Abstract
Three new Talaromyces species isolated from soil are reported here, namely T. dimorphus (ex-type strain AS3.15692 T), T. lentulus (ex-type strain AS3.15689 T) and T. mae (ex-type strain AS3.15690 T). T. dimorphus is characterized by biverticillate and monoverticillate penicilli, ampulliform phialides, slimy texture with sparse mycelial funicles and absent conidiogenesis on MEA. T. lentulus is featured by vivid yellow mycelium on Cz and MEA, absent conidiogenesis on CYA, and globose smooth-walled conidia. T. mae presents sparse conidia on CYA and YES, funiculous and floccose texture on MEA, and ovoid smooth-walled conidia. Both morphological and molecular characters show that T. dimorphus is unique and has no close relatives. Although T. lentulus and T. mae resembles T. adpressus and T. pinophilus very much, phylogenetic analyses of CaM, BenA, ITS and Rpb2 sequences all support their status as novel species.
Similar content being viewed by others
Introduction
The genus Talaromyces was established by Benjamin1 in year 1955 to include the species of certain penicillia producing teleomorphic gymnothecial ascocarps with asci borne in short chains or singly, anamorphic symmetrical biverticillate penicilli, and vivid yellow, orange or pink mycelium, which belong to Penicillium section Biverticillata-Symmetrica series Penicillium luteum according to Raper and Thom2. Stolk and Samson3 proposed a new genus, i. e. Hamigera to accommodate species with asci borne singly from crosiers, and left those whose asci borne in chains to Talaromyces. Pitt4 dealt with the teleomorphic and anamorphic species in different ways owing to the dual nomenclature, regarding the teleomorphic species of this group of moulds as Talaromyces and anamorphic ones as Penicillium subgenus Biverticillium, respectively. In the year of 2012, the dual naming system was repealed, using a single name for a single species instead5. Then, the genus Talaromyces consists of those species showing the above-mentioned characters, regardless of sexual or asexual states.
In the study of Samson et al.6, 71 species were listed in the genus Talaromyces. Houbraken et al.7 established a new genus, Rasamsonia to accommodate the thermotolerant and thermophilic species from Talaromyces and Geosmithia, so the genus Talaromyces only contains mesophilic species in Trichocomaceae. In the following years, many new taxa of Talaromyces were reported. For example, Visagie and Jacobs8 discovered 3 new species, Manoch et al.9 2 new species, Peterson and Jurjević10 1 new member, Sang et al.11 2 new taxa, Frisvad et al.12 1 new species. In the monographic work of 2014, Yilmaz et al.13 accepted 88 species and divided Talaromyces into 7 sections, namely sections Talaromyces, Helici, Purpurei, Trachyspermi, Bacillispori, Subinflati and Islandici. In the coming few years, Visagie et al.14 reported 5 new members of section Talaromyces. Yilmaz et al.15 found 4 new members of section Islandici. Wang et al.16 added 2 new ones to section Talaromyces. Romero et al.17 and Luo et al.18 each reported 1 novelty of section Trachyspermi. Wang et al.19 discovered 1 new species of section Talaromyces. Yilmaz et al.20 reported 3 new species of section Talaromyces, and 1 of section Bacillispori. Chen et al.21 discovered 9 new species, among which, 3 in section Talaromyces, 2 in section Helici, 3 in section Islandici and 1 in section Trachyspermi. Crous et al.22 discovered 1 novel species belonging to section Talaromyces. Wang et al.23 reported 2 species belonging to section Trachyspermi and section Talaromyces respectively. Guevara-Suarez et al.24 added 2 novelties to section Talaromyces, 1 to section Helici and 1 to section Trachyspermi. Peterson and Jurjević25 dicovered 11 novelties, among them 10 belong to sect. Islandici and 1 to sect. Subinflata.
In the survey of moulds around China, many isolates belonging to Talaromyces were discovered. Here, we report 3 new taxa of section Talaromyces, namely T. dimorphus sp. nov., T. lentulus sp. nov. and T. mae sp. nov.
Results
PCR amplification gave amplicons of CaM about 650 bp, BenA about 410 bp, ITS about 560 bp and Rpb2 about 850 bp. The trimmed alignments of CaM, BenA, ITS, Rpb2 and the combined CaM-BenA-ITS sequences were 494, 373, 453, 714 and 1329 characters with gaps, respectively.
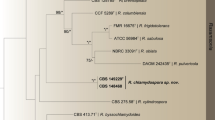
The phylogenetic trees generated by either the four individual loci or the concatenated CaM-BenA-ITS sequences show four isolates as three distinct, monophyletic species (Figs 1–3, S1–3).These phylograms all present that the three new members, i. e. T. dimorphorus, T. lentulus and T. mae belong to Sect. Talaromyces. T. dimorphorus is so distinctive that no close relatives are found in the section. T. adpressus, T. lentulus, T. mae and T. pinophilus are grouped in one clade with 100%, 99% and 99% bootstrap support according to CaM, Rpb2 and CaM-BenA-ITS sequences, respectively. But the phylogram resulted from BenA indicates that T. adpressus, T. lentulus, and T. sayulitensis are closely related with a bootstrap support of 80% while T. mae is in an outgroup clade to them, however, the ITS phylogram does not show that these species are related.
Description of Talaromyces dimorphus X.-Z. Jiang & L. Wang, sp. nov., Fig. 4
Fungal Names: FN 570521; MycoBank: MB 824518
Etymology: The specific epithet is derived from that both biverticillate and monoverticillate penicilli are commonly produced by the species.
Holotype: HMAS 247023
Colonies 16–18 mm diam on Cz at 25 °C after 7 d, thin, plane, margins submerged, irregular; velutinous; conidiogenesis moderate, near Grass Green (R. Pl. VI); mycelium white; no exudate and soluble pigment; reverse Water Green (R. Pl. XLI). Colonies 23–25 mm diam on CYA at 25 °C after 7 d, thin, radially sulcate; velutinous; conidiogenesis moderate, in central areas, coloured Pistachio Green to Leaf Green (R. Pl. XLI); mycelium white; no exudates and soluble pigment; reverse Reed Yellow to Olive Yellow (R. Pl. XXX). Colonies 38–39 mm diam on MEA at 25 °C after 7 d, low, plane; slimy texture overlaid with sparse, short and white mycelial funicles about 1–3 mm, and longer in centres; conidiogenesis absent; no exudate or soluble pigment; reverse Cream Color (R. Pl. XVI) and near Buckthorn Brown centrally. Colonies 29–30 mm diam on YES at 25 °C after 7 d, thin, radially sulcate; funiculose, but floccose centrally; conidiogenesis limited, Niagara Green (R. Pl. XXXIII); mycelium white; exudate and soluble pigment absent; reverse Buckthorn Brown (R. Pl. XV). On CYA at 37 °C after 7 d, no growth. On CYA at 5 °C after 7 d, no growth.
Conidiophores arising from funicles and surface hyphae; stipes (15–) 25–50 (−70) × 2.5–3.5 μm, smooth-walled; penicilli biverticillate and monoverticillate; metulae (2–) 4–6 per vertical, 9–13 × 2.5–3.5 μm; phialides 2–4 per verticil, ampulliform, 7–11 × 2.5–3.0 μm, with short and blunt collula; conidia ovoid to ellipsoidal, 2.5–3.5 μm, smooth-walled, borne in short divergent chains about 45–60 μm long.
Strains examined: China, Hainan, Jianfengling Forest Park, 18°43′12″N 108°49′48″E, 1300 m, from soil, 8 Nov. 2015, coll. X.-Z. Jiang, ex-type culture AS3.15692 = NN072337 (Holotype: HMAS 247023, from dried culture of ex-type AS3.15692 on CYA).
Notes: This new taxon is characterized by sparse slimy colonies on MEA, short stipes, biverticillate and monoverticillate penicilli, ampuliform phialides with short blunt collula and smooth-walled conidia.
Description of Talaromyces lentulus X.-Z. Jiang & L. Wang, sp. nov., Fig. 5
Fungal Names: FN 570522; MycoBank: MB 824519
Etymology: The specific epithet is derived from its late development of conidiogenesis on CYA and YES.
Holotype: HMAS 247024
Colonies 26–28 mm diam on Cz at 25 °C after 7 d, thin, plane, umbonate in centers; velutinous; conidiogenesis limited to moderate in central areas, near Spinach Green (R. Pl. V); mycelium Green Yellow (R. Pl. V); no exudate and soluble pigment; reverse Light Buff (R. Pl. XV), slightly with variegated Flesh Color (R. Pl. XIV). Colonies 26–27 mm diam on CYA at 25 °C after 7 d, thin, with few radial sulci; velutinous with sparsely overlaid mycelium; conidiogenesis absent, mycelium near Pale Salmon Color (R. Pl. XIV), slightly mingled with Naphthalene Yellow (R. Pl. XVI); exudate absent or limited, clear; no soluble pigment; reverse Cinnamon (R. Pl. XXIX). Colonies 43–44 mm diam on MEA at 25 °C after 7 d, moderately deep, plane; velutinous with sparse floccose mycelium overlaid; conidiogenesis moderate, near Grayish Olive to Light Grayish Olive (R. Pl. XLVI); mycelium Light Viridine Yellow (R. Pl. V); no exudate and soluble pigment; reverse Baryta Yellow (R. Pl. IV). Colonies 37–38 mm diam on YES at 25 °C after 7 d, slightly deep, irregularly plicate in centres; velutinous, and floccose with short loose funicles in centres; conidiogenesis sparse; mycelium Citron Yellow (R. Pl. XVI), Pale Pinkish Buff (R. Pl. XVI) in central areas; exudate and soluble pigment absent; reverse Mahogany Red to Burnt Sienna (R. Pl. II).On CYA at 37 °C after 7 d, colonies 18–21 mm diam, thin, plane; velutinous; no conidiogenesis, mycelium coloured Pale Salmon Color (R. Pl. XIV); exudate and soluble pigment; reverse Antique centrally and Pale Yellow-Orange in other areas (R. Pl. III). On CYA at 5 °C after 7 d, no growth.
Conidiophores arising from surface hyphae; stipes 240–380 × 2.5–3.0 μm, smooth-walled; penicilli biverticillate; metulae 4–6 per stipe, 10–11 × 2.0–2.5 μm; phialides 2–4 per metula, acerose with short collula, 9–10 × 1.5–2.0 μm; conidia globose, 2.5–3.0 μm, smooth-walled, conidial chains irregularly tangled into loose massed, about 40–60 μm long.
Strains examined: China, Shandong, Dongying, 37°43′12N 118°47′24E, 8 m, from soil, 15 Sep. 2015, coll. X.-Z. Jiang, ex-type culture AS3.15689 = NN071323 (Holotype: HMAS 247024 from dried culture of ex-type AS3.15689 on CYA).
Notes: This new species is characterized by vivid yellow mycelium, late development of conidiogenesis on CYA and YES, and good growth at 37 °C.
Description of Talaromyces mae X.-Z. Jiang & L. Wang, sp. nov., Fig. 6
Fungal Names: FN 570523; MycoBank: MB 824520
Etymology: named after Mrs. Xin-Yi Ma, who is the first scholar reporting Aspergillus and Penicillium species in China in the year of 1936.
Holotype: HMAS 247025
Colonies 18–19 mm diam on Cz at 25 °C after 7 d, thin, plane, slightly protuberate centrally; velutinous; conidiogenesis limited, near Serpentine Green (R. Pl. XVI); mycelium white at margins but Strontian Yellow in central areas (R. Pl. XLVI); no exudate and soluble pigment; reverse Cream Color (R. Pl. XVI). Colonies 22–24 mm diam on CYA at 25 °C after 7 d, thin, irregularly sulcate; velutinous with sparsely overlaid mycelium; conidiogenesis limited, in central areas, near Serpentine Green (R. Pl. XVI); mycelium white at margins while Straw Yellow (R. Pl. XVI) in other areas; clear exudate limited, no soluble pigment; reverse Baryta Yellow (R. Pl. IV) and with Salmon Color (R. Pl. XIV) centrally. Colonies 42–43 mm diam on MEA at 25 °C after 7 d, slightly deep, plane; funiculose and floccose with funicles about 1–3 mm; conidiogenesis sparse, near Light Elm Green (R. Pl. XVII); mycelium Light Dull Green-Yellow (R. Pl. XVII); no exudate and soluble pigment; reverse Cream Color (R. Pl. XVI). Colonies 33–34 mm diam on YES at 25 °C after 7 d, thin, slightly irregularly plicate; funiculose and floccose; conidiogenesis absent; mycelium Ivory Yellow to Primrose Yellow (R. Pl. XXX); exudates and soluble pigment absent; reverse Wax Yellow (R. Pl. XVI). On CYA at 37 °C after 7 d, colonies 17–18 mm diam, plane, slightly deep; velutinous, no conidiogenesis, exudate and soluble pigment. On CYA at 5 °C after 7 d, no growth.
Conidiophores arising from aerial hyphae and hyphal funicles; stipes (50–) 60–100 × 2.5–3.0 μm, smooth-walled; penicilli biverticillate; metulae 4–6 per stipe, 8–10 × 2.0–2.5 μm; phialides 2–4 per metula, acerose with short collula, 8–10 × 1.5–2.0 μm; conidia ovoid, 2.0–2.5 μm, walls smooth to finely rough, born in short irregularly tangled chains about 40–60 μm long.
Strains examined: China, Shanghai, Dongping Forest Park, 31°40′48″N 121°28′48″E, 3.5 m, from soil, 20 Sep. 2015, coll. X.-Z. Jiang, ex-type culture AS3.15690 = NN071328 (Holotype: HMAS 247025, from dried culture of ex-type AS3.15690 on CYA). Shandong: Dongying, 37°43′12″N 118°45′10″E, 8 m, from soil, 15 Sep. 2015, coll. X.-Z. Jiang, additional culture NN071327.
Notes: This new taxon is characterized by vivid yellow mycelium and hyphal funicles on MEA, good growth at 37 °C, and ovoid smooth-walled conidia.
Discussion
Sect. Talaromyces is the largest section of the genus Talaromyces, and of which many new species were discovered following the monographic study of Yilmaz et al.13, which now include 52 species until the publication of Guevara-Suarez24. Apart from the teleomorphs, the members in this section show a great diversity in morphological characters. For example, on CYA at 25 °C some species grow very fast such as T. rapidus (44–46 mm) while some ones grow considerable slowly such as T. mangshanicus (6–7 mm). The colony texture is also varied greatly from strict velutinous (e. g. T. qii) to floccose (e. g. T. derxii) and even synnematous (e. g. T. duclauxii) or funiculous (e. g. T. funiculosus). As for microscopic characters, some members bear typical compact biverticillate penicilli (e. g. T. beijingensis), whereas, some species produce divergent ones (e. g. T. flavovirens). In addition, though the majority typically have biverticillate penicilli, certain species commonly bear both biverticillate and monoverticillate ones, such as T. liani. Moreover, most of the members produce acerose phialides, while some bear typical ampuliform ones, e. g. T. stellenboschensis and T. mangshanicus.
One new member reported here, namely T. dimorphus commonly shows both bivertillate and monoverticillate penicilli with ampuliform phialides, and moderate growth on CYA at 25 °C. These characters much resemble those of T. veerkampii, whereas, the striking differences between them lie in the colony characters. For instance, T. veerkampii shows sparse sporulation on CYA at 25 °C, and dense sporulation on MEA, fast growth on YES (35–46 mm) with bronze-green colony reverse, while T. dimorphus produces moderate conidia on CYA at 25 °C, slimy and sparse colony texture on MEA overlaid by mycelial funicles, moderate growth on YES (29–30 mm) with brown reverse colour. Moreover, T. veerkampii grows well at 37 °C (18–23 mm)14, but T. dimorphus presents no growth at this temperature. In addition, the flask-shaped phialides of T. veerkampii bears gradually tapered thin collula, and the conidial walls are finely rough, while the new species produces phialides with short blunt necks, and its conidia are smooth-walled. The molecular evidence of the phylograms based on CaM, BenA, ITS and Rpb2 as well as the concatenated CaM-BenA-ITS sequences all shows that T. dimorphus is such a distinct species that no close relatives are found hitherto (Figs 1–3, S1–3).
In the phylograms according to CaM, Rpb2 and CaM-BenA-ITS sequences, T. adpressus, T. lentulus, T. mae and T. pinophilus are in one clade with strong bootstrap support (100%, 99% and 99%, respectively, Figs 1, S1–2), and the phylogram resulted from BenA shows that T. lentulus, T. adpressus, and T. sayulitensis are in one clade with a bootstrap support of 80%, while T. mae and T. pinophilus are in an outer clade to these 3 species (Fig. 2, S3). Moreover, the ITS tree does not show these 5 species are close-related (Fig. 3). Morphological resemblance is in accordance with the relationship among these closely related species shown by molecular evidence. Visagie et al.14 and Chen et al.21 reported T. sayulitensis and T. adpressus respectively, which are morphologically very similar to T. pinophilus. The two new members, T. lentulus and T. mae are hardly distinguished from T. pinophilus by morphological characters too. The most remarkable morphological difference between T. lentulus and T. pinophilus is the length of stipes, which in T. pinophilus, is much shorter (30–200 μm) than that of T. lentulus (240–380 μm). Moreover, the two species are well-distinguished by all the phylograms (Figs 1–3, S1–3). Further, when 5 additional T. pinophilus isolates apart from the ex-type isolate CBS 631.66 were included in BenA analysis, T. pinophilus and T. lentulus are well separated (Fig. S3), which presents that T. lentulus is not a distinctive isolate of T. pinophilus. T. lentulus can be distinguished from T. sayulitensis in that the new species shows moderate conidiogenesis on MEA, while T. sayulitensis hardly produces conidia on MEA, and T. lentulus grows more slowly (18–21 mm) than T. sayulitensis (32–40 mm) at 37 °C. Still, T. lentulus seldom bears penicilli with sub-terminal branches, whereas sub-terminal branches are sometimes found in T. sayulitensis. Again, the two species can be well distinguished phylogeneically (Figs 1–3, S1–3). The differences between T. lentulus and T. adpressus are ready. For instance, T. lentulus produces yellow mycelium on MEA and YES, but T. adpressus bears white mycelium on these culture media. Microscopically, T. lentulus has much longer and thinner stipes (240–380 × 2.5–3.0 μm) than T. adpressus does (100–200 × 3–4.5 μm).
The differences between the two new members, T. mae and T. lentulus are even subtle. In general, T. mae grows somewhat slowly than T. lentulus (Cz 18–19 mm; CYA 22–24 mm; 42–43 mm; YES 33–34; CYA 37 °C 17–18 mm vs. Cz 26–28 mm; CYA 26–27 mm; 43–44 mm; YES 37–38; CYA 37 °C 18–21 mm), and the most notable difference is that T. mae shows a funiculous texture on MEA but T. lentulus does not. Moreover, T. mae bears conidiophores on aerial and funiculous hyphae, and accordingly shorter stipes (60–100 μm) while T. lentulus produces conidiophores on surface hyphae with longer stipes (240–380 μm). More important, the molecular evidence unequivocally shows them as different species (Figs 1–3, S1–3), and T. mae is not a strain of T. pinophilus either (Fig. S3).
Although establishing new species based on one single isolate or specimen may mistake certain isolates (or populations) of a known species for novel species, this can be avoided by examining the clade containing the studied isolates and the isolates of a known species in the phylograms of different individual genes. Empirically, if the studied isolates in the same clade with the isolates of a known species all have very few substitutions (very short or no branch length in phylograms) from the nearest node in every gene tree and with a strong bootstrap support (over 80%), then the studied isolates can be regarded as the known species, such as the clade including six isolates of T. pinophilus in Fig. S3 or the T. ruglosus isolates in the study of Yilmaz et al.15. In this study, the two isolates AS3.15689 and AS3.15692 of the proposed new taxa T. lentulus and T. dimorphus, respectively, are well separated from other isolates of known species in all the trees, thus, their novelty status can be verified.
When we inferring the phylogenetic relationships using concatenated sequences of different genes, there is an assumption that all these genes have undergone the same evolutionary ways, but in nature, it is not the case. On the whole, different genes have different evolutionary ways, thus have different mutation models, so we preferred drawing phylograms based on individual gene sequences, which may result in incongruent relationships among certain species, though. For example, T. francoae, T. kendrickii, T. mangshanicus and T. qii and are closely related in CaM and BenA trees (Figs 1,2, S3), but in ITS tree, T. mangshanicus has no close relatives (Fig. 3), while in rpb2 tree T. aculeatus and T. mangshanicus are siblings (Fig. S1). When the ex-type Rpb2 sequences of T. aculeatus (KM023287) and T. mangshanicus (KX447527) are compared with each other, only 3 nucleotides are found different in the alignment of 852 nucleotides. The explanation may be that Rpb2 gene of these two species evolved in the same way, which is different from CaM, BenA and ITS. For another instance, T. aculeatus and T. apiculatus are close-related in CaM and BenA phylograms (Figs 1,2, S3), whereas, they are well separated in ITS and Rpb2 trees (Figs 3, S1). Thus, it seems normal that T. sayulitensis lies in the outgroup position to T. adpressus and T. lentulus in CaM tree (Fig. 1), but is closely related to T. adpressus and T. lentulus in BenA trees (Figs 2, S3). However, the 3 species T. adpressus, T. lentulus and T. sayulitensis are all separated in the ITS phylogram (Fig. 3). Notwithstanding this incongruence, the novelty status of the T. dimorphus, T. lentulus and T. mae can be verified by all the four individual gene trees.
Materials and Methods
Isolation of strains
Soil samples were collected and kept in sterilized plastic bags. The dilution plating method was used in the isolation of the fungi26. The strains of Talaromyces were deposited in Novozymes Culture Collection (Novozymes (China) Investment Co. Ltd., Beijing 100085, China) as NN072337, NN071323, NN071328 and NN071327, and the 3 ex-type cultures of T. dimorphus, T. lentulus and T. mae were also deposited in China General Microbiological Culture Collection (CGMCC) as AS3.15692 = NN072337, AS3.15689 = NN071323, AS3.15690 = NN071328, respectively. The dried specimens of the holotypes from the ex-type cultures were deposited in the Herbarium Mycologicum Academiae Sinicae as HMAS 247023, HMAS 247024, HMAS 247025, respectively.
Morphological studies
Colony characters were assessed using Czapek agar (Cz)2, Czapek yeast autolysate agar (CYA)4, 2% malt extract agar (MEA, malt extract (Difco, Lawrence, Kansas, USA)4, YES (yeast extract sucrose agar (Oxoid, Basingstoke, Hants, UK)27. Colour names followed those of Ridgway28. Microscopic mounts were prepared using material from colonies growing on MEA at 25 °C after 7 days mounted in 90% lactic acid without dye. Microscopic examination and photography were performed with an Axioplan2 imaging and Axiophot2 universal Microscope (Carl Zeiss (Shanghai) Co. Ltd., Shanghai, China).
Molecular studies
DNA extraction followed the method of Scott et al.29. Partial calmodulin gene (CaM) was amplified using the primers cmdAD1 and cmdQ130; partial β-tubulin gene (BenA) sequences were obtained with Bt2a and Bt2b31; the ITS1-5.8S-ITS2 region (ITS) of nuc rDNA was amplified using ITS5 and ITS432, and the partial DNA-dependent RNA polymerase II second largest subunit gene (Rpb2) sequences were obtained with sense primers rpb2T1: 5′-act ggt aac tgg ggt gag ca-3′or T2: 5′-acg ggt aac tgg ggt gaa ca-3′ with antisense primers rpb2E1: 5′-tc aca gtg agt cca ggt gtg-3′or E2: 5′-tc gca atg cgt cca ggt atg-3′. Polymerase chain reactions (PCR) were carried out in 20 µL reaction mixture containing 0.5 µL of each primer (10 pmol/µL), 1.0 µL of genomic DNA (10 ng/µL), 10 µL of 2 × PCR MasterMix buffer (0.05 u/µL Taq polymerase, 4 mM MgCl2, 0.4 mM dNTPs), and 8 µL of double distilled water (Tsingke Co. Ltd, Beijng, China). Amplifications were performed in a PTC-200 thermocycler (MJ Research, Watertown, Massachusetts, USA), the reaction program consisted of 94 °C for 3 min; 94 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s, 34 cycles; 72 °C for 5 min. After amplification the PCR amplicons were electrophoresed in a 2.0% agarose gel soaked in TAE buffer with a 100 bp DNA ladder (MBI Fermentas, Burlington, Ontario, CA) at 100 V for 15 min. The gel were then stained in an aqueous 0.5 μg/mL ethidium bromide water solution for 10 min and examined under 254 nm UV using a portable UV light in a dark room. Samples showing one single, obvious band of the anticipated length in the gel were then purified and sequenced on both strands with an ABI 3730 DNA analyzer (Applied Biosystems, Waltham, Massachusetts, USA). Raw sequences were proof-read and edited manually with BioEdit 7.0.933. Edited sequences were aligned using muscle in MEGA version 634. Forty strains of Talaromyces were included in CaM, BenA, ITS and the concatenated CaM-BenA-ITS phylogenetic analyses with sequences from ex-types. Only 18 sequences were obtained in Rpb2 analysis. The five sequence matrices were analyzed using Maximum Likelihood (ML) method and subjected to 1000 bootstrap replications, with substitution model and rates among sites K2+G+I for CaM, K2+G for BenA, Rpb2 and CaM-BenA-ITS, and T92+G+I for ITS. Gaps were treated as partial deletion according to Hall35. T. assiutensis CBS147.78 T was chosen as the outgroup (Table 1).
References
Benjiamin, C. R. Ascocarps of Aspergillus and Penicillium. Mycologia 47, 669–687 (1955).
Raper, K. B. & Thom, C. A manual of the Penicillia. 875 pp (Williams and Wilkins, Baltimore (1949).
Stolk, A. C. & Samson, R. A. Studies on Talaromyces and related genera I. Hamigera gen. nov. and Byssochlamys. Persoonia 6, 341–357 (1971).
Pitt, J. I. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. 634 pp (Academic Press, London (1980).
McNeill, J. et al. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 154 (Koeltz Scientific Books, Koenigstein (2012).
Samson, R. A. et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 70, 159–183 (2011).
Houbraken, J., Spierenburg, J. H. & Frisvad, J. C. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Anton. Leeuw. 101, 403–421 (2012).
Visagie, C. M. & Jacobs, K. Three new additions to the genus Talaromyces isolated from Atlantis sandveld fynbos soils. Persoonia 28, 14–24 (2012).
Manoch, L., Dethoup, T., Yilmaz, N., Houbraken, J. & Samson, R. A. Two new Talaromyces species from soil in Thailand. Mycoscience 54, 335–342 (2013).
Peterson, S. W. & Jurjević, Ž. Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces Clade 2a. PloS ONE 8(10), e78084 (2013).
Sang, H. et al. Two novel Talaromyces species isolated from medicinal crops in Korea. J. Microbiol. 51, 704–708 (2013).
Frisvad, J. C. et al. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 8(12), e84102 (2013).
Yilmaz, N., Visagie, C. M., Houbraken, J., Frisvad, J. C. & Samson, R. A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 78, 175–341 (2014).
Visagie, C. M. et al. Five new Talaromyces species with ampulliform-like phialides and globose rough walled conidia resembling T. verruculosus. Mycoscience 56, 486–502 (2015).
Yilmaz, N. et al. Taxonomic re-evaluation of species in Talaromyces section Islandici, using a polyphasic approach. Persoonia 36, 37–56 (2016).
Wang, Q.-M., Zhang, Y.-H., Wang, B. & Wang, L. Talaromyces neofusisporus and T. qii, two new species of section Talaromyces isolated from plant leaves in Tibet, China. Sci. Rep. 6, 18622 (2016).
Romero, A. M., Romero, A. I., Barrera, V. & Comerio, R. Talaromyces systylus, a new synnematous species from Argentinean semi-arid soil. Nova. Hedw. 102, 241–256 (2016).
Luo, Y., Lu, X., Bi, W., Liu, F. & Gao, W. Talaromyces rubrifaciens, a new species discovered from heating, ventilation and air conditioning systems in China. Mycologia 108, 773–779 (2016).
Wang, X.-C. et al. A new species of Talaromyces (Trichocomaceae) from the Xisha Islands, Hainan, China. Phytotaxa 267, 187–200 (2016).
Yilmaz, N. et al. Four novel Talaromyces species isolated from leaf litter from Colombian Amazon rain forests. Mycol. Prog. 15, 1041–1056 (2016).
Chen, A. J. et al. New Talaromyces species from indoor environments in China. Stud. Mycol. 84, 119–144 (2016).
Crous, P. W. et al. Fungal planet description sheets: 469–557. Persoonia 37, 252–253 (2016).
Wang, X.-C., Chen, K., Qin, W.-T. & Zhuang, W.-Y. Talaromyces heiheensis and T. mangshanicus, two new species from China. Mycol. Prog. 16, 73–81 (2017).
Guevara-Suarez, M. et al. Four new species of Talaromyces from clinical sources. Mycoses 2017, 1–22 (2017).
Peterson, S. W. & Jurjević, Ž. New species of Talaromyces isolated from maize, indoor air, and other substrates. Mycologia 109, 537–556 (2017).
Malloch, D. Moulds their isolation, cultivation and identification. 97 pp (University of Toronto Press, Toronto (1981).
Frisvad, J. C. & Samson, R. A. Polyphasic taxonomy of Penicillium subgen. Penicillium. A guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud. Mycol. 49, 1–173 (2004).
Ridgway, R. Color standards and color nomenclature. 53 pp (Published by the author, Washington DC (1912).
Scott, J., Malloch, D., Wong, B., Sawa, T. & Straus, N. DNA heteroduplex fingerprinting in Penicillium. In Integration of modern taxonomic methods for Penicillium and Aspergillus classification: (eds: Samson, R. A. & Pitt, J. I.) 225–236 (Harwood Academic Publishers, Amsterdam, 2000).
Wang, L. Four new records of Aspergillus section Usti from Shandong Province, China. Mycotaxon 120, 373–384 (2012).
Glass, N. L. & Donaldson, G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. App. Environ. Microbiol. 61, 1323–1330 (1995).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications: (eds: Innis, M. A. et al.) 315–322 (Academic Press, San Diego, 1990).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 41, 95–98 (1999).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Hall, B. G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 30, 1229–1235 (2013).
Acknowledgements
This work is supported by the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (CAS Grant No. QYZDY-SSW-SMC029) and National Natural Science Foundation of China (NSFC No. 31750001).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: L.W. Performed the experiments: X.Z.J., Z.D.Y., Y.M.R. Analyzed the data: X.Z.J., Y.M.R., L.W. Contributed reagents/materials/analysis tools: Y.M.R., L.W. Wrote the paper: L.W.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, XZ., Yu, ZD., Ruan, YM. et al. Three new species of Talaromyces sect. Talaromyces discovered from soil in China. Sci Rep 8, 4932 (2018). https://doi.org/10.1038/s41598-018-23370-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23370-x
This article is cited by
-
Two New Species of Talaromyces Sect. Trachyspermi Discovered in China
Mycopathologia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.