ICC, abnormal pH and Hispanic origin. Patients with ICC, but not LGD and HGD, exhibited increased genital inflammatory scores and elevated specific immune mediators. Notably, IL-36γ was significantly associated with ICC. Our study revealed local, host immune and microbial signatures associated with cervical carcinogenesis and provides an initial step to understanding the complex interplay between mucosal inflammation, HPV persistence and the VMB.
Similar content being viewed by others
Introduction
Cervical cancer is the most common human papillomavirus (HPV)-related malignancy with estimated 526,000 new cases and 239,000 deaths worldwide in 20151. Notably, incident rates of cervical cancer vary among different racial and ethnic groups2,3. In the United States, cervical cancer is more prevalent in Hispanic and African American women, compared to other races or ethnicities3. This disproportionate burden of cervical cancer in these populations has been primarily attributed to a lack of screening and an unequal access to health care; however, other ethnic-related factors might also contribute to these health disparities.
Although persistent infection with high-risk (HR) HPV genotypes is the primary cause of precancerous cervical intraepithelial neoplasia (CIN) and invasive cervical carcinoma (ICC), only a small portion of women infected with HPV progress to CIN, and if not treated, to ICC4, suggesting that other factors in the local microenvironment in conjunction with HPV play a role in cancer progression. An epidemiologic study associated Chlamydia trachomatis with the development of ICC5. We and others hypothesize a mechanistic role of vaginal microbiota (VMB) in both HPV persistence and cervical cancer progression6,7,8,9,10,11,12,13,14,15.
Lactobacilli protect the host against genital infections by producing lactic acid (which lowers vaginal pH below 4.5), secretion of antimicrobial compounds and competitive exclusion16,17,18,19. The VMB composition of reproductive age women is Lactobacillus-dominated (LD) with four predominant species (i.e., Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners or Lactobacillus jensenii), and this LD has been associated with vaginal health16,17,18,19. However, in some women, the VMB lacks a high proportion of lactobacilli and is dominated by a diverse mixture of anaerobic and microaerophilic bacteria (e.g., Gardnerella, Atopobium, Prevotella, Sneathia) commonly associated with bacterial vaginosis (BV). VMB composition varies between women of different ethnic/racial groups with higher prevalence of non-Lactobacillus-dominant (NLD) VMB in African American and Hispanic women16,20,21. However, the higher frequency of NLD VMB in these populations may reflect higher rates of asymptomatic BV19, and controlling for BV eliminates these differences22.
Several epidemiological studies reported an association between BV and HPV infection and persistence8,9,10. Women infected with HPV without cervical disease have been shown to exhibit more diverse VMB relative to HPV-negative women6,7. Two longitudinal studies also revealed that L. gasseri-dominant VMB was associated with HPV clearance, whereas enrichment in Atopobium spp. was associated with HPV persistence11,15. Two additional reports showed increasing VMB diversity in patients with CIN and ICC12,13. However, both studies had a very limited number of ICC cases and authors recommended larger cohort studies to validate their findings. In addition, these studies identified Sneathia spp. to be associated with HPV detection and/or cervical neoplasm12,13,15.
Furthermore, BV-associated bacteria have been associated with genital inflammation, specifically increased levels of proinflammatory cytokines, but not cervical immune cells. Lactobacillus dominance, particularly L. crispatus, has been associated with low levels of genital inflammation23,24,25,26,27,28,29. Inflammatory profiles differ between BV and classical sexually transmitted diseases such as gonorrhea, chlamydia or genital herpes25,28. Nevertheless, chronic genital inflammation may promote carcinogenesis similar to other mucosal sites. In clinical studies, HPV infection or clearance has not been associated with increased levels of genital inflammation, but rather VMB composition30,31. However, one study showed increased levels of proinflammatory cytokines in patients with cervical dysplasia32.
Herein, we performed a cross-sectional study in Arizona Hispanic and non-Hispanic women to identify associations between HPV genotype distribution, vaginal pH, VMB composition, levels of genital immune mediators, ethnicity and severity of cervical neoplasm. Our systematic approach allowed us to identify unique host immune and microbial signatures associated with cervical carcinogenesis in our cohort. To our knowledge this is the first report comprehensively exploring the complex interplay between HPV, VMB, genital immune mediators, disease progression and patient-specific factors such as ethnicity.
Results
Patient demographics
A total of 100 premenopausal women were recruited, enrolled in the study and classified into five groups: HPV-negative controls (Ctrl HPV−; n = 20), HPV-positive controls (Ctrl HPV+; n = 31), low-grade dysplasia (LGD; n = 12), high-grade dysplasia (HGD; n = 27) and invasive cervical carcinoma (ICC; n = 10). Table 1 shows the demographics. There was no significant difference among the groups in terms of age (P = 0.64), body mass index (BMI) (P = 0.96), HPV status [including HPV16, HPV18, high-risk (HR) and low-risk (LR) genotypes distribution] (P > 0.05), HPV risk profile (including infections with single or multiple HR genotypes as well as mixed infections with HR and LR genotypes) (P > 0.10), gravidity (P = 0.25), parity (P = 0.51) or previous loop electrosurgical excision procedure (LEEP) (P = 0.27). Forty-seven percent of patients were of Hispanic origin and 53% were of non-Hispanic origin. Hispanic ethnicity was not significantly different among the groups, ranging from 25.0% (5/20) in Ctrl HPV− to 63.0% (17/27) in HGD (P = 0.11). Two factors, pH and type of contraception, varied across the groups. The rates of hormonal contraceptive use were significantly different (P = 0.02) with the lowest rate in Ctrl HPV− (18.2%; 2/9) and the highest rate in ICC (80.0%, 4/5). However, contraception data were only available for 62% (62/100) of the women.
Increased vaginal pH correlates with severity of cervical neoplasm and Hispanic ethnicity
Vaginal pH was collected from 94% (94/100) of the women using nitrazine paper tests. Normal vaginal pH was defined as ≤4.5, and abnormal vaginal pH was defined as >4.5. Number of patients with abnormal pH significantly varied (P = 0.01, Table 1) and gradually increased among the groups (Fig. 1A). Ctrl HPV− group had the lowest rate of abnormal pH (55.0%, 11/20). Then, percentage of patients with abnormal pH increased to 77.8% (21/27) and 72.7% (8/11) in Ctrl HPV+ and LGD, respectively. The highest rates of abnormal pH were observed in HGD and ICC, respectively, 92.6% (25/27) and 100% (9/9), which was a significant increase when compared to Ctrl HPV− group. The differences in the percentage of women with normal and abnormal vaginal pH across groups remained statistically significant after adjustment for age and BMI (P = 0.003) (Supplementary Table S1). Moreover, vaginal pH (normal vs. abnormal) was not significantly associated with gravidity or parity (Supplementary Fig. S1). Mean pH values were also significantly different among the groups (P = 0.006) (Supplementary Fig. S2A). This analysis showed that vaginal pH level was increased at various stages of cervical carcinogenesis and abnormal vaginal pH is highly associated with cancer. Interestingly, analysis of vaginal pH across the groups in Hispanic and non-Hispanic patients revealed that 89.1% (41/46) of Hispanic women exhibit abnormal pH compared to 68.8% (33/48) of non-Hispanic women (P = 0.02) (Fig. 1B, Supplementary Fig. 2B). However, the difference in the percentage of women with normal and abnormal vaginal pH between ethnicities was attenuated after adjusting for age and BMI (P = 0.07), but it was still significant after adjusting only for age (P = 0.03) or only for BMI (P = 0.048).
Vaginal pH significantly increases with severity of cervical neoplasm and is overall higher in Hispanic women. Percentage of patients with normal pH (≤4.5) and abnormal pH level (>4.5) among the groups (A) and across the groups in Hispanic and non-Hispanic women (B). P values calculated using Fisher’s exact test.
HPV genotype distribution in our cohort is in accordance with the general population
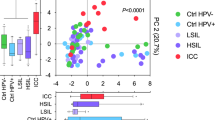
We determined the distribution of 37 HR and LR HPV genotypes in our cohort. The analysis showed that among HPV-positive women, 46.8% (37/79) of individuals were infected with single HR HPV genotype, 44.3% (35/79) were infected with multiple HR HPV genotypes, and only 1.3% (7/79) were infected with LR HP genotypes (Table 1). Furthermore, 91.1% (72/79) of women were positive for both HR and LR HPV genotypes (Table 1). The number of HPV genotypes detected ranged from 1 to 8 in each individual sample and the proportion of single and multiple type infections significantly varied among the groups (P = 0.02) (Fig. 2A). The most prevalent HPV genotypes in our cohort were HPV16 (64.6%, 51/79), HPV45 (21.5%, 21/79), HPV58 (20.3%, 16/79) and HPV31 (18.9%, 15/79) (Fig. 2B). While Ctrl HPV+ patients were co-infected with up to 8 different HPV genotypes (mean = 2.68 ± 1.83), the maximum number of concurrent HPV genotypes detected in ICC samples was only three (mean = 1.70 ± 0.94). This analysis showed that the distribution of HPV genotypes in our cohort was similar to larger epidemiologic studies33,34. Interestingly, when we analyzed the HPV distribution across the groups with regard to ethnicity, prevalence of two HR HPV genotypes was significantly higher in Hispanic compared to non-Hispanic patients. The percentage of HPV16 was 82.05% (32/39) in Hispanic compared to 47.5% (14/40) in non-Hispanic (P = 0.002), whereas percentage of HPV31 was 35.9% in Hispanic (14/39) compared to 2.5% (1/40) in non-Hispanic (P = 0.0001) (Fig. 2C). These data reveal higher prevalence of two HR HPV genotypes in Hispanic compared to non-Hispanic women.
High microbiome diversity and Lactobacillus depletion is associated with increased vaginal pH and correlates with the severity of cervical neoplasm
To examine the composition of VMB in patients at various stages of cervical carcinogenesis, we performed sequencing of the variable region 4 (V4) of 16S rRNA gene. The VMB analysis revealed that vaginal pH, ethnicity and age were significantly correlated with β-diversity differences (Supplementary Table S2). Furthermore, the principal coordinate analysis showed that samples did not cluster in weighted ordination according to severity of cervical neoplasm, but did separate according to vaginal pH, confirming that vaginal pH levels correlate with changes in the VMB composition (Supplementary Figs S3 and S4). Moreover, increasing severity of cervical neoplasm associated with decreasing relative abundance of Lactobacillus spp. and increasing abundance of a variety of microaerophiles and anaerobes (Fig. 3A). Rates of Lactobacillus-dominant (LD) VMB (defined as ≥80% relative abundance of Lactobacillus spp.) were significantly decreased, whereas dysbiotic non-Lactobacillus-dominant (NLD) VMB were increased in LGD (67%), HGD (56%) and ICC (80%) when compared to Ctrl HPV− (40%) or Ctrl HPV+ (32%) (P = 0.04) (Fig. 3B). We also observed that Hispanic patients overall exhibited a more diverse VMB compared to non-Hispanic patients (Fig. 3B). Since sequencing of V4 region is not sufficient to identify lactobacilli at the species level, we performed quantitative PCR analysis using primers specific to four predominant vaginal Lactobacillus species: L. crispatus, L. gasseri, L. iners and L. jensenii. The analysis revealed that L. iners and L. crispatus were the most prevalent lactobacilli in our cohort and the abundance of L. crispatus, but not other lactobacilli, was significantly increased in patients with HGD compared to Ctrl HPV+ (P = 0.01) (Supplementary Fig. S5). Furthermore, to identify differential taxa that may be related to increasing severity of cervical neoplasm, we tested for differences between the four patient groups compared to the HPV-negative control, using DESeq2 analysis35,36,37. We found that Sneathia spp. were significantly enriched, whereas Lactobacillus spp. were underrepresented in ICC, as well as, in the precancerous groups (LGD, HGD) and Ctrl HPV+ (Fig. 4A,D). Other BV-associated bacteria, Atopobium and Parvimonas were significantly enriched in both precancerous groups (LGD and HGD), whereas other BV-associated bacteria (e.g., Gardnerella, Prevotella, Megasphaera, and Shuttleworthia) were enriched only in HGD. To eliminate possible effect of confounders, we controlled our data for age, BMI and ethnicity. We did not adjust for pH, since increased vaginal pH directly correlated with changes in the VMB composition across our groups (Supplementary Figs S3 and S4). Following adjustment, we still observed enrichment in Sneathia spp. in both precancerous groups (LGD, HGD) (Fig. 4B,D). Atopobium and Parvimonas were not significantly enriched in any groups. Following adjustment, we also found L. iners to be enriched in HPV+, LGD and HGD groups. Interestingly, Sneathia and Atopobium were also enriched in women with abnormal pH or of Hispanic ethnicity, whereas Lactobacillus spp. were underrepresented in these women (Fig. 4C). This data indicate that Sneathia spp. or together with other BV-associated bacteria might play a role in cervical carcinogenesis.
Vaginal microbiota (VMB) composition changes to more diverse and less Lactobacillus-dominant with severity of cervical neoplasm that correlates with increased vaginal pH. Heat map reflects relative abundance of the most prevalent bacterial genera and pH level (A). Percentage of patients with Lactobacillus-dominant (LD) and non-Lactobacillus-dominant (NLD) VMB among the groups and ethnicities (B). P values calculated using Fisher’s exact test.
Lactobacillus is underrepresented whereas Sneathia is enriched in HPV-positive, LGD, HGD and ICC samples when compared to HPV-negative controls as well as in patients with abnormal pH and Hispanic women across the groups. Enrichment in bacterial taxa among the groups when compared to HPV-negative control prior (A) and after adjusting for age, BMI, and ethnicity (B). Enrichment in bacterial taxa in patients with abnormal pH vs. normal pH and Hispanic vs. non-Hispanic (C). Comparison of enrichment before and after adjustment for covariates (D). The bacterial enrichment was calculated using the DESeq. 2 package. The threshold for log2 fold difference was 2. Overall differences were tested using a likelihood ratio test, with pairwise comparisons using the Benjamini-Yekutieli false discovery adjustment. Red and blue bars/circles indicate enrichment or underrepresentation of taxa compared to HPV-negative control. The size of circles indicates the fold difference compared to HPV-negative control. Arrows indicate enrichment or underrepresentation in Lactobacillus and Sneathia spp.
Patients with invasive carcinoma, but not precancerous neoplasm exhibit increased levels of genital immune mediators and genital inflammatory score
To characterize local immune signatures in patients at various stages of cervical carcinogenesis, we measured levels of 22 immune mediators in cervicovaginal lavages (Fig. 5A). We found that seven proinflammatory and chemotactic cytokines: IL-36γ, TNFα, RANTES, MIP-1α, MIP-1β, RANTES, IP-10; two hematopoietic cytokines: Flt-3L, GM-CSF; three adaptive immune cytokines: IL-2, IL-4, sCD40L, and an anti-inflammatory cytokine IL-10, were significantly elevated in ICC, whereas the other immunoregulatory cytokine IL-1Ra was significantly reduced in ICC when compared to Ctrl HPV− (Fig. 5B). Within the ICC patients, there were also significant differences in levels between stage I patients vs. stage II-IV patients (Supplementary Fig. S6). None of the immune mediators tested were significantly increased in precancerous groups. The level of IFNγ was significantly decreased in Ctrl HPV+ and HGD and level of IL-1Ra was also lower in HGD (Supplementary Fig. S7). To reduce concerns about multiple comparisons, we utilized a previously described genital inflammation scoring system to show evidence of genital inflammation in our cohort28,31,38. Levels of seven cytokines (IL-1α, IL-1β, IL-8, MIP-1β, CCL20 (MIP-3α), RANTES, and TNFα) were used to determine inflammatory scores; patients were assigned one point for each mediator when the level was in the upper quartile. Using this scoring system, ICC, but not other precancerous groups, exhibited significantly elevated inflammatory score compared to Ctrl HPV− (P < 0.05) (Fig. 5C). As confirmation, we performed a principal components analysis on the 7 cytokines (Supplementary Fig. S8). The first principal component explained 59% of the variance. There was a statistically significant overall difference in the first principal component scores across the 5 severity groups (P = 0.0003). Pairwise comparisons demonstrated statistically significant differences between the Ctrl HPV− and ICC (P = 0.007), Ctrl HPV+ and ICC (P = 0.0003), and HGD and ICC (P = 0.0002). Similar differences were observed also after adjusting for age, BMI and ethnicity. Then, we tested various scoring systems for evaluation of evidence of genital inflammation, which defined genital inflammation as having 3/7, 4/7, 5/7, 6/7 and 7/7 cytokines in the upper quartile (Supplementary Fig. S8). Using 5/7 scoring system was the most sensitive to show evidence of genital inflammation in our patients (see the trend analysis in Supplementary Fig. S8), and also reflected elevated levels of other proinflammatory cytokines and chemokines tested, but not included in the score (Fig. 5A,C). Using this system, we showed that evidence of genital inflammation was significantly increased in ICC patients (60%) compared to Ctrl HPV− (5.0%; P = 0.02), Ctrl HPV+ (6.5%; P = 0.01) and HGD (3.7%; P = 0.006). Evidence of genital inflammation in other groups was not significantly increased compared to Ctrl HPV− (Fig. 5C). Since depletion of lactobacilli and enrichment in Sneathia correlated with the severity of cervical neoplasm, we examined associations between secreted immune mediators and Lactobacillus dominance (relative abundance ≥80%) or Sneathia presence (relative abundance ≥0.01%) (Supplementary Figs S9 and S10). For several of the mediators, the effect of the patient group differed in those with Lactobacillus dominance and Sneathia presence so stratified results are presented (i.e., there was a statistically significant interaction). Following adjustment for age, BMI and ethnicity, MIP-1β and IL-2 were positively associated with ICC in patients with LD VMB (Fig. 6A), whereas IP-10, RANTES, Flt-3L, IL-4 and sCD40L were positively associated with ICC in patients with NLD VMB when compared to Ctrl HPV− (Fig. 6B). IL-36γ was the only cytokine that was positively associated with ICC in patients with either LD or NLD VMB (Fig. 6F). On the other hand, TNFα, TNFβ, MIP-1α, GM-CSF and IL-10 were positively associated with ICC in all patients regardless of Lactobacillus dominance (Fig. 6C). All tested immune mediators were associated with Sneathia presence. After adjusting for covariates, IP-10, GM-CSF, and IL-2 were positively associated with ICC, whereas IFNα2 was negatively associated with ICC in patients with Sneathia-absent VMB when compared to Ctrl HPV− (Fig. 6D). IL-1β, TNFβ, MIP-1β and Flt-3L was positively associated with ICC, whereas IL-1Ra was negatively associated with ICC in patients with Sneathia-present VMB (Fig. 6E). IL-36γ, TNFα, MIP-1α, RANTES, sCD40L and IL-10 were positively associated with ICC in patients with either Sneathia-absent or present VMB (Fig. 6F). The analysis revealed unique immune signature associations between invasive carcinoma and VMB composition.
Patients with ICC exhibit significantly increased genital immune mediators and genital inflammation score. Heat map reflects relative levels of immune mediators across all the samples and inflammatory scores (A). Increasing brightness of red and blue indicate higher and lower concentration of each protein, respectively. Levels of immune mediators significantly altered in ICC when compared to Ctrl HPV− (B). Boxplot whiskers range between 10th and 90th percentiles, dots indicate outliers. P values were calculated using linear mixed effects models where group was the fixed effect and replicate was the random effect with Tukey adjustment. Level of inflammatory scores among the groups (P values calculated using Kruskal-Wallis test) and percentage of patients with genital inflammation, defined as having at least 5/7 of the following cytokines in the upper quartile: IL-1α, IL-1β, IL-8, MIP-1β, CCL20 (MIP-3α), RANTES, and TNFα (P values calculated using Fisher’s exact test) (C).
IL-36γ is significantly associated with ICC regardless of VMB after adjusting for age, BMI, ethnicity, and Lactobacillus dominance or Sneathia presence. β-coefficients of immune mediators that were not associated with (A) or associated with Lactobacillus dominance (defined as ≥80% relative abundance) (B,C). β-coefficients of immune mediators that were associated with Sneathia presence (defined as ≥0.01% relative abundance) (D,E). Dots indicate β-coefficients of linear regression analysis; error bars represent standard error (SE). Red and blue dots indicate positive or negative associations, respectively, that were significant compared to Ctrl HPV− after adjusting for covariates (P < 0.05). Venn diagram showing relations between VMB composition and associations of immune mediators with ICC and VMB composition (F).
Discussion
Persistent infection with HR HPV genotypes is the most important risk factor for cervical cancer precursors and ICC4. However, the cause of persistent HPV infection (as most infections are cleared) and progression to cervical cancer remain poorly understood39. In this report, we explored associations between HPV, vaginal pH, VMB composition, levels of secreted genital immune mediators and severity of cervical neoplasm to further investigate the complex interaction between these factors in the local microenvironment.
HPV genotype distribution in our cohort was similar to larger epidemiologic studies, as expected, with HPV16 being most prevalent (65% of HPV-positive samples)33,34. Other frequent genotypes detected included HPV45, HPV58 and HPV31. These HPV genotypes are targeted by the newest nonavalent HPV vaccine, but not the bi- and quadrivalent vaccines; however, the women in our cohort were not vaccinated. Infection with multiple genotypes were common. In addition, rates of co-infection with multiple genotypes decreased with the severity of neoplasm, in accordance with previous reports40. Interestingly, HPV16 and HPV31 were significantly more prevalent in Hispanic compared to non-Hispanic women. Others also reported racial/ethnic differences in HPV distribution among women with CIN34,41,42. In contrast to our data, Hariri et al. showed higher prevalence of HPV45 in Hispanic women and higher rates of HPV16/18-related lesions in non-Hispanic34. Notably, geographic differences in HPV distribution have been also reported33,42.
We found that abnormal vaginal pH (>4.5) correlated with the severity of cervical neoplasm. A large population-based study also showed that elevated vaginal pH is associated with oncogenic HPV detection, multiple HPV types infections and diagnosis of LGD43. Our data expand these findings showing positive association of abnormal pH with advanced cervical dysplasia and ICC. Walther-António et al. also reported high vaginal pH, combined with detection of Atopobium vaginae and Porphyromonas spp., to be associated with endometrial cancer44. Collectively, these data suggest that abnormal pH might be a marker of gynecologic cancer. Moreover, we also found that Hispanic women exhibited overall higher vaginal pH, in accordance with a previous report16. Elevated vaginal pH might be an additional risk factor for cervical cancer development/progression.
In addition, abnormal vaginal pH (>4.5) in our cohort correlated with decreased relative Lactobacillus abundance, in accordance with a previous report showing elevated vaginal pH in women with diverse NLD VMB16. In our study, NLD VMB significantly (P = 0.04) increased with severity of cervical neoplasm with significantly higher rates of diverse NLD VMB in patients with advanced cervical neoplasia and invasive carcinoma. A previous report also suggested that the lactobacilli depletion is associated with advancing CIN severity, but the analysis did not reach statistical significance12. Interestingly, we also observed that Hispanic women had higher ratios of diverse NLD VMB compared to non-Hispanic women. Higher rates of diverse NLD VMB in Hispanic women are consistent with a previous report16. However, the observed disproportion can be due to higher rates of asymptomatic BV22, which has been associated with HPV detection, as well as, delayed HPV clearance8,10. In contrast to other studies6,7,11,15,31, HPV positivity did not significantly alter the VMB composition in women without cervical dysplasia; however, the high proportion of Hispanic women in our cohort might contribute to this finding. Overall, our study indicates that diverse NLD VMB is also associated with cervical disease severity and progression.
With regard to specific bacterial taxa, the majority of identified bacteria that were enriched in HPV-positive women or women with LGD, HGD or ICC are commonly associated with BV (i.e., Gardnerella, Atopobium, Prevotella. Megasphaera, Parvimonas, Peptostreptococcus, Anaerococcus, Sneathia)45. However, we also identified bacterial genera associated with aerobic vaginitis (i.e. Streptococcus agalactiae) or other dysbioses (i.e. Clostridium) to be enriched in these women46. Moreover, we found novel bacterial taxa, i.e. Shuttleworthia, Gemella and Olsenella, to be also associated with HPV positivity and/or cervical neoplasia.
Interestingly, we found Sneathia spp. to be the only taxon that was significantly enriched in women infected with HPV, as well as, women with precancerous lesions and cervical cancer. Results of our study complement and extend previous findings7,13,15. Two previous studies showed higher levels of Sneathia in HPV-positive women compared to uninfected women and suggested that Sneathia might be a microbiological marker of HPV infection7,15. Mitra et al. also identified S. sanguinegens to be more prevalent in women with HGD compared to LGD12. Furthermore, Audirac-Chalifour et al. showed that Sneathia predominates in women with CIN, but not ICC13. Our study shows Sneathia to be associated with all stages of cervical carcinogenesis (i.e., Ctrl HPV+, LGD, HGD, ICC). Moreover, after controlling for known VMB confounders (age, BMI, ethnicity), our analysis revealed that precancerous LGD and HGD are enriched in Sneathia. This finding suggests that presence of Sneathia in VMB may also be a metagenomic marker for CIN progression. Lack of enrichment of Sneathia in invasive carcinoma following adjustment for covariates might be due to small ICC sample size. Alternatively, Sneathia might be outcompeted by other bacteria (“bacterial passengers”), having a competitive advantage in the tumor microenvironment, as hypothesized at other mucosal sites47.
Other novel taxon identified in this study to be associated with cervical dysplasia included Prevotella amnii, originally isolated from human amniotic fluid48. Previously, Prevotella spp. have been associated with HPV infection, but not identified to the species level7. We also found Atopobium and Parvimonas spp. to be enriched with low- and high-grade precancerous lesions. However, following adjustment, we did not detect this relationship, suggesting that enrichment in Atopobium and Parvimonas was associated with one of the covariates. Interestingly, following adjustment for covariates, we also found L. iners to be enriched in HPV-positive women, as well as, women with LGD and HGD. Others also reported L. iners to be associated with higher risk of cervical dysplasia14,49. L. iners can dominate VMB of healthy women; nevertheless, it is also found in the majority of women diagnosed with BV50. Moreover, L. iners-dominated VMB is more likely to transition to the dysbiotic VMB51. Our findings suggest that L. iners can contribute to the changes in VMB composition, which may lead to disease progression.
In this study, we systematically examined levels of secreted immune mediators in the cervicovaginal microenvironment at various stages of cervical carcinogenesis. Our analysis revealed increased levels of proinflammatory and chemotactic cytokines (IL-36γ, TNFα, RANTES, MIP-1α, MIP-1β, IP-10), hematopoietic cytokines (Flt-3L, GM-CSF), adaptive immune response cytokines (IL-2, IL-4, sCD40L), and an anti-inflammatory cytokine (IL-10) in women with ICC, but not with precancerous lesions. We did not observe an elevated proinflammatory response in HPV-positive women, which is in accordance with a recent report showing that neither HPV infection nor clearance is associated with broad differences in cervicovaginal cytokines31. We also evaluated a previously described inflammatory score system to avoid multiple comparisons28,31,38. We observed evidence of genital inflammation (defined by elevation of ≥5/7 cytokines) only in patients with ICC, but not with precancerous lesions, when compared to HPV-negative controls. Masson et al. showed that women with BV exhibited only moderately increased levels of pro-inflammatory cytokines, and decreased levels of chemotactic cytokines, whereas women with STI, such as chlamydia and gonorrhea, exhibit the highest levels of cervicovaginal cytokines25. We also did not observe an overt proinflammatory response in our STI-negative cohort, other than the cancer group. Shannon et al. used inflammatory scores and showed that only 45% women with the diverse NLD VMB demonstrate evidence of genital inflammation (defined by elevation of ≥3/7 cytokines). When we employed the same scoring system (≥3/7) for evidence of genital inflammation, we still did not observe any significant differences between precancerous groups and controls.
To decipher the relationship between VMB composition and immune mediators, we explored the associations between immune mediators and Lactobacillus, which are related to vaginal health, as well as, potentially pathogenic Sneathia. Several immune mediators, such as, TNFα, TNFβ, MIP-1α, GM-CSF and IL-10 were associated with cancer, but not Lactobacillus-dominance, suggesting that their elevated levels are cancer-driven. However, the associations of several other cytokines (IL-36γ, MIP-1β, RANTES, IP-10, IL-2, IL-4, Flt-3L, sCD40L) with cancer were dependent on the VMB composition. Interestingly, IL-36γ was highly associated with cancer, irrespective of VMB composition. IL-36γ, which belongs to the IL-1 family, is a proinflammatory cytokine expressed at various mucosal sites, including the female reproductive tract, and it functions as a putative “alarmin” in damaged tissue52,53. Our studies using a human 3-dimensional vaginal epithelial cell model showed that IL-36γ is a driver for epithelial and immune activation following microbial insult and might play a crucial role in the host defense against invading pathogens52. However, the role of IL-36γ in cancer is poorly understood. Wang et al. revealed that IL-36γ exerted anti-tumor effects in vivo and transformed the tumor microenvironment in favor of tumor eradication54. Moreover, the study showed that tumoral expression of IL-36γ in melanoma and lung cancer decreased tumor progression54. This might suggest that increased secretion of IL-36γ in the cervicovaginal microenvironment is a defense mechanism employed by the host to limit cervical cancer progression. On the other hand, in psoriasis, IL-36γ has been shown to promote the inflammatory response through the Wnt/β-catenin pathway, which is altered in many cancers55. This may suggest that IL-36γ is contributing to cervical carcinogenesis by promoting damaging inflammation. The impact of IL-36γ on cervical and other gynecologic cancers warrants further investigation.
A major strength of our study is a high proportion of Hispanic women, which allowed us to determine ethnic-specific factors contributing to health disparities. Compared to other reports, we systemically explored several factors (i.e. HPV, VMB, pH, ethnicity, level of secreted immune mediators), which may contribute to HPV persistence and/or disease progression. Moreover, we used sophisticated statistical analyses to adjust our microbiome and immune mediator data for known confounders. Our study has some limitations, including a relatively modest sample size and the lack of specific sexual behavior data, i.e. number of sexual partners or sexual debut age. We employed a cross-sectional study design; therefore, we focused on associations, not causation. Longitudinal studies are required to extend these findings and determine the extent to which a diverse NLD VMB or specific bacterial species are casual factors in cervical carcinogenesis or consequences of the disease.
In conclusion, we showed that vaginal pH was associated with ethnicity and significantly increased with the severity of cervical neoplasm. We also revealed that abnormal vaginal pH, Hispanic ethnicity and the severity of cervical neoplasm was significantly associated with depletion of lactobacilli. Furthermore, Lactobacillus dominance decreased with the severity of cervical neoplasm. We identified Sneathia spp., other BV-associated bacterial species and novel bacterial taxa to be associated with HPV infection and/or HPV-related cervical neoplasm. In addition, we found that women with ICC, but not women with precancerous lesions, exhibited evidence of genital inflammation. Notably, IL-36γ was identified as a unique immune mediator significantly associated with cervical cancer regardless of VMB composition. Overall, this study revealed unique immune and microbial signatures in the local microenvironment associated with cervical carcinogenesis in non-Hispanic and Hispanic women. Further studies, particularly those employing longitudinal designs, are required to better understand the molecular mechanisms involved in the complex role that bacterial communities and/or individual bacterial species may play in the development of cervical neoplasm and the progression to invasive disease.
Methods
Participant enrollment
Participants were consecutively recruited at three clinical sites located in Phoenix, AZ: St. Joseph’s Hospital, and Medical Center (SJHMC), University of Arizona (UA) Cancer Center and Maricopa Integrated Health System (MIHS) and screened based off eligibility criteria and approached to participate in the study. All patients provided informed written consent and all research and related activities involving human subjects were approved by the Institutional Review Boards at UA, SJHMC and MIHS and performed in accordance with federal guidelines and regulations. One hundred premenopausal, non-pregnant, women diagnosed with cervical dysplasia and ICC, as well as, healthy HPV-positive and HPV-negative women were enrolled and contributed to the study. Histology of colposcopy-directed biopsy samples was used to classify patients into groups. A two-tiered system of CIN was used: low-grade dysplasia (LGD) was defined as CIN1, and high-grade dysplasia (HGD) was defined as CIN2/3. If histology was not available (i.e. for healthy HPV-positive and HPV-negative controls), cytology was used for classification. Additionally, HPV status was determined by the Linear Array HPV Genotyping Tests (Roche, Indianapolis, IN), and used to subdivide healthy women into two groups: HPV-positive (Ctrl HPV+, including high- and low-risk HPV genotypes) and HPV-negative controls (Ctrl HPV−). Overall, patients were divided into five groups: women with LGD, women with HGD, women with ICC, including adenocarcinoma and squamous cell carcinoma, Ctrl HPV+ and Ctrl HPV−. Exclusion criteria for the study included: currently menstruating; currently on antibiotics, antifungals or antivirals or within the previous 3 months; current vaginal infection (including bacterial vaginosis), vulvar infection, urinary tract infection or sexually transmitted infection (chlamydia, gonorrhea, trichomoniasis, genital herpes) or within the previous 3 weeks; unusual or foul-smelling vaginal discharge; use of douching substances, vaginal applied medications and suppositories, feminine deodorant sprays, vaginal lubricants within 48 hours prior the visit; any skin condition in the genital area interfering with the study; sexual intercourse less than 48 hours prior the visit; type I or type II diabetes; hepatitis; being HIV-positive. The exclusion criteria were verified by physician’s pelvic exam, medical record and/or self-reported. The details of exclusion criteria are listed in the Supplementary Table S3.
Sample collection
Vaginal swabs and cervicovaginal lavages (CVL) were collected by a clinician. A speculum was inserted without lubricant and two vaginal swabs were collected. The first swab was collected by swabbing the lateral walls of the mid vagina using an eSwab collection system containing Amies transport medium (COPAN Diagnostics, Murrieta, CA). The second swab was used to measure vaginal pH using nitrazine paper, and recorded by the clinician according to the manufacturer’s instructions using a scale of 4.5–7.5. Following vaginal swab collection, CVLs were collected using 10 ml of sterile 0.9% saline solution (Teknova, Hollister, CA). Following the collection, both vaginal swab and CVL were immediately placed on ice and frozen at −80 °C within 1 hour of collection.
Sample processing
CVLs were thawed on ice and clarified by centrifugation (700 × g for 10 min at 4 °C). DNA was extracted from vaginal swabs using PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) following the manufacturer’s instructions. Both CVL and extracted DNA were aliquoted to avoid freeze/thaw cycles and stored at −80 °C for further analysis.
HPV genotyping
HPV genotyping was performed on DNA samples extracted from vaginal swabs using the Linear Array HPV Genotyping Tests (Roche) following the manufacturer’s instructions.
Measurement of immune mediators in CVL
Levels of 21 cytokines and chemokines (IL-1α, IL-1β, IL-6, IL-12p70, TNFα, TNFβ, IL-8, IP-10, MIP-1α, MIP-1β, RANTES, Flt-3L, GM-CSF, IFNα2, IFNγ, IL-2, IL-4, IL-17A, sCD40L, IL-1Ra, IL-10) were determined in CVL aliquots using the Milliplex MAP Human Cytokine/Chemokine and Th17 Magnetic Bead Panels (Millipore, Billerica, MA) in accordance with the manufacturer’s protocol. Level of IL-36γ was measured in CVL aliquots by enzyme-linked immunosorbent assay using Human IL-36γ ELISA kit (RayBiotech, Norcross, GA) in accordance with manufacturer’s instructions. A four-parameter logistic regression curve fit was used to determine the concentration. All samples were assayed in duplicate.
16S rRNA sequencing analysis
16S rRNA sequencing analysis was performed by the Second Genome Inc. (San Francisco, CA). Briefly, the V4 region of bacterial 16S rRNA operon was amplified from the genomic DNA obtained from vaginal swabs and sequenced on the MiSeq platform (Illumina, San Diego, CA). The samples were analyzed using USEARCH (for details see Supplementary Methods).
Differential abundance analysis
The operation taxonomic units (OTUs) present at the species level were evaluated for their differential presence across the patient groups. Initially, the R package, DESeq236,37, was used to perform a likelihood ratio test with shrinkage to the base 2 log fold difference to determine whether an OTU had a significantly different normalized count in one of the four groups compared to the normalized count in the HPV-negative control. Subsequently, pairwise comparisons versus the HPV-negative control were performed using the Benjamini-Yekutieli false discovery adjustment (adjusted P < 0.05). A similar analysis was performed with adjustment for age, BMI (≤25, >25), and ethnicity (Hispanic, non-Hispanic).
Quantitative real-time PCR analysis
Relative abundance of four Lactobacillus spp. was determined by quantitative real-time PCR analysis, performed on an Applied Biosystems QuantStudio6 Flex Real Time PCR System (Life Technologies, Grand Island, NY) using DNA extracted from vaginal swabs, TaqMan Assays specific for L. crispatus, L. gasseri, L. iners, L. jensenii and panbacterial 16S rRNA genes and TaqMan Vaginal Microbiota Amplification Control and TaqMan Fast Advanced Master Mix (Life Technologies). Relative abundances were calculated using a standard curve method and the 16S rRNA gene level as an internal standard.
Statistical analyses
Differences in the demographic variables between patient groups were tested using analysis of variance model (ANOVA) for continuous variables and Fisher’s exact test for categorical variables. The difference in normal (≤4.5) versus abnormal pH levels (>4.5) between patient groups was tested using Fisher’s exact test, overall and adjusted for age and BMI using logistic regression. Lactobacillus relative abundance was classified as Lactobacillus-dominant (LD) versus non-Lactobacillus-dominant (NLD) based on a cut-point of 80%. This cut-point classified roughly equal number of patients in the LD (n = 51) and NLD (n = 49) groups. Differences across patient groups were tested using Fisher’s exact test. Comparison of the abundance of the four predominant Lactobacillus species was tested using the nonparametric Kruskal-Wallis test.
For the immune mediator data, the values above the detection limit were substituted with the maximum observation values of the target and values below the detection limit were substituted with 0.5 of the minimum detectable concentration provided in manufacturer’s instructions. The log transformation was applied to normalize the data. The difference in the concentration between the patient groups was tested using a linear mixed effects model where group was a fixed effect and replicate was the random effect. The β-coefficient and standard error were estimated to show the direction of association. If the overall difference was significant (P < 0.05), paired tests were performed with Tukey adjustment. Comparisons were adjusted for BMI, age and ethnicity in the linear mixed effects models. The β-coefficients therefore represent the expected change in each immune mediator for that patient group relative to the HPV-negative control for two women with the same values of the clinical covariates. Preliminary analyses demonstrated that there were statistically significant interactions between Lactobacillus dominance (LD) and the patient groups on the levels of the immune mediators (P < 0.05). Thus, whether there were significant differences between the severity groups depended on LD on non-Lactobacillus-dominance (NLD). For these immune mediators, comparison between the groups was performed separately for LD and NLD. To reduce concerns about multiple comparisons, we evaluated a previously described scoring system based on the levels of 7 cytokines. We assessed various scoring systems (e.g. 3/7, 4/7, 5/7, 6/7, 7/7) and computed overall P values using Fisher’s exact test (Supplementary Fig. S8A). Additionally, we tested for trend using logistic regression (treating the number of cytokines as a continuous variable). As confirmation, we performed a principal components analysis and assessed differences between the severity groups overall and after adjusting for age, BMI and ethnicity (Supplementary Fig. S8B). The overall assessment was based on ANOVA with pairwise comparisons adjusted using Tukey’s procedure. All analyses were performed using SAS 9.4.
References
Global Burden of Disease Cancer, C. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3, 524–548, https://doi.org/10.1001/jamaoncol.2016.5688 (2017).
Siegel, R. L. et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 65, 457–480, https://doi.org/10.3322/caac.21314 (2015).
Viens, L. J. et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep 65, 661–666, https://doi.org/10.15585/mmwr.mm6526a1 (2016).
Shulzhenko, N., Lyng, H., Sanson, G. F. & Morgun, A. Menage a trois: an evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol 22, 345–353, https://doi.org/10.1016/j.tim.2014.02.009 (2014).
Lehtinen, M. et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex Transm Infect 87, 372–376, https://doi.org/10.1136/sti.2010.044354 (2011).
Gao, W., Weng, J., Gao, Y. & Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect Dis 13, 271, https://doi.org/10.1186/1471-2334-13-271 (2013).
Lee, J. E. et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8, e63514, https://doi.org/10.1371/journal.pone.0063514 (2013).
Watts, D. H. et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis 191, 1129–1139, https://doi.org/10.1086/427777 (2005).
Gillet, E. et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis 11, 10, https://doi.org/10.1186/1471-2334-11-10 (2011).
Guo, Y. L., You, K., Qiao, J., Zhao, Y. M. & Geng, L. Bacterial vaginosis is conducive to the persistence of HPV infection. Int J STD AIDS 23, 581–584, https://doi.org/10.1258/ijsa.2012.011342 (2012).
Brotman, R. M. et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 210, 1723–1733, https://doi.org/10.1093/infdis/jiu330 (2014).
Mitra, A. et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep 5, 16865, https://doi.org/10.1038/srep16865 (2015).
Audirac-Chalifour, A. et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS One 11, e0153274, https://doi.org/10.1371/journal.pone.0153274 (2016).
Oh, H. Y. et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect 21, 674 e671–679, https://doi.org/10.1016/j.cmi.2015.02.026 (2015).
Di Paola, M. et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci Rep 7, 10200, https://doi.org/10.1038/s41598-017-09842-6 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108(Suppl 1), 4680–4687, https://doi.org/10.1073/pnas.1002611107 (2011).
Hickey, R. J., Zhou, X., Pierson J. D., Ravel, J., Forney L. J. Understanding vaginal microbiome complexity from an ecological perspective. Translational Research (2012).
Nunn, K. L. & Forney, L. J. Unraveling the Dynamics of the Human Vaginal Microbiome. The Yale journal of biology and medicine 89, 331–337 (2016).
Martin, D. H. & Marrazzo, J. M. The Vaginal Microbiome: Current Understanding and Future Directions. The Journal of infectious diseases 214(Suppl 1), S36–41, https://doi.org/10.1093/infdis/jiw184 (2016).
Zhou, X. et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1, 121–133, https://doi.org/10.1038/ismej.2007.12 (2007).
Fettweis, J. M. et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160, 2272–2282, https://doi.org/10.1099/mic.0.081034-0 (2014).
Beamer, M. A. et al. Bacterial species colonizing the vagina of healthy women are not associated with race. Anaerobe 45, 40–43, https://doi.org/10.1016/j.anaerobe.2017.02.020 (2017).
Gosmann, C. et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46, 29–37, https://doi.org/10.1016/j.immuni.2016.12.013 (2017).
Anahtar, M. N. et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965–976, https://doi.org/10.1016/j.immuni.2015.04.019 (2015).
Masson, L. et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 90, 580–587, https://doi.org/10.1136/sextrans-2014-051601 (2014).
Kyongo, J. K. et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol 22, 526–538, https://doi.org/10.1128/CVI.00762-14 (2015).
Jespers, V. et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 7, 11974, https://doi.org/10.1038/s41598-017-12198-6 (2017).
Shannon, B. et al. Distinct effects of the cervicovaginal microbiota and herpes simplex type 2 infection on female genital tract immunology. J Infect Dis 215, 1366–1375, https://doi.org/10.1093/infdis/jix088 (2017).
Masson, L. et al. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect 92, 186–193, https://doi.org/10.1136/sextrans-2015-052072 (2016).
Kriek, J. M. et al. Female genital tract inflammation, HIV co-infection and persistent mucosal Human Papillomavirus (HPV) infections. Virology 493, 247–254, https://doi.org/10.1016/j.virol.2016.03.022 (2016).
Shannon, B. et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol 10, 1310–1319, https://doi.org/10.1038/mi.2016.129 (2017).
Mhatre, M. et al. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm Dis 39, 591–597, https://doi.org/10.1097/OLQ.0b013e318255aeef (2012).
Clifford, G. M. et al. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 14, 1157–1164, https://doi.org/10.1158/1055-9965.EPI-04-0812 (2005).
Hariri, S. et al. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 206, 1878–1886, https://doi.org/10.1093/infdis/jis627 (2012).
Weiss, S. et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5, 27, https://doi.org/10.1186/s40168-017-0237-y (2017).
McMurdie, P. J. & Holmes, S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10, e1003531, https://doi.org/10.1371/journal.pcbi.1003531 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol 15, 550, https://doi.org/10.1186/s13059-014-0550-8 (2014).
Arnold, K. B. et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 9, 194–205, https://doi.org/10.1038/mi.2015.51 (2016).
Chase, D., Goulder, A., Zenhausern, F., Monk, B. & Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol 138, 190–200, https://doi.org/10.1016/j.ygyno.2015.04.036 (2015).
Azuma, Y. et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin Oncol 44, 910–917, https://doi.org/10.1093/jjco/hyu112 (2014).
Vidal, A. C. et al. HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA. Cancer Causes Control 25, 1055–1062, https://doi.org/10.1007/s10552-014-0406-2 (2014).
Niccolai, L. M. et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: Associations with race, ethnicity, and poverty. Cancer 119, 3052–3058, https://doi.org/10.1002/cncr.28038 (2013).
Clarke, M. A. et al. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis 12, 33, https://doi.org/10.1186/1471-2334-12-33 (2012).
Walther-Antonio, M. R. et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med 8, 122, https://doi.org/10.1186/s13073-016-0368-y (2016).
Onderdonk, A. B., Delaney, M. L. & Fichorova, R. N. The Human Microbiome during Bacterial Vaginosis. Clin Microbiol Rev 29, 223–238, https://doi.org/10.1128/CMR.00075-15 (2016).
Donders, G. G. G., Bellen, G., Grinceviciene, S., Ruban, K. & Vieira-Baptista, P. Aerobic vaginitis: no longer a stranger. Res Microbiol 168, 845–858, https://doi.org/10.1016/j.resmic.2017.04.004 (2017).
Tjalsma, H., Boleij, A., Marchesi, J. R. & Dutilh, B. E. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol 10, 575–582, https://doi.org/10.1038/nrmicro2819 (2012).
Lawson, P. A., Moore, E. & Falsen, E. Prevotella amnii sp. nov., isolated from human amniotic fluid. Int J Syst Evol Microbiol 58, 89–92, https://doi.org/10.1099/ijs.0.65118-0 (2008).
Piyathilake, C. J. et al. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev Res (Phila) 9, 357–366, https://doi.org/10.1158/1940-6207.CAPR-15-0350 (2016).
Srinivasan, S. et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7, e37818, https://doi.org/10.1371/journal.pone.0037818 (2012).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4, 132ra152, https://doi.org/10.1126/scitranslmed.3003605 (2012).
Winkle, S. M., Throop, A. L. & Herbst-Kralovetz, M. M. IL-36gamma Augments Host Defense and Immune Responses in Human Female Reproductive Tract Epithelial Cells. Front Microbiol 7, 955, https://doi.org/10.3389/fmicb.2016.00955 (2016).
Gresnigt, M. S. & van de Veerdonk, F. L. Biology of IL-36 cytokines and their role in disease. Semin Immunol 25, 458–465, https://doi.org/10.1016/j.smim.2013.11.003 (2013).
Wang, X. et al. IL-36gamma transforms the tumor microenvironment and promotes type 1 lymphocyte-mediated antitumor immune responses. Cancer Cell 28, 296–306, https://doi.org/10.1016/j.ccell.2015.07.014 (2015).
Wang, W., Yu, X., Wu, C. & Jin, H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int J Med Sci 14, 1002–1007, https://doi.org/10.7150/ijms.20809 (2017).
Acknowledgements
We would like to thank Dr. David Greenspan, Dr. Bradley Monk, Kelli Williamson, Ann De Jong, Eileen Molzen, Liane Fales, Maureen Sutton for the kind assistance in patient recruitment and sample collection, Shripad Sinari for the assistance in microbiome data analysis and the patients that enrolled in this study. This study was generously supported by the Flinn Foundation Grant # 1917 (to M.M.H.-K. and D.M.C.) and partially by the National Institutes of Health NIAID Grant 1R15AI113457-01A1 (to M.M.H.-K.) and the National Institutes of Health NCI Grant P30 CA023074.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the study. M.M.H.-K. and D.M.C. conceived and designed the study. A.G., D.B. and D.M.C. participated in the patient recruitment, sample and data collection. P.L. performed the experiments. All authors contributed to analysis and interpretation of the data. H.C. and D.J.R. performed the statistical analysis. P.L., M.M.H.-K., D.M.C. and D.J.R. wrote and critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Łaniewski, P., Barnes, D., Goulder, A. et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep 8, 7593 (2018). https://doi.org/10.1038/s41598-018-25879-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25879-7
This article is cited by
-
Targeting inflammation as cancer therapy
Journal of Hematology & Oncology (2024)
-
HPV-associated cervicovaginal microbiome and host metabolome characteristics
BMC Microbiology (2024)
-
Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer
Infectious Agents and Cancer (2024)
-
Aerobic vaginitis is associated with carbonic anhydrase IX in cervical intraepithelial neoplasia
Scientific Reports (2024)
-
The emerging role of the gut microbiome in cancer cell plasticity and therapeutic resistance
Cancer and Metastasis Reviews (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.