Abstract
Anopheles gambiae and An. coluzzii, the two most important malaria vectors in sub-Saharan Africa, are recently radiated sibling species that are reproductively isolated even in areas of sympatry. In females from these species, sexual transfer of male accessory gland products, including the steroid hormone 20-hydroxyecdysone (20E), induces vast behavioral, physiological, and transcriptional changes that profoundly shape their post-mating ecology, and that may have contributed to the insurgence of post-mating, prezygotic reproductive barriers. As these barriers can be detected by studying transcriptional changes induced by mating, we set out to analyze the post-mating response of An. gambiae and An. coluzzii females captured in natural mating swarms in Burkina Faso. While the molecular pathways shaping short- and long-term mating-induced changes are largely conserved in females from the two species, we unravel significant inter-specific differences that suggest divergent regulation of key reproductive processes such as egg development, processing of seminal secretion, and mating behavior, that may have played a role in reproductive isolation. Interestingly, a number of these changes occur in genes previously shown to be regulated by the sexual transfer of 20E and may be due to divergent utilization of this steroid hormone in the two species.
Similar content being viewed by others
Introduction
Although overall malaria mortality rates have significantly declined since 2010 due to increased prevention and control measures, Sub-Saharan Africa continues to carry a disproportionately high share of the global malaria burden, bearing more than 90% of the 212 million cases and of the estimated 429,000 deaths caused by Plasmodium parasites1. One of the main reasons for this higher burden in the African continent is the presence of a very efficient mosquito vectorial system, principally represented by the two most recently radiated species of the Anopheles gambiae complex, i.e. Anopheles gambiae and An. coluzzii2,3. These species are sympatric in West and Central Africa4,5, but differ in their larval ecology, with An. gambiae being more adapted to temporary rain-dependent and An. coluzzii to permanent anthropogenic breeding sites6,7,8,9. Due to their major role as malaria vectors, the two species are the target of several studies aimed at developing novel approaches for the control of disease transmission in sub-Saharan Africa, with the view to complement or strengthen current insecticide-based control methods10,11,12.
One of these novel approaches consists in manipulating the mosquito reproductive success. A recent study showed that application of non-steroidal agonists of the steroid hormone 20-hydroxyecdysone (20E) on An. gambiae virgin females virtually sterilizes them by preventing their insemination and reducing egg development10. This hormone is a potent regulator of gene transcription during both juvenile development and oogenesis in adults13,14, and in some anopheline species is synthetized in the male accessory glands (MAGs) and transferred during mating to the female atrium together with other seminal secretions embedded in a gelatinous structure named the mating plug15,16,17,18,19,20. Several studies have shown that in species of the Afrotropical An. gambiae complex, sexual transfer of 20E is essential for proper induction of female post-mating behaviors, such as refractoriness to further mating, enhanced egg production, triggered egg laying and increased fertility17,18,19,21. In addition, 20E injections in the G3 strain, which is a mixture of An. gambiae and An. coluzzii, induce a broad transcriptional response in the female reproductive tract, closely overlapping with the vast response induced by mating in the same strain, where hundreds of genes are up-and down-regulated at different time points after copulation18,22. However, the extent to which the response to mating is conserved between An. gambiae and An. coluzzii females is currently unknown.
In Drosophila, multiple lines of evidence point to a role of female post-mating biology in the insurgence of post-mating, prezygotic reproductive barriers. For instance, in crosses between recently diverged species, failure in sperm transfer and/or storage in hetero-specific crosses was attributed to mating-induced changes23,24. Additionally, processing of the insemination “plug” that forms in Drosophila females immediately after mating takes longer in hetero- than in homo-specific crosses25,26. It has also been postulated that fast evolving male-female molecular interactions or post-mating changes in transcript abundance may represent signatures of natural selection shaping the evolutionary arms race between the sexes27,28,29,30.
Post-mating events may have also played a role in the recent divergence between An. gambiae and An. coluzzii. While hybrid males from most crosses between species of the An. gambiae complex are sterile, males from crosses between An. gambiae and An. coluzzii do not show signatures of genetic incompatibilities and are fully fertile, with no obvious loss in fitness under laboratory conditions31. Nevertheless, hybrids between these two species are rarely observed in most areas of sympatry5,32. Where the two species are sympatric, e.g. in Burkina Faso, spatial and temporal segregation of the swarms is significantly contributing to assortative mating33,34, while close-range mate recognition cues, such as species-specific flight tones and/or contact cuticular pheromones, are believed to reinforce pre-mating isolation35,36,37,38,39,40,41. Inter-specific mating couples have, however, been repeatedly collected in the field42,43, suggesting the co-occurrence of intrinsic and/or extrinsic post-mating isolation mechanisms. While the latter have been shown to play a role5, intrinsic post-mating isolation mechanisms have never been investigated.
Here we report the first data on the transcriptional changes induced by mating in An. gambiae and An. coluzzii females captured in natural mating swarms from Burkina Faso. Our results corroborate previous data obtained under laboratory conditions18,22, allow the identification of factors potentially important for mating, fertility and reproductive success in each species, and provide novel insights on inter-specific differences that shape their reproductive ecology and may help unravel the mechanisms of their reproductive isolation.
Results
Collection of mating couples from natural swarms
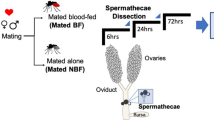
In order to analyze the natural post-mating response of females from the two anopheline sibling species, we collected 91 An. gambiae and 75 An. coluzzii mating couples from different swarms in the villages of Soumousso and Vallèe du Kou (Burkina Faso) (Fig. 1). Females of each couple were then dissected at either 1 day or 4 days post mating (PM), to capture the short-term as well as the lasting, long-term response to copulation. We dissected the lower reproductive tract (LRT) comprising atrium and spermatheca, and the rest of the body (carcass).
Schematic map of the collection sites in Burkina Faso. For each of the two sites (Vallèe du Kou and Soumousso), the total number of mosquitoes collected is indicated. The relative percentage of species is reported in the pie charts for both larval and adult samples, and species are color-coded as described in the figure. Anopheles arabiensis were not studied further. The map and the drawings have been generated using Illustrator CC 2017 (Adobe).
Virgin females were instead produced by collecting larvae from natural breeding sites, and LRTs and carcasses were dissected from resulting adult females at 2 and 5 days post emergence. Because the age of mated females could not be determined as they were caught in natural mating swarms, we chose these time points for tissue collection in virgins to approximately age-match these samples to the ones dissected from mated females, given that it is generally believed that females mate on the second night after emergence44.
Post-mating transcriptional response in the lower reproductive tract (LRT) of field An. gambiae and An. coluzzii females
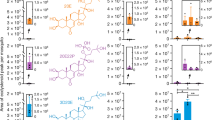
In our analysis of the post-mating response in the LRT, we focused on ten genes shown to be strongly up- or down-regulated after mating in laboratory experiments and thus likely to be involved in the reproductive processes triggered by copulation18,22 (Tables 1, 2; Fig. 2). These included 9 genes whose function in An. gambiae has not been determined yet – i.e. one ABC transporter (AGAP011518), three serine proteases (AGAP005194, AGAP005195, AGAP005196), one amino protease (AGAP000885), two metallopeptidases (AGAP001791 and AGAP009791), a protease inhibitor (AGAP009766), and a putative anti-microbial Andropin-like gene (AGAP009429)45. The last gene was the mating induced stimulator of oogenesis (MISO, AGAP002620), which is induced by the sexual transfer of the steroid hormone 20E and is implicated in the increase in egg development experienced by mated An. gambiae females after blood feeding17.
Gene expression levels after mating in the female lower reproductive tract (LRT). Gene expression levels are shown as Rpl19 normalized values (±Standard Error). (A) An. gambiae female LRT were tested as virgins (cyan dots) or at 1 day and 4 days post mating (blue dots). (B) An. coluzzii female LRT were tested as virgins (light green dots) or at 1 day and 4 days post mating (dark green dots). Red asterisks indicate a significant difference between mated and virgin females: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.001.
In An. gambiae females, changes in gene expression were detected in five proteases (ANOVA analysis; AGAP000885 P = 0.0038, AGAP001791 P = 0.0042; AGAP005195 P = 0.0179; AGAP005196 P = 0.0023; AGAP009791 P = 0.0030), all downregulated at 1 day PM (post-hoc analysis with FDR correction; AGAP000885 P = 0.0092, AGAP001791 P = 0.0096; AGAP005195 P = 0.0242; AGAP005196 P = 0.0056; AGAP009791 P = 0.0064). Moreover, four of these genes showed reduced expression levels also at 4 days PM (post-hoc analysis with FDR correction; AGAP000885 P = 0.0085, AGAP001791 P = 0.0276; AGAP005195 P = 0.0368; AGAP009791 P = 0.004) (Table 1, Fig. 2).
In An. coluzzii females, transcript levels were reduced for three proteases. AGAP001791 was downregulated at 4 days PM (ANOVA P = 0.04; post-hoc analysis with FDR correction P = 0.05), AGAP005194 at 1 day PM (ANOVA P = 0.0042; post-hoc analysis with FDR correction P = 0.0021), and AGAP009791 at both time points analyzed (ANOVA P = 0.0013; post-hoc analysis with FDR correction: 1 PM P = 0.0069; 4 days PM P = 0.0046). Furthermore, MISO was strongly up-regulated at 1 day PM (ANOVA P < 0.0001; post-hoc analysis with FDR correction P < 0.0001), while the ABC transporter AGAP011518 was downregulated at 4 days PM (ANOVA P = 0.0069; post-hoc analysis with FDR correction P = 0.0022) (Table 2, Fig. 2).
With the exception of AGAP009766 and Andropin-like, which were previously shown to be upregulated after mating, results were consistent with those obtained in the laboratory, showing that the transcriptional response to mating is mostly conserved after colonization18,22.
Post-mating transcriptional response of genes related to reproduction, mating behavior and immunity in the carcass of field An. gambiae and An. coluzzii females
We next analyzed the expression of genes in the female carcass, initially focusing on seven factors that may be related to reproductive success or mating behavior (Table 1 and Table 2; Fig. 3). Our analysis included Vitellogenin (Vg, AGAP004203), which encodes a yolk protein that is needed for egg development46 and which in Aedes aegypti mosquitoes is strongly upregulated by 20E synthetized after blood feeding47, and other six genes shown to be differentially expressed in An. gambiae and An. coluzzii virgin females48 that we reasoned might be associated with assortative mating behavior. These included: the sex determining gene doublesex (dsx, AGAP004050), the antennal carrier protein AP-1, (AGAP004799), the odorant binding protein 25 (OBP25, AGAP012320), the cuticular protein CPF3 (AGAP004690), the glutathione S transferases – epsilon class 2 (GST-E2, AGAP009194); and lingerer (AGAP004817). dsx regulates the terminal sexual differentiation in most insects49, and specifically it determines the differentiation of neurons that control male courtship behavior50 and female sexual receptivity51,52,53. In Drosophila, lingerer is involved in the control of male copulatory organs during courtship54. CPF3 is a non-canonical cuticular protein with no chitin-binding capacity, which may be part of the epicuticle55 where it could bind to sex pheromones such as cuticular hydrocarbons (CHCs)48. Odorant binding proteins such as AP-1 and OBP25 transfer odorants to specific receptors56 and might play a role in female mate choice by helping the identification of co-specific males. GST-E2 might instead be involved in the metabolism of chemical stimuli from antennae and other sensory organs57, thus regulating the availability of stimulants such as CHCs.
Gene expression levels after mating in the female carcass. Gene expression levels are shown as Rpl19 normalized values (±Standard Error). (A) An. gambiae female carcasses were tested as virgins (cyan dots) or at 1 day and 4 days post mating (blue dots). (B) An. coluzzii female carcasses were tested as virgins (light green dots) or at 1 day and 4 days post mating (dark green dots). Red asterisks indicate a significant difference between mated and virgin females: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.001.
While no changes were detected in An. coluzzii (Table 2, Fig. 3), in An. gambiae mean gene expression levels were different for five of the seven genes analyzed (dsx ANOVA P = 0.0408; CPF3 ANOVA P = 0.0007; AP-1 ANOVA P = 0.040; OBP25 ANOVA P = 0.0037; Vg ANOVA P < 0.0001). dsx, CPF3 and AP-1 were downregulated at 1 day PM (post-hoc analysis with FDR correction dsx P = 0.014; CPF3 P = 0.0013; AP-1 P = 0.0123) and AP-1 was down-regulated also at 4 days PM (P = 0.0342). OBP25 and Vg were instead upregulated at 1 day PM (post-hoc analysis with FDR correction OBP25 P = 0.0109; Vg P < 0.0001) (Table 2, Fig. 3).
We finally tested whether mating induces a differential immune response in the two species, possibly driven by diverging sexually transmitted pathogens58,59. To this aim, we evaluated the expression levels in the female carcass of five immunity-related genes: the thioester containing protein 1 (TEP1), which is a complement-like factor, homologous to the human C3, that binds and mediates killing of pathogens including Plasmodium parasites60; the leucine-rich immune protein 1 (LRIM1), which circulates in the hemolymph as a disulphide-bounded complex with the leucine-rich protein APL1C and interacts with TEP1 controlling its activity61,62; and the antimicrobial peptides cecropin 1 (CEC1), CEC3, and gambicin (GAMB)63,64. Only TEP1 showed to be upregulated in An. gambiae females at 1 day PM (ANOVA P = 0.0297; post-hoc test with FDR correction P = 0.0044).
Discussion
Our results on the transcriptional response to mating in An. gambiae and An. coluzzii females collected from natural mating swarms largely corroborate previous data obtained under laboratory conditions17,18,22, demonstrating the opportunity of studying complex phenomena such as mating and post-mating behavior in laboratory colonies. This result is remarkable when considering that gene expression is age-dependent65,66,67 and that in our study it was not possible to precisely age-match mated females to virgin ones. For this reason - as well as for the limited number of samples we analyzed due to intrinsic difficulties in collecting couples from natural mating swarms - we observed some variability in our results that probably limited our power to detect subtler, age-dependent changes.
Despite these limitations, some interesting differences were detected in the post-mating responses of the two species samples. Although field An. gambiae and An. coluzzii males and females from the same geographic areas studied here share largely overlapping reproductive microbiomes42, we detected a mating-induced regulation of TEP1, a key immune gene, in the carcass of An. gambiae females. This species-specific upregulation may be due to sexual transfer of microorganisms populating the An. gambiae male reproductive tract, similarly to what observed in D. melanogaster where mating anticipates immune reactions to sexually transmitted pathogens possibly as a mechanism to enhance fecundity68.
Perhaps more interestingly, our data also highlight differential mating-induced changes in genes involved in oogenesis, which may reflect inter-specific differences in the physiological processes leading to egg development. First, we show that MISO – an atrial gene strongly induced by sexual transfer of 20E that regulates the number of eggs developed by females after mating and blood feeding17,19 – was significantly upregulated only in An. coluzzii at 1 day PM, although a trend towards an increase was also observed in An. gambiae at the same time point (Tables 1 and 2, Fig. 2). Given that MISO interacts in the atrium with 20E transferred during mating17, the differential transcriptional dynamics of this gene in the two species suggests that the timing of release of the steroid hormone from the mating plug may be regulated in a species-specific fashion. Second, we reveal that another 20E-induced gene important for oogenesis, Vg, is differentially regulated in the female carcass of the two anophelines. This yolk protein precursor, produced in the fat body and incorporated in the developing eggs via receptor-mediated endocytosis69, was strongly upregulated in An. gambiae at 1 day PM. This difference may reflect a reduced reliance of An. coluzzii females on mating for oogenesis, possibly due to an increased ability to store nutritional reserves during larval development70, and may provide some cues on why females of this species are competent to start egg development as virgins, while An. gambiae females generally need a mating-induced boost to promote the same process44,70,71. Even if the two species have a similar competence for Plasmodium transmission in the laboratory72,73, the fact that An. gambiae females often require multiple blood feedings to complete oogenesis70 may have important implication for malaria transmission in field settings, as it may increase its chances to become infected with Plasmodium parasites earlier in adult life and be associated with higher infection prevalence, as observed in some regions74,75.
We also detected differences in the regulation of the atrial proteolytic machinery which may be involved in the digestion of the mating plug and other seminal secretions. While the protease AGAP009791 was significantly repressed in both species at both time points analyzed, other proteases were downregulated in a time- and species-specific manner. Specifically, AGAP001791 was repressed at both time points in An. gambiae but only at 4 days PM in An. coluzzii; at 1 days PM AGAP000885, AGAP005195 and AGAP005196 were downregulated in An. gambiae, with AGAP000885 and AGAP005195 repressed also at 4 days PM in this species, while AGAP005194 levels were downregulated in An. coluzzii at 1 day PM only. As postulated for MISO, the differential expression of proteolytic enzymes is consistent with the occurrence of species-specific timing of digestion of seminal secretions, which is associated with fertility in Drosophila as well as An. gambiae20,25,26 and, when perturbed, can lead to speciation23,24. Intriguingly, several codons - including those close to the catalytic portion - of the genes encoding the atrial proteases AGAP005194, AGAP005195 and AGAP005196 are evolving under long-term and episodic positive selection in the An. gambiae complex76, supporting the hypothesis that timely and proper mating plug digestion might drive the emergence of post-mating pre-zygotic barriers in species of this complex. Similar to the activation of MISO and Vg, the post-mating downregulation of the proteolytic machinery appears to depend on the sexual transfer of 20E, as all six proteases analyzed here were repressed in the atrium of virgin laboratory females following 20E injection18.
Finally, the expression of four genes encoding for factors possibly involved in mating behavior (dsx, CPF3, AP-1 and OBP25) was regulated by mating in An. gambiae females only. dsx is a key gene in the sexual differentiation cascade, and is produced as sex-specific isoforms77,78 that in Drosophila govern multiple aspects of reproductive biology, including the female receptivity to mating and the development and the activity of neural circuit that regulate sex-specific sexual behavior51,52,53. Furthermore, in the fruit fly dsx controls the expression of genes that synthetize female-specific long-chain cuticular hydrocarbons (CHC), notably the desaturase DESAT-F, that are potent pheromones for male courtship behavior79. It is therefore possible that dsx may affect the synthesis of CHC pheromones also in An. gambiae females, consistent with the observation that An. gambiae and An. coluzzii have, indeed, slightly different CHC profiles that are altered after mating36,38. Interestingly, CPF3, another gene related to CHC, is also downregulated in An. gambiae females. This cuticular protein is likely expressed in the epicuticle, where it is postulated to bind to cuticular pheromones such as CHCs48. Because post-mating changes in the CHCs profiles affect female attractiveness in many monandrous insect species80,81, the An. gambiae-specific downregulation of genes related to chemical contact cues might reflect the occurrence of different post-mating signals in the two species.
Interestingly, both cuticular proteins (CPs) and CHCs have been linked to 20E function, as this ecdysteroid reduces CP expression levels during development82 and is involved in CHC production in adult Drosophila83. Although a link between expression of the genes studied here and male-transferred 20E has yet to be confirmed in field setting, the different post-mating regulation of genes shown in laboratory conditions to be controlled by this steroid hormone17,18,21 supports the hypothesis of divergent male 20E effects in the two species, consistently with the finding of differential 20E levels in the MAGs of An. coluzzii and An. gambiae males in Burkina Faso84. It is intriguing to speculate that the transcriptional differences observed here could represent signatures of a divergent evolutionary arms race between the sexes, which in turn may have led to changes in key reproductive processes and possibly to the development of mechanisms of sexual isolation85,86.
Materials and Methods
Mosquito sample preparation
Anopheles gambiae and An. coluzzii were collected in September 2009 in the Western part of Burkina Faso, i.e. in the village of Soumousso (11°00′46′N, 4°02′45W) and in Vallèe du Kou (11°24′N, 4°24′W), located 55 km east and 30 km north-west of Bobo-Dioulasso, respectively. While in Soumousso larval breeding sites are mostly temporary, rain-dependent puddles more favorable to An. gambiae, the irrigation scheme in Vallèe du Kou largely favors An. coluzzii.
Virgin females were obtained from larvae collected in natural breeding sites (3 in Soumousso and 6 in Vallèe du Kou) and reared to the adult stage in natural climatic conditions and photoperiod using cages placed in the outdoor space available at the IRSS laboratory in Bobo-Dioulasso. In order to ensure females would not mate, pupae were individually transferred in single cups and their sex determined at emergence. Using this method, adult females and males were never in contact with each other.
Mating couples were collected from naturally occurring swarms as previously described33,39,87, allowed to complete copulation, transferred to single cups using mouth aspirators, and brought to the laboratory in sealed containers avoiding shifts in temperature and humidity.
Virgin and mated females were maintained in individual cups and DNA was extracted from single legs removed from live specimens for genotyping88 prior to dissections of reproductive organs. These were carried out under a dissecting stereo-microscope (5x magnification lens) at different time intervals, i.e. 2 and 5 days post emergence in the case of virgin females, and 24 hours and 4 days post-mating in the case of mated ones (to analyze both short-term and long-term response to mating). The time points for virgin females were selected to match as much as possible the age of mated females based on data showing that most females mate on the second night after emergence44. The lower reproductive tract (LRT, comprising atrium and spermathecae) and the rest of the body (carcass) of single females were stored separately in RNAlater solution (Ambion) and pools of five individual tissues/species/time interval were obtained for each time point (Table S1).
RNA extraction and cDNA synthesis
For tissue-specific analysis, total RNA was extracted using TRI Reagent (Helena Biosciences). The amount of RNA for female carcasses was limited to 1 μg. All samples were treated with DNase I (Invitrogen), according to manufacturer’s guidelines. cDNAs were synthesized in 100 μl reactions using 1x First Strand buffer, 5 mM DDT, 0.5 mM dNTPs, 2.5 μM random hexamers, 40 units RNaseOut recombinant ribonuclease inhibitor, and 125 units of M-MLV reverse transcriptase (all reagents from Invitrogen).
Quantitative Reverse Transcription PCR with SYBR green detection
Samples were run in 15 μl reaction volume using 1x Fast SYBR Green Master Mix (Applied Biosystems). Gene expression was quantified in duplicates on a StepOnePlus Real-Time thermocycler (Applied Biosystems) using the following program: 95 °C for 15 min, then 40 cycles (95 °C for 15 sec, 60 °C for 60 sec) followed by a dissociation curve analysis. Primers used for qRT-PCR are listed in Table S2. Three technical replicates were used for each biological replicate for each gene. A standard curve against serial dilutions of cDNA templates (mated and virgin) was used for each gene to determine the linear range of the assay.
Statistical analysis
Gene expression levels were normalized using deltaCt method against the ribosomal gene RpL19 (AGAP004422), which is expressed at high levels and does not respond to mating18,22. To test for mating-induced changes in gene expression, the two species were studied separately. As we do not know if the primers anneal with the same efficiency or if the reference gene is expressed at the same levels in the two species, we did not perform cross-species comparisons. An ANOVA was first used to test whether each gene showed significant changes in the two time points analyzed, including in the analysis both mated and virgin samples. If the global F test gave positive results, pairwise posthoc contrast tests (Tukey-Kramer procedure) have been used to determine differences between mated and virgin females at each time point analyzed. To control for possible Type I error arising through use of multiple ANOVA tests, the P values were corrected by applying a False Discovery Rate procedure (FDR).
References
World Malaria Report. World Malaria Report 2016, http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1 (2017).
Fontaine, M. C. et al. Mosquito genomics. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science 347, 1258524, https://doi.org/10.1126/science.1258524 (2015).
Kamali, M., Xia, A., Tu, Z. & Sharakhov, I. V. A new chromosomal phylogeny supports the repeated origin of vectorial capacity in malaria mosquitoes of the Anopheles gambiae complex. PLoS Pathog 8, e1002960, https://doi.org/10.1371/journal.ppat.1002960 (2012).
della Torre, A., Tu, Z. & Petrarca, V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem Mol Biol 35, 755–769, https://doi.org/10.1016/j.ibmb.2005.02.006 (2005).
Pombi, M. et al. Dissecting functional components of reproductive isolation among closely related sympatric species of the Anopheles gambiae complex. Evol Appl 10, 1102–1120, https://doi.org/10.1111/eva.12517 (2017).
Costantini, C. et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol 9, 16, https://doi.org/10.1186/1472-6785-9-16 (2009).
Gimonneau, G. et al. Larval habitat segregation between the molecular forms of the mosquito Anopheles gambiae in a rice field area of Burkina Faso, West Africa. Med Vet Entomol 26, 9–17, https://doi.org/10.1111/j.1365-2915.2011.00957.x (2012).
Kamdem, C. et al. Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS One 7, e39453, https://doi.org/10.1371/journal.pone.0039453 (2012).
Simard, F. et al. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol 9, 17, https://doi.org/10.1186/1472-6785-9-17 (2009).
Childs, L. M. et al. Disrupting Mosquito Reproduction and Parasite Development for Malaria Control. PLoS Pathog 12, e1006060, https://doi.org/10.1371/journal.ppat.1006060 (2016).
Bai, H., Gelman, D. B. & Palli, S. R. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag Sci 66, 936–943, https://doi.org/10.1002/ps.1962 (2010).
Galizi, R. et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun 5, 3977, https://doi.org/10.1038/ncomms4977 (2014).
Raikhel, A. S., Brown, M. & Belles, X. In Comprehensive Molecular Insect Science Vol. 3 (eds L. Gilbert, S. Gill, & K. Iatrou) 433–491 (Elsevier, 2005).
Yamanaka, N., Rewitz, K. F. & O’Connor, M. B. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 58, 497–516, https://doi.org/10.1146/annurev-ento-120811-153608 (2013).
Pondeville, E., Maria, A., Jacques, J. C., Bourgouin, C. & Dauphin-Villemant, C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci USA 105, 19631–19636, https://doi.org/10.1073/pnas.0809264105 (2008).
Baldini, F., Gabrieli, P., Rogers, D. W. & Catteruccia, F. Function and composition of male accessory gland secretions in Anopheles gambiae: a comparison with other insect vectors of infectious diseases. Pathog Glob Health 106, 82–93, https://doi.org/10.1179/2047773212y.0000000016 (2012).
Baldini, F. et al. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol 11, e1001695, https://doi.org/10.1371/journal.pbio.1001695 (2013).
Gabrieli, P. et al. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc Natl Acad Sci USA 111, 16353–16358, https://doi.org/10.1073/pnas.1410488111 (2014).
Mitchell, S. N. et al. Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347, 985–988, https://doi.org/10.1126/science.1259435 (2015).
Rogers, D. W. et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol 7, e1000272, https://doi.org/10.1371/journal.pbio.1000272 (2009).
Shaw, W. R. et al. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc Natl Acad Sci USA 111, 5854–5859, https://doi.org/10.1073/pnas.1401715111 (2014).
Rogers, D. W. et al. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA 105, 19390–19395, https://doi.org/10.1073/pnas.0809723105 (2008).
Bono, J. M., Matzkin, L. M., Kelleher, E. S. & Markow, T. A. Postmating transcriptional changes in reproductive tracts of con- and heterospecifically mated Drosophila mojavensis females. Proc Natl Acad Sci USA 108, 7878–7883, https://doi.org/10.1073/pnas.1100388108 (2011).
Bono, J. M. et al. Molecular evolution of candidate genes involved in post‐mating‐prezygotic reproductive isolation. J Evol Biol 28, 403–414, https://doi.org/10.1111/jeb.12574 (2015).
Kelleher, E. S. & Markow, T. A. Reproductive tract interactions contribute to isolation in Drosophila. Fly (Austin) 1, 33–37, https://doi.org/10.4161/fly.3840 (2007).
Knowles, L. L. & Markow, T. A. Sexually antagonistic coevolution of a postmating-prezygotic reproductive character in desert Drosophila. Proc Natl Acad Sci USA 98, 8692–8696, https://doi.org/10.1073/pnas.151123998 (2001).
Leder, E. H. et al. The evolution and adaptive potential of transcriptional variation in sticklebacks–signatures of selection and widespread heritability. Mol Biol Evol 32, 674–689, https://doi.org/10.1093/molbev/msu328 (2015).
Whitehead, A. & Crawford, D. L. Neutral and adaptive variation in gene expression. Proc Natl Acad Sci USA 103, 5425–5430, https://doi.org/10.1073/pnas.0507648103 (2006).
Pavey, S. A., Collin, H., Nosil, P. & Rogers, S. M. The role of gene expression in ecological speciation. Ann N Y Acad Sci 1206, 110–129, https://doi.org/10.1111/j.1749-6632.2010.05765.x (2010).
Arnqvist, G., Edvardsson, M., Friberg, U. & Nilsson, T. Sexual conflict promotes speciation in insects. Proc Natl Acad Sci USA 97, 10460–10464, https://doi.org/10.1073/pnas.97.19.10460 (2000).
Diabate, A., Dabire, R. K., Millogo, N. & Lehmann, T. Evaluating the effect of postmating isolation between molecular forms of Anopheles gambiae (Diptera: Culicidae). J Med Entomol 44, 60–64, https://doi.org/10.1603/0022-2585(2007)44[60:ETEOPI]2.0.CO;2 (2007).
Lee, Y. et al. Spatiotemporal dynamics of gene flow and hybrid fitness between the M and S forms of the malaria mosquito, Anopheles gambiae. Proc Natl Acad Sci USA 110, 19854–19859, https://doi.org/10.1073/pnas.1316851110 (2013).
Sawadogo, S. P. et al. Differences in timing of mating swarms in sympatric populations of Anopheles coluzzii and Anopheles gambiae s.s. (formerly An. gambiae M and S molecular forms) in Burkina Faso, West Africa. Parasit Vectors 6, 275, https://doi.org/10.1186/1756-3305-6-275 (2013).
Diabate, A. et al. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. Proc Biol Sci 276, 4215–4222, https://doi.org/10.1098/rspb.2009.1167 (2009).
Anyanwu, G. I., Molyneux, D. H. & Phillips, A. Variation in cuticular hydrocarbons among strains of the Anopheles gambiae sensu stricto by analysis of cuticular hydrocarbons using gas liquid chromatography of larvae. Mem Inst Oswaldo Cruz 95, 295–300 (2000).
Caputo, B. et al. Comparative analysis of epicuticular lipid profiles of sympatric and allopatric field populations of Anopheles gambiae s.s. molecular forms and An. arabiensis from Burkina Faso (West Africa). Insect Biochem Mol Biol 37, 389–398, https://doi.org/10.1016/j.ibmb.2007.01.002 (2007).
Chung, H. & Carroll, S. B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 37, 822–830, https://doi.org/10.1002/bies.201500014 (2015).
Polerstock, A. R., Eigenbrode, S. D. & Klowden, M. J. Mating Alters the Cuticular Hydrocarbons of Female Anopheles gambiae sensu stricto and Aedes aegypti (Diptera: Culicidae). J Med Entomol 39, 545–552, https://doi.org/10.1603/0022-2585-39.3.545 (2002).
Diabate, A. et al. Mixed swarms of the molecular M and S forms of Anopheles gambiae (Diptera: Culicidae) in sympatric area from Burkina Faso. J Med Entomol 43, 480–483, https://doi.org/10.1603/0022-2585(2006)43[480:MSOTMM]2.0.CO;2 (2006).
Pennetier, C., Warren, B., Dabire, K. R., Russell, I. J. & Gibson, G. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr Biol 20, 131–136, https://doi.org/10.1016/j.cub.2009.11.040 (2010).
Simoes, P. M., Gibson, G. & Russell, I. J. Pre-copula acoustic behaviour of males in the malarial mosquitoes Anopheles coluzzii and Anopheles gambiae s.s. does not contribute to reproductive isolation. J Exp Biol 220, 379–385, https://doi.org/10.1242/jeb.149757 (2017).
Segata, N. et al. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers. Sci Rep 6, 24207, https://doi.org/10.1038/srep24207 (2016).
DABIRE, K. R. et al. Assortative mating in mixed swarms of the mosquito Anopheles gambiae s.s. M and S molecular forms, in Burkina Faso, West Africa. Med Vet Entomol 27, 298–312, https://doi.org/10.1111/j.1365-2915.2012.01049.x (2013).
Charlwood, J. D. et al. ‘A mate or a meal’–pre-gravid behaviour of female Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Malar J 2, 9, https://doi.org/10.1186/1475-2875-2-9 (2003).
Dottorini, T. et al. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA 104, 16215–16220, https://doi.org/10.1073/pnas.0703904104 (2007).
Sappington, T. W. & Raikhel, A. S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol 28, 277–300, https://doi.org/10.1016/S0965-1748(97)00110-0 (1998).
Deitsch, K. W., Chen, J. S. & Raikhel, A. S. Indirect control of yolk protein genes by 20-hydroxyecdysone in the fat body of the mosquito, Aedes aegypti. Insect Biochem Mol Biol 25, 449–454, https://doi.org/10.1016/0965-1748(94)00082-A (1995).
Cassone, B. J. et al. Differential gene expression in incipient species of Anopheles gambiae. Mol Ecol 17, 2491–2504, https://doi.org/10.1111/j.1365-294X.2008.03774.x (2008).
Garrett-Engele, C. M. et al. intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development 129, 4661–4675 (2002).
Dauwalder, B. The roles of fruitless and doublesex in the control of male courtship. Int Rev Neurobiol 99, 87–105, https://doi.org/10.1016/b978-0-12-387003-2.00004-5 (2011).
Rezaval, C. et al. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol 22, 1155–1165, https://doi.org/10.1016/j.cub.2012.04.062 (2012).
Zhou, C., Pan, Y., Robinett, C. C., Meissner, G. W. & Baker, B. S. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163, https://doi.org/10.1016/j.neuron.2014.05.038 (2014).
Jang, Y. H., Chae, H. S. & Kim, Y. J. Female-specific myoinhibitory peptide neurons regulate mating receptivity in Drosophila melanogaster. Nat Commun 8, 1630, https://doi.org/10.1038/s41467-017-01794-9 (2017).
Kuniyoshi, H. et al. lingerer, a Drosophila gene involved in initiation and termination of copulation, encodes a set of novel cytoplasmic proteins. Genetics 162, 1775–1789 (2002).
Togawa, T., Augustine Dunn, W., Emmons, A. C. & Willis, J. H. CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem Mol Biol 37, 675–688, https://doi.org/10.1016/j.ibmb.2007.03.011 (2007).
Hekmat-Scafe, D. S., Scafe, C. R., McKinney, A. J. & Tanouye, M. A. Genome-Wide Analysis of the Odorant-Binding Protein Gene Family in Drosophila melanogaster. Genome Res 12, 1357–1369, https://doi.org/10.1101/gr.239402 (2002).
He, P. et al. A reference gene set for sex pheromone biosynthesis and degradation genes from the diamondback moth, Plutella xylostella, based on genome and transcriptome digital gene expression analyses. BMC Genomics 18, 219, https://doi.org/10.1186/s12864-017-3592-y (2017).
Miest, T. S. & Bloch-Qazi, M. Sick of mating: sexual transmission of a pathogenic bacterium in Drosophila melanogaster. Fly (Austin) 2, 215–219, https://doi.org/10.4161/fly.6726 (2008).
Knell, R. J. & Webberley, K. M. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol Rev Camb Philos Soc 79, 557–581, https://doi.org/10.1017/S1464793103006365 (2004).
Blandin, S. et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661–670, https://doi.org/10.1016/S0092-8674(04)00173-4 (2004).
Fraiture, M. et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5, 273–284, https://doi.org/10.1016/j.chom.2009.01.005 (2009).
Povelones, M., Upton, L. M., Sala, K. A. & Christophides, G. K. Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog 7, e1002023, https://doi.org/10.1371/journal.ppat.1002023 (2011).
Zheng, X. L. & Zheng, A. L. Genomic organization and regulation of three cecropin genes in Anopheles gambiae. Insect Mol Biol 11, 517–525, https://doi.org/10.1046/j.1365-2583.2002.00360.x (2002).
Vizioli, J. et al. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA 98, 12630–12635, https://doi.org/10.1073/pnas.221466798 (2001).
Carlson, K. A. et al. Genome-Wide Gene Expression in relation to Age in Large Laboratory Cohorts of Drosophila melanogaster. Genet Res Int 2015, 835624, https://doi.org/10.1155/2015/835624 (2015).
Wang, M. H. et al. Gene expression-based biomarkers for Anopheles gambiae age grading. PLoS One 8, e69439, https://doi.org/10.1371/journal.pone.0069439 (2013).
Cook, P. E. & Sinkins, S. P. Transcriptional profiling of Anopheles gambiae mosquitoes for adult age estimation. Insect Mol Biol 19, 745–751, https://doi.org/10.1111/j.1365-2583.2010.01034.x (2010).
Zhong, W. et al. Immune anticipation of mating in Drosophila: Turandot M promotes immunity against sexually transmitted fungal infections. Proc Biol Sci 280, 20132018, https://doi.org/10.1098/rspb.2013.2018 (2013).
Snigirevskaya, E. S., Hays, A. R. & Raikhel, A. S. Secretory and internalization pathways of mosquito yolk protein precursors. Cell Tissue Res 290, 129–142, https://doi.org/10.1007/s004410050915 (1997).
Mouline, K. et al. Physiology and development of the M and S molecular forms of Anopheles gambiae in Burkina Faso (West Africa). Med Vet Entomol 26, 447–454, https://doi.org/10.1111/j.1365-2915.2012.01018.x (2012).
Gillies, M. T. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Ann Trop Med Parasitol 48, 58–74, https://doi.org/10.1080/00034983.1954.11685599 (1954).
Ndiath, M. O. et al. Dynamics of transmission of Plasmodium falciparum by Anopheles arabiensis and the molecular forms M and S of Anopheles gambiae in Dielmo, Senegal. Malar J 7, 136, https://doi.org/10.1186/1475-2875-7-136 (2008).
Gneme, A. et al. Equivalent susceptibility of Anopheles gambiae M and S molecular forms and Anopheles arabiensis to Plasmodium falciparum infection in Burkina Faso. Malar J 12, 204, https://doi.org/10.1186/1475-2875-12-204 (2013).
Sanford, M. R. et al. Plasmodium falciparum infection rates for some Anopheles spp. from Guinea-Bissau, West Africa. F1000Research 3, 243, https://doi.org/10.12688/f1000research.5485.2 (2014).
Carnevale, P. et al. Diversity of malaria in rice growing areas of the Afrotropical region. Parassitologia 41, 273–276 (1999).
Mancini, E. et al. Molecular evolution of a gene cluster of serine proteases expressed in the Anopheles gambiae female reproductive tract. BMC Evol Biol 11, 72, https://doi.org/10.1186/1471-2148-11-72 (2011).
Scali, C., Catteruccia, F., Li, Q. & Crisanti, A. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. J Exp Biol 208, 3701–3709, https://doi.org/10.1242/jeb.01819 (2005).
Bopp, D., Saccone, G. & Beye, M. Sex determination in insects: variations on a common theme. Sex Dev 8, 20–28, https://doi.org/10.1159/000356458 (2014).
Shirangi, T. R., Dufour, H. D., Williams, T. M. & Carroll, S. B. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 7, e1000168, https://doi.org/10.1371/journal.pbio.1000168 (2009).
Oppelt, A. & Heinze, J. Mating is associated with immediate changes of the hydrocarbon profile of Leptothorax gredleri ant queens. J Insect Physiol 55, 624–628, https://doi.org/10.1016/j.jinsphys.2009.03.010 (2009).
Polidori, C. et al. Post-mating shift towards longer-chain cuticular hydrocarbons drastically reduces female attractiveness to males in a digger wasp. J Insect Physiol 100, 119–127, https://doi.org/10.1016/j.jinsphys.2017.05.001 (2017).
Charles, J. P. The regulation of expression of insect cuticle protein genes. Insect Biochem Mol Biol 40, 205–213, https://doi.org/10.1016/j.ibmb.2009.12.005 (2010).
Chiang, Y. N. et al. Steroid Hormone Signaling Is Essential for Pheromone Production and Oenocyte Survival. PLoS Genet 12, e1006126, https://doi.org/10.1371/journal.pgen.1006126 (2016).
Mamai, W. et al. Metabolomic and ecdysteroid variations in Anopheles gambiae s.l. mosquitoes exposed to the stressful conditions of the dry season in Burkina Faso, West Africa. Physiol Biochem Zool 87, 486–497, https://doi.org/10.1086/675697 (2014).
Fang, S., Takahashi, A. & Wu, C. I. A mutation in the promoter of desaturase 2 is correlated with sexual isolation between Drosophila behavioral races. Genetics 162, 781–784 (2002).
Greenberg, A. J., Moran, J. R., Fang, S. & Wu, C. I. Adaptive loss of an old duplicated gene during incipient speciation. Mol Biol Evol 23, 401–410, https://doi.org/10.1093/molbev/msj045 (2006).
Baldini, F. et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun 5, 3985, https://doi.org/10.1038/ncomms4985 (2014).
Santolamazza, F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J 7, 163, https://doi.org/10.1186/1475-2875-7-163 (2008).
Acknowledgements
The authors wish to thank members of the Catteruccia laboratory, particularly W. Robert Shaw and Perrine Marcenac, for their critical reading of the manuscript, and Manuela Bernardi for graphical help. The work was supported by the EC FP7 Collaborative Project 223601 ‘Malvecblok’ to FC and AdT, and FC has been sponsored on research related to this topic by the European Research Council FP7 ERC Starting Grant project ‘Anorep’ (ID: 260897). This publication is also partially supported by the National Institutes of Health (NIH) (award number: R01 AI104956) and a Bill & Melinda Gates Foundation (BMGF) and the Howard Hughes Medical Institute (HHMI) grant (ID: OPP1158190) to FC. The findings and conclusions within are those of the authors and do not necessarily reflect positions or policies of the NIH, BMGF, or HHMI.
Author information
Authors and Affiliations
Contributions
J.T., B.C., A.D., R.D., A.d.T. and F.C. designed the experiments. J.T., B.C. and P.B. performed the experiments. J.T., P.G. and A.S. analyzed the data. F.C. and P.G. wrote the manuscript. J.T. and P.G. contributed equally to this study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thailayil, J., Gabrieli, P., Caputo, B. et al. Analysis of natural female post-mating responses of Anopheles gambiae and Anopheles coluzzii unravels similarities and differences in their reproductive ecology. Sci Rep 8, 6594 (2018). https://doi.org/10.1038/s41598-018-24923-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24923-w
This article is cited by
-
Genome-wide identification and expression analysis of the mating-responsive genes in the male accessory glands of Spodoptera litura (Lepidoptera: Noctuidae)
Journal of Genetic Engineering and Biotechnology (2023)
-
Gene expression and alternative splicing dynamics are perturbed in female head transcriptomes following heterospecific copulation
BMC Genomics (2021)
-
20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors
Parasites & Vectors (2021)
-
Mating-regulated atrial proteases control reinsemination rates in Anopheles gambiae females
Scientific Reports (2020)
-
Evolution of sexually-transferred steroids and mating-induced phenotypes in Anopheles mosquitoes
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.