Abstract
Here we report Sr and Zn isotope ratios of teeth of medieval to early modern Breton people a population whose diet is known from historical, archeological and collagen isotope data. Most of the population, buried in the Dominican convent of Rennes, France, consists of parliamentary nobles, wealthy commoners and ecclesiastics, who had a diet rich in animal products. Our aim is to assess how the Zn isotope ratios of their teeth compare to those of other French historical populations previously studied, which were characterized by cereal-based diets, and those of modern French individuals, who daily eat animal products. We describe a clear offset (∼0.35‰) between local and non-local human individuals in Zn isotope ratios. The δ66Zntooth values of local individuals overlap that of modern French people, and are lower than those of local carnivores. Non-local δ66Zn values are similar to those of historical individuals analyzed previously. We conclude the lower Zn isotope ratios of local humans relative to the associated fauna can be explained by the consumption of carnivorous fish and pork, in agreement with historical, zooarchaeological and collagen (C, N, S) isotope data. Zn isotopes could therefore be a tracer of fish and/or substantial meat consumption in ancient populations.
Similar content being viewed by others
Introduction
The origin of Zn isotopic variability in human tissues remained unknown until Van Heghe et al. (2012)1, reported the strong impact of meat and fish consumption on blood Zn isotope ratios (66Zn/64Zn expressed as δ66Zn values), a preliminary conclusion quickly confirmed by Costas-Rodriguez et al. (2014)2. A parallel study on African food webs did not quantify the exact relationship between diet and bone Zn isotope ratios3, however by focusing on a much smaller geographical area, the sensitivity of Zn isotopes to diet was demonstrated4: Zn isotope ratios of bones and teeth clearly differ between carnivores and herbivores, with carnivores exhibiting the lowest ratios. The dependence of Zn isotope ratios on trophic level has also been confirmed in a marine ecosystem5.
The isotopic composition of Zn in animal tissues is controlled by two dietary factors: the isotopic fractionation that occurs during intestinal absorption and the Zn isotope ratios of the food products. Dietary Zn mainly comes from animal products, notably because Zn – and preferentially its lighter isotopes - from plants tends to precipitate with the phytates in the gastro intestinal tract6. This precipitation is likely to trigger isotopic fractionation inducing the preferential absorption of heavy Zn isotopes. Additionally, plant products usually have the most elevated δ66Zn values2. As a consequence, herbivore tissues exhibit higher Zn isotope ratios compared to carnivore or omnivore tissues3,4,5. Muscles are 66Zn depleted relative to the average isotopic composition of the body and no isotope fractionation of Zn is expected during meat consumption3. Carnivores therefore have lower δ66Zn values than their prey: the higher the trophic level of an animal is, the lower are the Zn isotope ratios of its body tissues5.
Zn isotope ratios of dental enamel from populations from different locations and historical periods were recently compared7. The study highlighted a very surprising trend: the δ66Zn dental values of preindustrial populations were much higher than those of modern individuals. Two explanations were then hypothesized to explain such a pattern. The observed trend could be due to:
-
1)
an increase in fish and meat consumption in the 20th century8. As mentioned above, elevated Zn isotope ratios are expected in tissues of individuals with plant-based diets1,2,3,4. Conversely, consumption of high trophic level food products, such as carnivorous fish (e.g. tuna, salmon, cod, pike) is expected to generate low Zn isotope ratios of mammal tissues.
-
2)
the release of anthropogenic Zn in modern environments by industries and/or the use of manuring products. The anthropogenic Zn can indeed exhibit low Zn isotope ratios9,10 and enter modern food webs9.
In order to decide between these two hypotheses, we analyzed a preindustrial population (13th to 18th century) characterized by diets with significant meat and fish consumption11. This allows us to test if historic human population living before the release of anthropogenic Zn into modern environments exhibit δ66Zn values closer to other preindustrial populations characterized by a cereal-based diet, or are more like modern individuals who also had diets with intensive meat and fish consumption. We studied the wealthy/elite late medieval to early modern population of the former Dominican convent of Rennes (Brittany, France, Fig. 1), for which we already reconstructed the diet using C, N and S isotopes11. This population includes ecclesiastics, nobles and commoners who had to obey fasting rules and therefore ate fish instead of meat for one third of the year12. The medieval and early modern diets of the aristocracy also included a very important amount of meat on non-fasting days. The C, N and S isotope ratios determined from the bones and teeth on the individuals buried in Rennes’s Dominican convent as well as the zooarchaeological study performed on this site and the nearby refuse midden, indicate a substantial consumption of animal products: terrestrial herbivores and omnivores, including suckling pigs, eels and marine fish11.
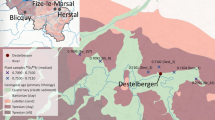
Location of Rennes and its Dominican convent. (A) Historical border of Brittany in the 15th century (blank map fron Daniel Dalet, http://www.histgeo.ac-aix-marseille.fr). (B) Location of the convent and the refuse dump outside of the walls in the 16th century (after the city map drawn by Hévin in 1685, image free of rights, http://www.wiki-rennes.fr/Fichier:Plan_Hevin.jpg). Both maps were created using the software Adobe Illustrator CS6.
Since the environmental context can impact local Zn isotope ratios4,9,13, the geographical origin of the individuals was assessed using Sr isotope ratios in human dental enamel as well as previously published S isotope data obtained on tooth collagen (Supplementary Information 1). Potential impacts of marine food consumption on S and Sr isotope ratios are assessed using previously published δ13C values11 by evaluating the absence or presence of a correlation between these isotope ratios.
In this paper, we present stable Zn and radiogenic Sr isotope data from the analysis of dental enamel of 54 individuals from the Dominican convent of Rennes. The associated fauna (6 terrestrial animals) was also analyzed to better interpret the diet of this population and compared the isotopic data of C, N and S previously conducted on the collagen from the same teeth.
Results
A socio-economic group was attributed to the different individuals depending on their burial location11. The majority are elite and wealthy individuals who are buried in the church and its chapels (group Privileged, A), but three subgroups can be defined within this group. The first subgroup (A’) includes the aristocrats identified by discriminatory funeral practices (embalming and/or lead coffins)11,14. A second subgroup is identified as slightly less favored as the others (B’) and includes the individuals buried in the nave of the church. The rest of the individuals buried in the church choir and the chapels corresponds to the last subgroup (A-A’). The ‘non-privileged’ individuals are buried outside the walls of the convent in the cloister garden and in the immediate exteriors of the convent (group Non-privileged, B-B’). In addition, the individuals from the chapter house are likely to be Dominican ecclesiastics (group Ecclesiastics, C). Finally, men with blade injuries found in mass graves out of the convent’s walls were probably soldiers (group Soldiers, D). The distribution of the individuals sampled among socio-economic group, phase, age at death and sex are given in the Table 1. Results for Sr and Zn isotope ratios measured in animal and human bones and teeth are given for each individual in the Supplementary Information Tables S2 and S3 and summarized in the Table 2. Isotope compositions of external standards are also described in the Supplementary information (Table S3) and are in agreement with expected values.
Sr isotope analyses
The Sr isotope ratios of terrestrial mammal teeth (n = 6) range from 0.7115 and 0.7146. Terrestrial domestic animals (pig, cat, dog, cows and sheep) being raised in the city or in the nearby country15, have local bioavailable 87Sr/86Sr values. This range also fits with the local values expected from the IRHUM database16 (Isotopic Reconstruction of Human Migration), ranging from 0.710 to 0.716 (Fig. 2A). The IRHUM database documents a really limited sea spray effect on the coasts of Brittany (Fig. 2B). The individuals exhibiting, lower, “non-local” tooth values are supposed to have spent the end of their childhood or the beginning of their adolescence in a sedimentary or volcanic region. Seventeen percent of the individuals analyzed are exhibiting non-local 87Sr/86Sr (87Sr/86Sr < 0.710 based on the subdivisions from the IRHUM database reproduced in Fig. 2), and are therefore likely to have spent their childhood outside of Brittany (9/54 teeth). By combining S isotopes that provide information on the relative distance to the coast – or more exactly, the existence of a marine influence on local values17 - to Sr isotopes, tracers of the local geology, 6 categories of geographical origins were defined/can be defined (Table 3). Two individuals exhibit inland S isotope values (NC-non-coastal) - therefore are not compatible with the observed Breton range- but 87Sr/86Sr > 0.710 (R-radiogenic) –compatible with the local range (R_NC). One individual is characterized by a coastal (C, δ34S > 11‰)11,17 but non-local Sr (NR-non-radiogenic, 87Sr/86Sr < 0.710) isotope signature (NR_C). Four individuals have both non-local S and Sr isotope values (inland δ34S value and 87Sr/86Sr < 0.710. NR_NC). Finally, for the remaining four individuals with non-local Sr isotope ratios, the S isotope signature of their teeth was not available (NR_X). As previously observed with S isotopes, all women and nobles tested (group 2 A’ and 3 A’, Table S1) fall into the “probable local” group (nwomen = 10, nnobles = 8). All soldiers showed non-local S isotope values in teeth or bones (nsoldiers = 3) but only one of the three teeth analyzed exhibit a 87Sr/86Sr value incompatible with the local range. One ecclesiastic from the second phase, already spotted as non-local with its δ34S value11, is also characterized by a non-local 87Sr/86Sr, whereas other ecclesiastics exhibit values compatible with the local range (6/7 ecclesiastics). The other non-local individuals mostly consist of people from the less-privileged groups (1B, 2B, and 3B’) (4 non-privileged for 9 non-local individuals).
Sr isotope ratios in Brittany (according to IRHUM database) and in dental enamel of Rennes individuals. J: juveniles. F: female. I: Indeterminate. PM: probable male. M: male. C+O=carnivorous and omnivorous animals. H: herbivores. The error bars are smaller than the symbol size. The map of Brittany and associated Sr isotope ratios was created using Adobe Illustrator CS6 based on the information available on the IRHUM database website16 (http://80.69.77.150/, blank map fron Daniel Dalet, http://www.histgeo.ac-aix-marseille.fr).
Zn isotope analyses
As previously observed, Zn isotope ratios of dental enamel do not correlate with Zn concentrations7,18. This shows the absence of a mixing line between diagenetic and biogenic endmembers, and therefore argues for the absence of soil contamination19. Typical analytical uncertainties on δ66Zn are 0.04‰. The cattle and sheep δ66Zn values range from 0.85 to 1.09‰ (n = 3). They are higher than the cat (δ66Zn = 0.67‰) and the dog (δ66Zn = 0.50‰) values, an observation which is in agreement with the trophic level effect observed in previous studies4,5. The suckling pig has a δ66Zn value similar to that of carnivores (δ66Zn = 0.65‰). This observation is not surprising considering that (1) the written record of the hospital documents the feeding of the pigs with leftovers, which included fish and meat20 (2) we documented the high trophic level of those pigs in our previous study using C, N and S isotopes11. The isotope ranges are consistent with previous observations in other terrestrial mammal food webs3,4, as well as the trophic level offset between herbivores and omnivores/carnivores (0.35‰ in Rennes, 0.45‰ in terrestrial Kenyan mammal food web4, 0.3‰ in Arctic marine mammal food web5). Human δ66Zn values are generally lower than those for animals. Individuals showing 87Sr/86Sr compatible with the local range have significantly lower Zn isotope ratios than those of non-local individuals (Table 2, normal distribution, two-tailed, n = 54, t-test p = 1 × 10−7), whose δ66Zn values overlap with those of omnivorous/carnivorous mammals (Fig. 3). Contrary to what was previously observed for δ15N values, there are no differences between the Zn isotope ratios of men and women. Among the individuals who exhibit 87Sr/86Sr compatible with the local range, we did not notice differences between socio-economic groups for both δ15N or δ66Zn values (Table 4). The δ66Zn values of the locals from Rennes overlap with values of modern humans from France, whereas non-local individuals have δ66Zn values similar to that observed in historical populations from South-Eastern France7 (Fig. 3).
Isotope ratios in teeth of humans and animals buried in Rennes, Brittany. C, N and S isotope ratios were measured in dentine collagen and published in a previous work11. Zn and Sr isotope ratios were measured in dental enamel. Error bars are represented when they exceed the symbol size.
Correlation between isotope tracers
In order to investigate the influence of different food categories, including marine fish, on the isotope signatures of Sr and Zn, we explored the presence or absence of correlation between these isotope ratios and other dietary proxies (C, N and S stable isotope signature). Among the 54 human teeth analyzed in this study, we previously measured C and N isotope ratios for 47 of them, and S isotope ratios for 29 of them. No correlation has been observed between 87Sr/86Sr and Sr concentrations, δ15N, δ13C as well as δ34S values. δ66Zn values also do not correlate with δ15N, δ13C and δ34S values. A correlation however seems to appear between Zn and N isotope values when animals are taken into account (n = 51, Pearson’s correlation test, R2 = 0.21, p = 6 × 10−4, Fig. 3B). Sr and Zn isotope values do not correlate when animal and human teeth are considered together (n = 54, Pearson’s correlation test, R2 = 0.01, p = 0.60). However, the correlation does exist in human teeth, but only when non-local individuals are included (Fig. 3).
Discussion
Geographical origin of Rennes’ humans and animals
Two of the three herbivores analyzed exhibit 87Sr/86Sr higher than the expected local range according to the IRHUM database16 (Fig. 2), but such values exist in the surroundings of Rennes. In agreement with historical evidence, cattle and sheep were probably raised in the countryside, whereas dogs, cats and pigs were urban animals15,20 (Fig. 2). The local range defined by all domestic animal Sr isotope signatures therefore represents Rennes and the nearby countryside.
The local 87Sr/86Sr range defined here is compatible with the one predicted for the whole Armorican Massif, which is also the case for the local S isotope signatures11. Individuals exhibiting S and Sr isotope signatures compatible with the local ranges are therefore likely to be originating from Brittany. Individuals showing lower 87Sr/86Sr isotope signature are likely to come from sedimentary regions, such as the Parisian and Aquitaine Basins, South-Eastern England or South-Eastern France. Strontium isotope data confirm a local origin of women and nobles buried in the Dominican convent, as well as the presence of non-local individuals among the medieval ecclesiastics, commoners and soldiers buried in mass graves (Fig. 4). Two individuals buried in the church have high 87Sr/86Sr isotope signatures but non-coastal δ34S values, suggesting they have spent their childhood out of Brittany.
Burial location and associated tooth Sr, Zn and N isotope ratios in teeth of humans buried in the Dominican convent of Rennes and animals from the nearby refuse dump. N isotope ratios were previously published11. Blue color: privileged groups, red: ecclesiastics, yellow: commoners. Grey area: range of local 87Sr/86Sr.The map of the convent was drawn using the software Adobe Illustrator CS6 after the one created using the software Autocad for the excavation reports (Le Cloirec, 2016). The map corresponds to the convent during the 14th–16th centuries.
Zn isotope ratios: a promising new dietary tracer?
The range of δ66Zn values measured in teeth showing Sr isotope ratios compatible with local signatures overlaps with that of modern (20th century) individuals but strongly differs from previous historical periods (17th to 19th centuries) (Fig. 5). However, individuals exhibiting Sr isotope signatures compatible with other rock types such as volcanic or sedimentary rocks - that is to say lower than the local range - have similar Zn isotope ratios to the abovementioned historical populations. A large part of the preindustrial teeth previously studied7 belonged to individuals coming from sedimentary regions (Supplementary Information 1). The Zn isotope composition of igneous rocks and clastic sediments is fairly constant (0.2 < δ66Zn < 0.4‰)13, but siliceous and calcareous sediments can show higher Zn isotope ratios13,21, especially in limestones, for which the δ66Zn values can reach up to 1.35‰22,23. To verify if the Zn isotope differences between individuals showing high 87Sr/86Sr and those having low 87Sr/86Sr ratios could be simply related to the Zn isotope composition of the bedrock, we compared the Zn isotope ratios of all human historical teeth from this study and a previous one on Zn isotope ratios in teeth of French individuals7, to the expected values of the associated bedrock (Supplementary Information 1). We found that humans coming from regions with chalky bedrock indeed exhibited the highest Zn isotope ratios, but were not significantly different from the humans coming from regions with igneous or sandstone bedrocks. Moreover, individuals coming from igneous inland regions still had higher δ66Zn values than the local Bretons from this study (Supplementary Information 1). If the geology can have an impact on local human teeth ratios3,4, it seems that biological processes in soils tend to overprint these original ratios24.
Based on the absence of relationship between Zn isotope ratios of teeth and geology, as well as the established difference between δ66Zn values of herbivores and omnivores/carnivores1,2,4, diet is the most likely factor to account for the peculiar isotope signature of the local Bretons from Rennes. The δ66Zntooth values of the humans are clearly lower than that of the associated fauna, including the cat and the dog, and overlap those of modern individuals (carnivores, Figs 3, 5). As mentioned above, this low Zn isotope ratio could be the signature of a substantial animal product consumption of high trophic level, which would explain the lower Zn isotope signatures than the local carnivores. The French historical populations, which have been previously studied, characterized by high Zn isotope ratios, were workers coming from inland regions (e.g. French Alps, Rhone Valley, Jura, Pyrenees, Vosges), with limited access to fish consumption7,25. Whereas, Rennes’s individuals are likely to have had a frequent meat and fish consumption: nobles and middle class to display their wealth, ecclesiastics to respect fasting rules, but also urban workers who were complaining of too much salted fish consumption15. The Bretons were also known to have a substantial meat consumption relative to human populations from other French regions12. Therefore, high δ66Zn values of non-local individuals, mostly buried out of the convent walls and therefore likely to be commoners, could be explained by a cereal-based diet. Cereals, as most plant foods, have indeed much higher δ66Zn values than animal products2, and were the main dietary source of the non-privileged French population of that time12, especially outside of Brittany15. To explain the fact that Rennes humans exhibit lower Zn isotope ratios than dogs and cats, the existence of high trophic level food consumption, namely carnivorous fishes and/or suckling pigs, must be invoked. The pigs from the refuse dump of Rennes were bred in a hospital yard and fed with leftovers which daily included meat and fish20. Zinc isotope studies in archeological contexts being in their infancy, it is not possible to say at this stage if different signatures of freshwater or marine fish consumption can be expected. The absence of a correlation between carbon isotope ratios and 87Sr/86Sr values documents a weak contribution- albeit existing, according to zooarcheological evidence - of marine fish in Rennes human diets. We documented previously very high δ15N values in tooth and bone collagen of humans and animals, and could not conclude if these high N isotope ratios were more due to the consumption of herbivorous mammals, suckling animals or eels11. Given the fact that eels are migratory aquatic organisms and constitute 45% of the fish remains found in the refuse midden close to the convent as well as the refectory soil20,26 and that suckling pigs were a sought-after food at that time11, the Zn isotope ratios could reflect the signature of a substantial eel and pork consumption. Animal product consumption in Rennes’ diets is in every instance more important than in those of the historical French populations previously studied12,15,16,25,26, which explains the low Zn isotope ratios.
Absence of correlation between dietary tracers
If both Zn isotope ratios and N isotope ratios are indicators of the trophic level effect, one should expect a correlation between these two tracers. This correlation exists when all species are considered together (Fig. 3B), but is absent among human values. This pattern has already been observed in marine mammals5: an interspecific correlation between δ66Zn and δ15N values was reported but did not exist between individuals from the same species. Zn isotope ratios were measured in tooth enamel, whereas N isotope ratios were measured in the dentine. These two dental hard tissues with different formation times could therefore record different diets. We however reject a change of diet between the time of formation of the dental enamel and dentine sampled as being the explanation of the absence of correlation between δ66Zn and δ15N values, since the lack of correlation has already been reported for bones and blood18,27.
The two isotope tracers are therefore both influenced by trophic level, but other dietary factors may influence one of the isotope ratios and not the other one. For example, N isotope ratios, measured in collagen, mostly provide information on the protein portion of the diet28, whereas Zn, measured in bioapatite, is likely to reflect the bulk diet – albeit mostly the animal product portion. A trophic level effect is also observed in breastfeeding individuals29 for N isotope signatures but is not expected for Zn isotopes: the M1 milk teeth - which form their enamel during the first year of life - that we measured in this study, and that has also been previously analyzed7 did not differ from M2 and M3 permanent teeth. Moreover, milk products do not show depleted Zn isotope signatures compared to meat or fish2. Finally, environmental factors are likely to differentially influence the local Zn and N isotope signatures of the soils and plants4,9,13,30,31, which could account for the lack of correlation between these two dietary indicators.
Diet, mobility and social status
Colleter et al.11 reported dietary differences related to social status for N isotopes, although they were more obvious among adults (bone values) than during childhood (dentine values). When the whole population is considered together, dietary and mobility differences can also be observed for Zn isotopes relative to the burial location: individuals buried in the church and the chapels (groups A and B’) are, in general, more local and to also have a diet richer in animal products (according to δ66Zn and δ15N values, Figs 3 and 4) than individuals buried in the exteriors. However, when only individuals with local 87Sr/86Sr ratios are considered, the isotope differences between burial locations disappear for δ66Zn and δ15N values (Fig. 4, Table 2). In the present study, most of the non-local individuals were buried in the exteriors. It is therefore difficult to know whether social status isotope differences are due to dietary or environmental factors, or if it is related to a sample size bias. Nevertheless, as mentioned before, a substantial animal product consumption of the urban workers, likely to be buried in the exteriors, is consistent with historical writings15. A gender difference is clearly existing for N isotopes in teeth11 but not for Zn isotopes. A female diet including a significant proportion of young animals but a similar amount of animal products relative to the male diet could explain such an isotopic pattern.
Conclusions
The Zn stable isotope compositions measured in the teeth of individuals buried in the Dominican convent of Rennes show a remarkable pattern: (1) local privileged individuals exhibit overlapping values with modern individuals from France, which could be explained by substantial meat and/or fish consumption (2) individuals identified as migrants using Sr isotopes have Zn isotope ratios similar to those of poor French individuals previously analyzed from the 17th to the 19th centuries. This is partially explained by a limited influence of the geology on the Zn isotope composition of food products eaten during the childhood combined with a reduced meat consumption of migrating individuals. Local Rennes humans exhibit lower Zn isotope ratios than the associated fauna, including carnivores, which can possibly be explained by carnivorous fish and pork consumption. Fasting rules indeed imposed the consumption of fish a day out of three in medieval and early modern Western Europe. Given the historical, zooarcheological and other isotope data obtained from Rennes Dominican’s convent, this carnivorous fish consumption could mostly consist of codfish and eels. Zn isotopes have therefore a strong potential to trace the consumption of high trophic level food, and potentially fish, in ancient human diets.
Methods
Material
Rescue excavations in the city center of Rennes recently permitted the study of the implantation and evolution of a mendicant convent which has been described by Le Cloirec (2016)26. The Dominican convent was founded outside the walls of the city in 1368. Between the end of the 14th and the 18th century, the settlement was an important place of pilgrimage – because of the presence of a “miraculous painting” in the convent - and burial, especially for the parliamentary nobility14,15,32. A multi-isotope study previously documented the diet of this population11. Three phases of burial are differentiated on the site. The first phase (13th c., phase 1) predates the construction of the convent. The second (phase 2) goes from the end of the 14th century to the 16th century. The last period (phase 3) covers the 17th and 18th centuries and therefore corresponds to the modern period. Additional information is available in the Table S1 of the Supplementary data. In total, 54 human molars (M2 and M3) were sampled. The faunal remains (16th century, end of the phase 2, Fig. 1) consist in the most common species found in the refuse midden of the hospital concomitant and contemporaneous to the convent.
Methods
All the analyses were conducted in the laboratories of the Department of Human Evolution at the Max Planck Institute for Evolutionary Anthropology (MPI-EVA) in Leipzig, Germany, in accordance with approved guidelines and regulations.
The samples were mechanically cleaned using a dental drill equipped with a diamond tip. Two small pieces were sampled (5–20 mg). Dentine was removed with a diamond-tipped burr. For Sr isotope analyses, samples were digested in nitric acid and purified using the ion exchange method described in Maréchal et al. (1999)33. Each batch of preparation included 13 samples. One blank and one external standard SRM 1486 (bone meal) were analyzed to verify that no contamination or purification problem happened during the column chromatography. For Zn isotope analyses, samples were digested in hydrochloric acid (HCl), evaporated and dissolved in hydrobromic acid (HBr, 1.5 M). Each batch of preparation included a blank and an external standard (in house standard AZE bone powder and/or the SRM 1400 bone ash, Table S4). Zinc was then purified using a protocol adapted from Moynier et al. (2006)34 previously described in Jaouen et al. (2016b)5. The Sr and Zn isotope analyses were conducted using a Thermo Fisher Neptune MC-ICP-MS at the Max Planck Institute for Evolutionary Anthropology (Leipzig. Germany). δ66Zn values are expressed relative to the standard JMC-Lyon. External reproducibility is 0.04‰ for δ66Zn values and 0.000024 for Sr ratios (1 SD). The protocol followed for isotope and concentration analyses was previously described for Sr35,36 and for Zn5.
Data availability statement
All data generated or analyzed during this study are included in this published article and the Supporting Information file.
References
Van Heghe, L., Engström, E., Rodushkin, I., Cloquet, C. & Vanhaecke, F. Isotopic analysis of the metabolically relevant transition metals Cu, Fe and Zn in human blood from vegetarians and omnivores using multi-collector ICP-mass spectrometry. J. Anal. At. Spectrom. 27, 1327–1334 (2012).
Costas-Rodríguez, M., Van Heghe, L. & Vanhaecke, F. Evidence for a possible dietary effect on the isotopic composition of Zn in blood via isotopic analysis of food products by multi-collector ICP-mass spectrometry. Metallomics 6, 139–146 (2014).
Jaouen, K., Pons, M.-L. & Balter, V. Iron, copper and zinc isotopic fractionation up mammal trophic chains. Earth Planet. Sci. Lett. 374, 164–172 (2013).
Jaouen, K., Beasley, M., Schoeninger, M., Hublin, J.-J. & Richards, M. P. Zinc isotope ratios of bones and teeth as new dietary indicators: results from a modern food web (Koobi Fora, Kenya). Sci. Rep. 6 (2016).
Jaouen, K., Szpak, P. & Richards, M. P. Zinc isotope ratios as indicators of diet and trophic level in arctic marine mammals. PloS One 11, e0152299 (2016).
Lönnerdal, B. O. Dietary factors influencing zinc absorption. J. Nutr. 130, 1378–1383 (2000).
Jaouen, K., Herrscher, E. & Balter, V. Copper and zinc isotope ratios in human bone and enamel. Am. J. Phys. Anthropol. 162, 491–500 (2017).
Aymard, M. Les pratiques de l’alimentation carnée en France. in Le mangeur et l'animal, Mutation de l'élevage et de la consommation.Autrement, Paris. 172p (Paillat M., 1997).
Cloquet, C., Carignan, J. & Libourel, G. Isotopic composition of Zn and Pb atmospheric depositions in an urban/periurban area of northeastern France. Environ. Sci. Technol. 40, 6594–6600 (2006).
John, S. G., Park, J. G., Zhang, Z. & Boyle, E. A. The isotopic composition of some common forms of anthropogenic zinc. Chem. Geol. 245, 61–69 (2007).
Colleter, R. et al. Social status in late medieval and early modern Brittany: insights from stable isotope analysis. Anthropological and Archaeological Sciences (in press). https://doi.org/10.1007/s12520-017-0547-9.
Quellier, F. La table des Français: une histoire culturelle (XVe-début XIXe siècle). 274p. (PU Rennes, 2007).
Moynier, F., Vance, D., Fujii, T. & Savage, P. The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 82, 543–600 (2017).
Colleter, R. et al. Procedures and Frequencies of Embalming and Heart Extractions in Modern Period in Brittany. Contribution to the Evolution of Ritual Funerary in Europe. PloS One 11, e0167988 (2016).
Croix, A. La Bretagne aux 16e et 17e siècles: la vie, la mort, la foi. 2, 571 p. (Maloine, 1981).
Willmes, M. et al. The IRHUM (Isotopic Reconstruction of Human Migration) database. Earth Syst. Sci. Data 6, 117 (2014).
Nehlich, O. The application of sulphur isotope analyses in archaeological research: a review. Earth-Science Reviews 142, 1–17 (2015).
Jaouen, K. et al. Fe and Cu stable isotopes in archeological human bones and their relationship to sex. Am. J. Phys. Anthropol. 148, 334–340 (2012).
Reynard, B. & Balter, V. Trace elements and their isotopes in bones and teeth: Diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 416, 4–16 (2014).
Clavel, B. Données archéozoologiques et fouilles d’hôpitaux: l’exemple de l’hôpital sainte Anne (Rennes, Ille-et-Vilaine). In Les établissements hospitaliers en France du Moyen Âge au XIXe siècle 393p. (Le Clech-Charton S., 2010).
Cloquet, C., Carignan, J., Lehmann, M. F. & Vanhaecke, F. Variation in the isotopic composition of zinc in the natural environment and the use of zinc isotopes in biogeosciences: a review. Anal. Bioanal. Chem. 390, 451–463 (2008).
Luck, J. M., Ben, O. D., Albarede, F. & Telouk, P. Zn and Cu Isotopes Tracers of Metal Origin in the Dissolved and Particulate Loads of Rain. Geochim Cosmochim Acta A 7619 (1999).
Pichat, S., Douchet, C. & Albarède, F. Zinc isotope variations in deep-sea carbonates from the eastern equatorial Pacific over the last 175 ka. Earth Planet. Sci. Lett. 210, 167–178 (2003).
Fekiacova, Z., Cornu, S. & Pichat, S. Tracing contamination sources in soils with Cu and Zn isotopic ratios. Sci. Total Environ. 517, 96–105 (2015).
Herrscher, E., Bocherens, H., Valentin, F. & Colardelle, R. Comportements alimentaires au Moyen Âge à Grenoble: application de la biogéochimie isotopique à la nécropole Saint-Laurent (XIII e–XV e siècles, Isère, France). Comptes Rendus Académie Sci.-Ser. III-Sci. Vie 324, 479–487 (2001).
Le Cloirec, G. L’étude archéologique du couvent des jacobins de Rennes (35), du quartier antique à l’établissement religieux: rapport final d’opération archéologique. 3835 p (Excavation report) (2016).
Jaouen, K. et al. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics 5, 1016–1024 (2013).
Hedges, R. E. M. & Reynard, L. M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 34, 1240–1251 (2007).
Fogel, M. L., Tuross, N. & Owsley, D. W. Nitrogen isotope tracers of human lactation in modern and archaeological populations. Carnegie Inst. Wash. Yearb. 88, 111–117 (1989).
Stevens, R. E. & Hedges, R. E. M. Carbon and nitrogen stable isotope analysis of northwest European horse bone and tooth collagen, 40,000BP–present: Palaeoclimatic interpretations. Quat. Sci. Rev. 23, 977–991 (2004).
Drucker, D. G., Bocherens, H. & Billiou, D. Evidence for shifting environmental conditions in Southwestern France from 33 000 to 15 000 years ago derived from carbon-13 and nitrogen-15 natural abundances in collagen of large herbivores. Earth Planet. Sci. Lett. 216, 163–173 (2003).
Bordeaux, C. Moi, Claude Bordeaux–: journal d’un bourgeois de Rennes au 17ème siècle. 255 p. (Apogee, 1992).
Deniel, C. & Pin, C. Single-stage method for the simultaneous isolation of lead and strontium from silicate samples for isotopic measurements. Anal. Chim. Acta 426, 95–103 (2001).
Moynier, F., Albarède, F. & Herzog, G. F. Isotopic composition of zinc, copper, and iron in lunar samples. Geochim. Cosmochim. Acta 70, 6103–6117 (2006).
Britton, K., Grimes, V., Dau, J. & Richards, M. P. Reconstructing faunal migrations using intra-tooth sampling and strontium and oxygen isotope analyses: a case study of modern caribou (Rangifer tarandus granti). J. Archaeol. Sci. 36, 1163–1172 (2009).
Hartman, G. & Richards, M. Mapping and defining sources of variability in bioavailable strontium isotope ratios in the Eastern Mediterranean. Geochim. Cosmochim. Acta 126, 250–264 (2014).
Acknowledgements
We would like to thank Annabell Reiner for technical support, and the Max Planck Society for funding this study. MLP acknowledges salary support from an ERC Starting Grant (HabitablePlanet; 306655). We would also like to thank Emily I. Stevenson and the two reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
K.J. and M.P.R. designed the research; K.J., M.L.P., A.P., B.C. and R.C. performed the research; K.J., M.L.P., A.P., R.C., B.C., M.P.R. analyzed data; K.J., R.C., M.L.P., J.J.H., N.T., E.C. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaouen, K., Colleter, R., Pietrzak, A. et al. Tracing intensive fish and meat consumption using Zn isotope ratios: evidence from a historical Breton population (Rennes, France). Sci Rep 8, 5077 (2018). https://doi.org/10.1038/s41598-018-23249-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23249-x
This article is cited by
-
Isotope metallomics approaches for medical research
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.