Abstract
The aim of the study is to evaluate the efficacy of postoperative treatments based on pathological response for cervical cancer patients who received neoadjuvant chemotherapy (NACT) followed by radical surgery. Firstly, a total of 756 cervical squamous cell cancer (SCC) patients with FIGO IB2-IIB were included in this retrospective study. Then data from a prospective cohort of 393 patients was employed for further validation. Overall survival (OS) and disease-free survival (DFS) were assessed. In the retrospective study, SCC patients who accepted adjuvant chemotherapy after radical surgery had a relatively better OS than those who received no therapy (P = 0.08, HR = 0.57). The result was more noticeable in the prospective cohort study (P = 0.006, HR = 0.28). In the combined analysis, adjuvant chemotherapy improved clinical outcomes compared with no therapy (P = 0.002 and 0.04 for OS and DFS). Particularly for patients with extra-cervical residual disease, adjuvant chemotherapy improved OS (log-rank P = 0.008, 0.004 and 0.001 in the retrospective, prospective and combined studies). Optimal response patients had good outcomes even without therapy. Our study indicates that adjuvant chemotherapy can benefit clinical outcomes for SCC patients with NACT followed by radical surgery, especially those with extra-cervical residual disease. For optimal response patients, there may be no need for further treatment. This finding needs to be validated in more future studies.
Similar content being viewed by others
Introduction
Cervical cancer is the third most frequent female malignancy worldwide. Approximately 500,000 new cases of cervical cancer occur each year, with 80% of these being in developing countries1. In recent years, NACT followed by radical surgery has emerged as a new option for treating cervical cancer2,3,4. In China, NACT followed by radical surgery was used for several years in patients with FIGO stage IB2-IIB cervical cancer3.
To improve long-term survival and decrease recurrence, researchers have evaluated postoperative treatment after radical surgery for cervical cancer patients5,6,7,8,9,10. Conversely, few data are available on adjuvant postoperative therapy in patients treated with NACT and radical hysterectomy. Angioli R et al.11 evaluated the efficacy of adjuvant chemotherapy for cervical cancer patients with NACT followed by radical surgery. All enrolled patients received 3 cycles of platinum-based chemotherapy every 3 weeks. They concluded that the adjuvant chemotherapy regimen after neoadjuvant chemotherapy and radical surgery represented a valid treatment for patients with locally advanced cervical cancer. Huali Wang et al.12 found that adjuvant radiotherapy and chemotherapy effectively decreased the recurrence rate for patients who accepted NACT followed by radical surgery. Maki Matsumura et al.12 found that NACT followed by surgery with postoperative chemotherapy but without radiotherapy offered a viable option for treating stage IB2-IIB cervical cancer.
Several studies indicated that the pathological response seen in surgical specimens strongly predicts survival for patients with NACT followed by radical hysterectomy13,14,15. However, few data exist on the options and efficacy of postoperative treatments based on the pathological response of the surgical specimens. To date, we have only found one study on this topic. Landoni F. et al.14 indicated that optimal responders for FIGO stage IB2-IIB cervical cancer after chemo-surgical treatment do not need further treatment, and adjuvant chemotherapy after surgery could benefit patients with suboptimal response and intra-cervical residual disease. However, adjuvant treatments did not appear to benefit the clinical outcomes of patients with extra-cervical residual disease compared with those who received no further treatment. However, no related data exist on postoperative treatment based on the pathological response for SCC patients in China. Thus, we designed this study to investigate the effectively of pathological response and postoperative treatments of IB2-IIB SCC patients who received NACT followed by radical surgery.
Results
Patient characteristics
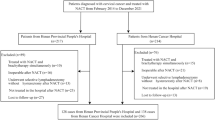
In the retrospective analysis, we included 756 SCC patients with stage IB2-IIB who received neoadjuvant chemotherapy followed by radical hysterectomy (Table 1). The median age was 44 years (range 39–50). Of the 756 patients, 58 experienced optimal response. Intra-cervical and extra-cervical residual disease occurred in 363 and 163 patients, respectively, and 172 had no response. Post-surgery, 307, 299 and 144 patients received no further treatment, adjuvant chemotherapy and adjuvant radiotherapy (including concurrent chemo-radiotherapy), respectively.
Among the total 567 cervical cancer patients in the prospective cohort, 393 SCC patients were included. All underwent neo-adjuvant platinum-based chemotherapy and radical surgery (Supplementary Figure 1). The mean age was 45 years (40–49). Of the 393 patients, 83, 257 and 52 patients received no further treatment, adjuvant chemotherapy and adjuvant radiotherapy, respectively. The details are shown in Table 1.
Cox analysis for post-surgery treatments and survival
Based on univariate Cox regression analysis in the retrospective study, patients who accepted adjuvant chemotherapy had a relatively better OS than those who did not receive therapy (P = 0.08, 0.57 [0.31, 1.08]). This result was more noticeable in the prospective study (P = 0.006, 0.28 [0.12, 0.69]). In addition, patients with chemotherapy after surgery had a lower recurrence rate than those without therapy in the prospective study (P = 0.04, 0.44 [0.2, 0.95]). Multivariate Cox regression analysis revealed that patients had better survival rates with adjuvant chemotherapy after surgery than those without therapy, particularly OS, in the prospective study (P = 0.02, 0.39 [0.17, 0.86] and P = 0.002, 0.22 [0.088, 0.57] for DFS and OS). Combined analysis revealed that adjuvant chemotherapy played a role in OS and DFS compared with no therapy (P = 0.04, 0.002 in the univariate Cox regression, and P = 0.02 and 0.001 in the multivariate Cox regression analyses for DFS and OS) (Supplementary Tables).
We also investigated the postoperative treatment effect on survival at different levels of pathological response in surgical specimens using the Kaplan-Meier method. In the retrospective study, optimal response patients did not differ significantly in OS and DFS among adjuvant chemotherapy, radiotherapy and no therapy (log-rank P = 1.00, 1.00 for DFS and OS). For optimal response patients, even without therapy, the DFS and OS rates both reached over 90% (Fig. 1). For patients with intra-cervical residual disease patients from the retrospective study, the prospective cohort and the combination of the two studies, there was no statistical difference in outcomes among the three treatments (Fig. 2). For extra-cervical residual disease patients, survival differed among the three postoperative treatments (log-rank P = 0.03 and 0.008 for DFS and OS) (Fig. 3). These patients had the best clinical outcomes after receiving adjuvant chemotherapy, and these results were validated in the prospective study. The corresponding log-rank P-values were 0.90 and 0.92 for optimal response patients and 0.007 and 0.004 for extra-cervical residual disease patients for DFS and OS, respectively, in the prospective study. For patients with no response patients from the retrospective study, the prospective cohort and the combination of the two studies, there was no statistical difference in outcomes among the three treatments, too (Fig. 4).
Kaplan-Meier survival estimates for optimal-response patients with cervical cancer from the retrospective study, the prospective cohort and the two studies combined. Disease-free survival (DFS) curves of evaluated patients in the retrospective study (A), the prospective cohort (B) and the combined results (C). Overall survival (OS) curves of evaluated patients in the retrospective study (D), the prospective cohort (E) and the combined results (F).
Kaplan-Meier survival estimates for patients with intra-cervical residual disease from the retrospective study, the prospective cohort and the two studies combined. Disease-free survival (DFS) curves of evaluated patients in the retrospective study (A), the prospective cohort (B) and the combined results (C). Overall survival (OS) curves of evaluated patients in the retrospective study (D), the prospective cohort (E) and the combined results (F).
Kaplan-Meier survival estimates for patients with extra-cervical residual disease from the retrospective study, the prospective cohort and the two studies combined. Disease-free survival (DFS) curves of evaluated patients in the retrospective study (A), the prospective cohort (B) and the combined results (C). Overall survival (OS) curves of evaluated patients in the retrospective study (D), the prospective cohort (E) and the combined results (F).
Kaplan-Meier survival estimates for patients with no response from the retrospective study, the prospective cohort and the two studies combined. Disease-free survival (DFS) curves of evaluated patients in the retrospective study (A), the prospective cohort (B) and the combined results (C). Overall survival (OS) curves of evaluated patients in the retrospective study (D), the prospective cohort (E) and the combined results (F).
We used joint analysis of the retrospective study and prospective cohort to analyze the postoperative treatment effect on survival. The results from the retrospective and prospective studies were combined. Univariate Cox analysis revealed the HRs for adjuvant chemotherapy and no therapy were 0.62 (95% CI, 0.39, 0.98, I2 = 10.8%) and 0.45 (95% CI, 0.27, 0.75, I2 = 41.5%) for DFS and OS, respectively. Multivariate Cox analysis revealed the HRs to be 0.58 (95% CI, 0.35, 0.94, I2 = 29%) and 0.44 (95% CI, 0.25, 0.75, I2 = 68.1%) for DFS (Fig. 5) and OS, respectively (Fig. 6).
Univariate and multivariate Cox regression for DFS and OS revealed that lymph node metastasis was the most important risk factor for survival. In the combined univariate analysis, the P values reached 0.000001 (HR = 3.26) and 0.00001 (HR = 3.42) for DFS and OS, and in the combined multivariate analysis, the P values were 0.002 and 0.00001 for DFS and OS, respectively (Supplementary Tables).
Discussion
The treatment approach for FIGO stage IB2-IIB cervical cancer after surgery remains controversial. Adjuvant radiotherapy (including CCRT) is used in many countries, as recommended by the National Comprehensive Cancer Network (NCCN) clinical practice guidelines for cervical cancer with pathological risk factors following radical hysterectomy16. Gynecologic Oncology Group study 92 also found that radiotherapy after radical surgery significantly increased progression-free survival in patients with IB cervical cancer with negative and inter-medium-risk factors17. Postoperative radiotherapy with or without CCRT is recommended for patients with intermediate- or high-risk factors. Nobuhiro Takeshima et al. investigated the efficacy of chemotherapy alone as a postoperative adjuvant therapy for intermediate- and high-risk cervical cancer and found that adjuvant chemotherapy alone may help to treat patients with cervical cancer10. However, other studies found no statistical significance among adjuvant chemotherapy, radiotherapy and observation. Manfred Lahousen et al. concluded that adjuvant chemotherapy and radiation did not improve survival or recurrence rates in high-risk cervical cancer patients after radical hysterectomy, and the most important treatment for these patients appeared to be radical abdominal hysterectomy with systematic pelvic lymphadenectomy18.
NACT followed by radical hysterectomy has recently become an encouraging alternative therapeutic choice for patients with stage IB2-IIB cervical cancer. Some authors evaluated the possibility of adding adjuvant therapy for cervical cancer after NACT followed by radical surgery. Other studies concluded that adjuvant therapy yields promising results with a 5-year OS ranging from 72 to 83% with reduced extra-pelvic recurrences from adding adjuvant chemotherapy after radical surgery19. Huali Wang et al. studied adjuvant treatment for patients who accepted NACT followed by radical surgery and found that 179 of 256 patients received postoperative radiotherapy with a recurrence rate of 4.47%, which differed significantly from the 15.58% recurrence rate in those without radiotherapy (P = 0.000). Among 246 patients who needed further postoperative chemotherapy, 182 who received postoperative chemotherapy had a recurrence rate of 2.747%, while 64 patients who did not receive the chemotherapy had a recurrence rate of 10.93% (P = 0.007). These authors concluded that postoperative radiotherapy and chemo-adjuvant therapy both decreased the recurrence rate20. F. Landoni concluded that additional chemotherapy cycles benefitted patients with suboptimal response and intra-cervical residual disease, and both adjuvant chemotherapy and adjuvant radiation treatment did not improve the clinical outcome for patients with extra-cervical residual disease compared to those who received no further treatment14.
In our retrospective study, adjuvant chemotherapy supported OS in SCC patients compared with no therapy, which was also validated in a prospective cohort in which the advantage was more noticeable (P = 0.006, HR = 0.28) with a reduced recurrence rate (P = 0.04). Adjuvant radiotherapy did not significantly differ from no therapy for SCC patients in either study. In the combined univariate analysis, the HRs for adjuvant radiotherapy and no therapy were 0.91 (95% CI, 0.28, 0.89, I2 = 79.7%) and 1.21 (95% CI, 0.73, 2.00, I2 = 0.0%) for DFS and OS, respectively. In the Cox multivariate analysis, the HRs were 0.77 (95% CI, 0.46, 1.30, I2 = 0.0%) and 0.64 (95% CI, 0.37, 1.12, I2 = 0.0%), respectively, for DFS (Supplementary Figure 2) and OS (Supplementary Figure 3). For optimal response patients, no matter any treatments can obtain a very good clinical outcome. That is because that they mostly had no risk factors which we investigated in our previous study21. Intra-cervical residual disease patients had relatively worse survival and showed no differences among the three treatments (log-rank P = 0.82, 0.42, 0.69 for the DFS rate and 0.82, 0.59, 0.59 for the OS rate in the retrospective study, prospective cohort and combined analysis, respectively) (Fig. 2). Adjuvant chemotherapy showed a clear advantage on survival in extra-cervical residual disease patients compared to those without therapy. In addition, the clinical outcomes of the no-response patients were the worst. Adjuvant chemotherapy and adjuvant radiotherapy did not improve the DFS and OS rate compared with no therapy (log-rank P = 0.77, 0.24, 0.76 for the DFS rate and 0.98, 0.07, 0.65 for the OS rate in the retrospective study, prospective cohort and combined analysis, respectively) (Fig. 3).
Lymph node metastasis is the most important risk factor for cervical cancer patients8,18,22,23. Although it is not included in the current 2009 FIGO staging system, lymph node status is crucial for prognostically characterizing and managing cervical carcinoma. Kim et al. reported an association between the number of metastatic lymph nodes and the survival of patients treated with radical surgery compared to patients treated with NACT plus radical surgery8. In our study, lymph node metastasis was the most important independent risk factor for survival.
A strength of our study is that we performed the research with retrospective and prospective cohort studies with sufficiently large sample sizes.
In conclusion, we evaluated the correlation between clinical outcomes and postoperative treatments based on the pathological response for IB2-IIB2 cervical cancer patients with NACT followed by radical surgery. We found that patients who accepted adjuvant chemotherapy after surgery experienced beneficial clinical outcomes, especially those with extra-cervical residual disease. In addition, optimal response patients may not need further treatment.
Materials and Methods
Patients
This study included a retrospective cohort and a prospective cohort. The retrospective study data dated from 1999 to 2008. The prospective cohort began in 2002 until the present. The prospective cohort was performed at the Departments of Obstetrics and Gynecology at our institutions, and the registration number from Clinicaltrials.gov is NCT01628757. The inclusion criteria for the study included squamous cell carcinoma, age ≥18 and <70 years, accepted NACT followed by radical surgery and FIGO stage IB2-IIB. The study was conducted in accordance with approved guidelines. The clinical investigation followed the Declaration of Helsinki. All experimental protocols were approved by the Ethics Committee of Tongji Medical College at Huazhong University of Science and Technology. All eligible patients provided written informed consent before entering this study.
Neoadjuvant chemotherapy
NACT used in our study was a cisplatin-based regimen, typically administered in 1–2 courses, depending on patient tolerance and response, and a few patients received an additional 1–2 cycles. The maximum duration was less than 2 months to avoid a delay in curative treatment.
Pathological response
The pathological responses were retrospectively assessed as follows: PCR was defined as the complete disappearance of the tumor in the cervix and negative nodes; PR1 (partial response one) is defined as residual disease with less than 3 mm stromal invasion, including in situ carcinoma with or without lymphatic metastasis; and PR2 (partial response two) is defined a persistent residual disease with more than 3 mm stromal invasion in the surgical specimen. PR2 was divided into two parts: intra-cervical residual disease (persistent residual disease with >3 mm stromal invasion on the surgical specimen and negative nodes) and extra-cervical residual disease (persistent residual disease with >3 mm stromal invasion on the surgical specimen and positive nodes or positive parametria and/or surgical margins with negative nodes). We chose the 3-mm threshold as the lowest limit of an OPR (PCR + PR1) because it represents the maximal extension of FIGO stage IA1 cervical cancer. The histopathological diagnosis was confirmed by two pathologists per patient.
Postoperative treatments
Adjuvant chemotherapy (cisplatin-based) after NACT followed by surgery was administered for 2 to 6 cycles per the same regimen as the NACT treatments. Adjuvant radiotherapy after NACT following surgery consisted of external beam irradiation and intra-cavity brachytherapy with or without concurrent chemotherapy (cisplatin, 30 mg/m2 every 6 weeks).
Follow-up study
Patient follow-up was designed to be conducted every 3 months during the first year and every 6 months over the next four years after surgery. As per our database, follow-up was not performed for a few patients due to phone number changes. Efforts were made to contact them by their given address; however, we were still unable to connect with some patients, thus their data were excluded from the survival analysis.
Statistical analysis
Survival was compared and estimated using the Kaplan-Meier method. The median follow-up time was calculated as the median observation time among all patients. The SPSS 13.0 software package was used to perform all statistical analyses. All reported P-values are two-sided, and we considered P-values less than 0.05 to be significant.
References
Jemal, A. et al. Global Cancer Statistics. CA Cancer J Clin. 61, 69–90 (2011).
Bogani, G. et al. A Prospective Case-Control Study On the Impact of Neoadjuvant Chemotherapy On Surgery-Related Outcomes of Laparoscopic Radical Hysterectomy. Anticancer Res. 34, 5703–5708 (2014).
Hu, T. et al. Matched-Case Comparison of Neoadjuvant Chemotherapy in Patients with FIGO Stage IB1-IIB Cervical Cancer to Establish Selection Criteria. Eur J Cancer. 48, 2353–2360 (2012).
Rotman, M. et al. A Phase III Randomized Trial of Postoperative Pelvic Irradiation in Stage IB Cervical Carcinoma with Poor Prognostic Features: Follow-Up of a Gynecologic Oncology Group Study. Int J Radiat Oncol Biol Phys. 65, 169–176 (2006).
Chai, Y. et al. Radical Hysterectomy with Adjuvant Radiotherapy Versus Radical Radiotherapy for FIGO Stage IIB Cervical Cancer. BMC Cancer. 14, 63 (2014).
Davila, F. R. et al. Post-Operative Radiotherapy in Patients with Early Stage Cervical Cancer. Gynecol Oncol. 134, 52–59 (2014).
Folkert, M. R. et al. Postoperative Pelvic Intensity-Modulated Radiotherapy and Concurrent Chemotherapy in Intermediate- and High-Risk Cervical Cancer. Gynecol Oncol. 128, 288–293 (2013).
Goff, B. A. et al. Impact of Surgical Staging in Women with Locally Advanced Cervical Cancer. Gynecol Oncol. 74, 436–442 (1999).
Scandurra, G. et al. Efficacy and Tolerability of Paclitaxel, Ifosfamide, and Cisplatin as a Neoadjuvant Chemotherapy in Locally Advanced Cervical Carcinoma. J Gynecol Oncol. 26, 118–124 (2015).
Stehman, F. B. et al. Carcinoma of the Cervix Treated with Radiation Therapy. I. A Multi-Variate Analysis of Prognostic Variables in the Gynecologic Oncology Group. Cancer-am Cancer Soc. 67, 2776–2785 (1991).
Angioli, R. et al. Neoadjuvant Chemotherapy Plus Radical Surgery Followed by Chemotherapy in Locally Advanced Cervical Cancer. Gynecol Oncol. 127, 290–296 (2012).
Landoni, F. et al. Is there a Role for Postoperative Treatment in Patients with Stage Ib2-IIb Cervical Cancer Treated with Neo-Adjuvant Chemotherapy and Radical Surgery? An Italian Multicenter Retrospective Study. Gynecol Oncol. 132, 611–617 (2014).
Gadducci, A. et al. Pathological Response On Surgical Samples is an Independent Prognostic Variable for Patients with Stage Ib2-IIb Cervical Cancer Treated with Neoadjuvant Chemotherapy and Radical Hysterectomy: An Italian Multicenter Retrospective Study (CTF Study). Gynecol Oncol. 131, 640–644 (2013).
Lahousen, M. et al. Chemotherapy Versus Radiotherapy Versus Observation for High-Risk Cervical Carcinoma After Radical Hysterectomy: A Randomized, Prospective, Multicenter Trial. Gynecol Oncol. 73, 196–201 (1999).
Matsumura, M. et al. Neoadjuvant Chemotherapy Followed by Radical Hysterectomy Plus Postoperative Chemotherapy but No Radiotherapy for Stage IB2-IIB Cervical Cancer–Irinotecan and Platinum Chemotherapy. Gynecol Oncol. 119, 212–216 (2010).
Reducing Uncertainties About the Effects of Chemoradiotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 18 Randomized Trials. J Clin Oncol. 26, 5802–5812 (2008).
Panici, P. B. et al. Neoadjuvant Chemotherapy and Radical Surgery in Locally Advanced Cervical Cancer. Prognostic Factors for Response and Survival. Cancer-Am Cancer Soc. 67, 372–379 (1991).
Kim, H. S. et al. Significance of Numbers of Metastatic and Removed Lymph Nodes in FIGO Stage Before Surgery. Gynecol Oncol. 121, 551–557 (2011).
Angioli, R. et al. A Randomized Controlled Trial Comparing Four Versus Six Courses of Adjuvant Platinum-Based Chemotherapy in Locally Advanced Cervical Cancer Patients Previously Treated with Neo-Adjuvant Chemotherapy Plus Radical Surgery. Gynecol Oncol. 139, 433–438 (2015).
Wang, H. et al. Clinicopathological Risk Factors for Recurrence After Neoadjuvant Chemotherapy and Radical Hysterectomy in Cervical Cancer. World J Surg Oncol. 11, 301 (2013).
Huang, K. et al. Optimal Pathological Response Indicated Better Long-Term Outcome Among Patients with Stage IB2 to IIB Cervical Cancer Submitted to Neoadjuvant Chemotherapy. Sci Rep. 6, 28278 (2016).
Kim, H. S. et al. Significance of Numbers of Metastatic and Removed Lymph Nodes in FIGO Stage IB1 to IIA Cervical Cancer: Primary Surgical Treatment Versus Neoadjuvant Chemotherapy. Gynecol. Oncol. 121, 551–557 (2011).
Sedlis, A. et al. A Randomized Trial of Pelvic Radiation Therapy Versus No Further Therapy in Selected Patients with Stage IB Carcinoma of the Cervix After Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 73, 177–183 (1999).
Acknowledgements
We thank all participants who were recruited for the studies and were evaluated in this analysis. This study was endorsed by the Key Basic Research and Development Program Foundation of China (973 Program; No. 2015CB553903 and 2009CB521806) and was supported by grants from the National Natural Science Foundation of China (No. 81302267; 81230038; 81090414; 81101964; 81272422; 81472444; 81572571; 91529102; 81572725; 81501530 and 81172464).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Shuang Li, Shixuan Wang, Ding Ma, Haiying Sun, and Kecheng Huang. Performed the experiments: Haiying Sun, Kecheng Huang, Fangxu Tang, Xiong Li, Xiaoli Wang, Sixiang Long, Shasha Zhou, Suolangquzhen, Jianwei Zhang, and Ruoqi Ning. Analyzed the data: Haiying Sun, Kecheng Huang, and Shuang Li. Contributed reagents/materials/analysis tools: Haiying Sun and Kecheng Huang. Wrote the manuscript: Haiying Sun and Kecheng Huang. Haiying sun revised the mauscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, H., Huang, K., Tang, F. et al. Adjuvant chemotherapy after surgery can improve clinical outcomes for patients with IB2-IIB cervical cancer with neoadjuvant chemotherapy followed by radical surgery. Sci Rep 8, 6443 (2018). https://doi.org/10.1038/s41598-018-24413-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24413-z
This article is cited by
-
CD34 and Bcl-2 as predictors for the efficacy of neoadjuvant chemotherapy in cervical cancer
Archives of Gynecology and Obstetrics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.