Abstract
This study aimed to evaluate the effect of brain atrophy on the functional outcome of patients with moderate-volume basal ganglia hemorrhage. Of 1003 patients with spontaneous intracerebral hemorrhage, 124 with moderate-volume basal ganglia hemorrhage (hematoma volume of 20–50 mL) were enrolled. The intercaudate distance (ICD) and sylvian fissure ratio (SFR) were used as linear brain atrophy parameters. The patients were divided into groups with favorable and unfavorable outcomes, according to the Glasgow Outcome Scale score, 90 days after symptom onset. Demographic and radiographic features, including the ICD and SFR, were compared between the two groups. Among the 124 patients, 74 (59.7%) exhibited a favorable outcome. The ICD and SFR values were significantly greater for the favorable group than for the unfavorable group. Multivariate analysis indicated that young age, high Glasgow Coma Scale score at admission, small hematoma volume, and increased ICD (odds ratio [OR], 1.207; 95% confidence interval [CI], 1.004–1.451) and SFR (OR, 1.046; 95% CI, 1.007–1.086, per 0.001) values had a beneficial effect on functional outcome. In conclusion, brain atrophy exhibits protective effects in patients with moderate-volume basal ganglia hemorrhage, and is an important factor for predicting functional outcome.
Similar content being viewed by others
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a major public health problem accounting for 10–15% of all primary strokes1. Notably, deep-seated basal ganglia hemorrhage, the most common form of spontaneous ICH, is associated with a high mortality risk, resulting in death or dependency in >70% of the patients1,2,3,4. Variables such as advanced age, poor initial neurological state, large hematoma volume, and intraventricular extension are independent predictors of poor outcomes following spontaneous ICH5,6,7,8.
Recently, interest in the role of brain atrophy in reducing regional mass effect of space-occupying lesions has risen9,10,11,12. Brain atrophy may prevent an increase in intracranial pressure (ICP) and cerebral herniation in patients with large cerebral infarctions, by providing additional intracranial space for compensation9,10,11,12. However, the potential impact of brain atrophy on spontaneous ICH has not been clearly elucidated. While recent research suggests that reduced cerebral volume impedes functional recovery following supratentorial ICH13, no studies have reported the effect of brain atrophy specifically on the outcome of basal ganglia hemorrhage. Therefore, we investigated the contribution of brain atrophy to the favorable functional outcome of patients with moderate-volume basal ganglia hemorrhage.
Methods
Patient selection and data acquisition
We retrospectively reviewed 1003 consecutive patients with spontaneous ICH treated in our institute, between March 2006 and September 2016. Of these, 124 were included in this study, and all were diagnosed with moderate-volume (20–50 mL) basal ganglia hemorrhage, based on computed tomography (CT) scans. The remaining 879 patients were excluded for the following reasons: (1) hemorrhage associated with antecedent disease, including arteriovenous malformation, cavernous malformation, moyamoya disease, tumor bleeding, or hemorrhagic transformation of cerebral infarction (n = 73); (2) ICH at locations other than the basal ganglia (n = 331); (3) hemorrhage extension to the ventricular space (n = 124); (4) hematoma volume <20 mL (n = 246) or >50 mL (n = 73); (5) age >80 years (n = 18) or a premorbid bedridden state (n = 1); and (6) lost to follow-up (n = 6). Patients who underwent repeated surgery (n = 2), developed severe medical complications (n = 2), and whose relatives refused treatment (n = 3) were also excluded.
All the patients’ medical records from hospital charts and radiographic studies were reviewed. The functional outcome was evaluated using the Glasgow Outcome Scale (GOS) at 90 days after symptom onset. A GOS score of 4–5 was considered a favorable outcome, while 1–3 was considered unfavorable. Outcome measurement was performed during outpatient visits or via telephone interviews.
This study was approved by the institutional review board of Hanyang University Medical Center. Owing to the retrospective nature, the need for informed consent was waived.
Image analysis
For most patients, hematoma volume was measured from initial CT scans; follow-up CT was used when hematoma expansion was detected. To estimate hematoma volume, we used the ABC/2 formula, where A represents the largest diameter on axial CT slices, B represents the diameter perpendicular to A on the same slice, and C represents the number of slices with a visible hematoma, multiplied by the slice thickness14. A volume of 20–50 mL was considered moderate15.
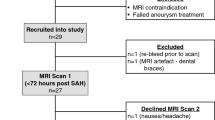
We used the intercaudate distance (ICD) and sylvian fissure ratio (SFR) on CT scans as parameters of brain atrophy. The ICD, which represents central atrophy, was defined as the minimum distance between the caudate indentations on the frontal horns of the lateral ventricles16,17. The SFR, which represents cortical atrophy, was defined as the average maximum width of both sylvian fissures in the section where they appeared widest, divided by the transpineal inner table diameter (Fig. 1A)16,17. However, as hematoma on the morbid side can obscure measurements, only the unaffected hemisphere was assessed17. Therefore, the ICD was replaced by the hemi-ICD: the minimum distance between the caudate indentation on the unaffected side and the septum pellucidum, multiplied by 2 (Fig. 1B). The width of the sylvian fissure on the unaffected side only was used for calculating the SFR (Fig. 1C). These measurements were independently conducted by a neurosurgeon and a neuroradiologist, blinded to the clinical outcome. The mean value of each measurement was used to enhance precision.
The intercaudate distance (ICD) and sylvian fissure ratio (SFR) as linear brain atrophy parameters. (A) Schematic figure showing the classic measurement technique for the ICD (a) and SFR [(b1 + b2)/2c]. The figure is adapted by permission from Jinsoo Rhu, the copyright holder. (B and C) Linear measurements from computed tomography images. The ICD is estimated by initial measurement of the hemi-ICD on the unaffected side and multiplication of the derived value by 2. For measurement of the SFR, the maximal width of the sylvian fissure on the unaffected side is divided by the transpineal inner table diameter.
Patient treatment
All patients were hospitalized within 3 days of symptom onset, and received acute stage treatment in a neurosurgical intensive care unit. A CT scan was obtained for all patients upon admission. A second scan was obtained after 4–8 hours, unless the neurological status deteriorated. Systolic blood pressure was maintained <140 mmHg using antihypertensive agents. Osmotic diuretics (mannitol and glycerol) were administrated to control increased ICP. Prophylactic antiepileptic drugs were not used, and any anticoagulation or antiplatelet medications were discontinued for at least 5 days from the time of admission. Based on the Glasgow Coma Scale (GCS) score, hematoma volume, mass effect, and presence of herniation, surgical treatment involving stereotactic catheter insertion or hematoma evacuation with craniotomy was considered. All patients underwent a comprehensive rehabilitation program during or after hospitalization.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 (SPSS, Chicago, Illinois). Student’s t-test or Mann–Whitney U test was used to compare continuous variables, and chi-square or Fisher’s exact test was used to compare categorical variables. Continuous variables were expressed as the mean ± standard deviation or median (interquartile ranges), while discrete variables were expressed as a count with percentage. Pearson’s correlation analysis was used to investigate the correlation between two continuous variables. To identify independent predictors of the functional outcome, backward logistic regression analysis was performed. Variables were considered for multivariate analysis only if they exhibited a P-value <0.2 in univariate analysis. A P-value <0.05 was considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Results
Overall clinical outcome and group comparisons
Of the 124 patients (36 women; 55.7 ± 11.8 years) with moderate-volume basal ganglia hemorrhage, 74 (59.7%) showed favorable functional outcomes based on the GOS score at 90 days. Table 1 shows the baseline characteristics of the patients according to functional outcome. Brain atrophy parameters were significantly greater for the favorable group than for the unfavorable group. However, due to severe brain edema affecting the contralateral hemisphere, ICD and SFR measurements were not obtained for 7 and 3 patients, respectively.
Prognostic factors for favorable outcome
The results of the logistic regression analysis are summarized in Table 2. In multivariate analysis, GCS score at admission (odds ratio [OR], 1.311; 95% confidence interval [CI], 1.019–1.686), hematoma volume (OR, 0.941; 95% CI, 0.888–0.997), the ICD (OR, 1.207; 95% CI, 1.004–1.451), and the SFR (OR, 1.046; 95% CI, 1.007–1.086, per 0.001) values exhibited significant and independent correlations with a favorable functional outcome. Although the effect of age was not significant in univariate analysis, it was significantly associated with a favorable outcome in multivariate analysis (OR, 0.869; 95% CI, 0.812–0.931).
Brain atrophy and clinical features
The ICD was applied to estimate the influence of brain atrophy on clinical features. With the median ICD value being used as a fiducial point, all patients (except 7 with missing ICD data) were dichotomized into two groups (the atrophy group, with an ICD ≥13.22 mm; and the non-atrophy group, with an ICD <13.22 mm). Clinical and radiographic features were compared between the two groups (Fig. 2 and Supplementary Table S1).
Clinical and radiological features of the atrophy and non-atrophy groups. (A) Scatterplot showing an increase in the intercaudate distance (ICD) with advancing age. The dotted line represents the Loess (locally weighted scatterplot smoothing) curve, and the shaded bands around the curve indicate 95% confidence limits. (B) Relationship between the hematoma volume and midline shift. (C) Distribution of the Glasgow Coma Scale (GCS) scores at admission for the two groups. (D) Treatment modalities for the two groups.
The mean age was significantly different between the atrophy and non-atrophy groups (60.5 ± 12.1 vs. 50.7 ± 10.1 years; P < 0.001), and the ICD increased with advancing age (Fig. 2A, correlation coefficient = 0.432; P < 0.001). Figure 2B presents the relationship between hematoma volume and midline shift. The mean midline shift was significantly smaller in the atrophy group than in the non-atrophy group (3.3 ± 2.2 vs. 6.1 ± 3.1 mm; P < 0.001). Additionally, there was no apparent correlation between the increase in midline shift and the hematoma volume in the atrophy group (correlation coefficient = 0.190; P = 0.150), whereas the midline shift was significantly augmented by the hematoma volume in the non-atrophy group (correlation coefficient = 0.631; P < 0.001). Figure 2C and D show the distribution of initial GCS scores and the treatment modalities used for the two groups. The admission GCS score was higher in the atrophy group than in the non-atrophy group (median, 14, interquartile range [IQR], 13–14 vs. 11.5, 9–13; P < 0.001). Moreover, the proportion of patients with a GCS score ≥13 was higher in the atrophy group than in the non-atrophy group (78.0 vs. 34.5%; P < 0.001), and patients in the former group exhibited a greater tendency of receiving nonsurgical treatment compared with those in the latter (44.1 vs. 13.8%; P < 0.001).
Discussion
This study was performed to assess the contribution of advanced brain atrophy to favorable functional outcome in patients with moderate-volume basal ganglia hemorrhage. Several studies have found that brain atrophy prevents malignant outcomes by reducing the mass effect in patients with large cerebral infarctions9,10,11,12. Furthering these findings, the protective effect of brain atrophy against space-occupying lesions has been demonstrated in this study. Both the ICD and SFR were significantly correlated with a favorable outcome. This indicates that the relative increase in the intracranial volume reserve, caused by brain atrophy, may prevent an increase in ICP and subsequent brain herniation, thereby protecting against cerebral edema.
Conversely, Herweh et al. suggested cerebral atrophy as an independent risk factor for poor outcome following spontaneous ICH and reported that preexisting neurodegenerative changes may result in an unfavorable outcome in such cases13. This apparent discrepancy in results can be attributed to the difference in study design, in terms of patient inclusion. Herweh et al. included hemorrhages in any supratentorial region that exhibited a wider volumetric range and smaller mean volume (mean, 12.8; range, 0.3–153.4 mL) compared with those included in this study13. Since local mass effects have different impacts depending on their locations, swelling in the basal ganglia is more strongly associated with a poor outcome compared with swelling at other locations9. Consequently, superficially located smaller hematomas are considered to result in a less prominent mass effect compared with the lesions included in this study.
Previously identified prognostic factors (age, hematoma volume, and admission GCS score) are also well-reflected in our study5,6,7,8,18,19. Interestingly, age did not exhibit significant effect in univariate analysis, although it was strongly associated with clinical outcome19. This may be due to age-related differences in brain atrophy. As brain atrophy is mostly influenced by age20,21, it seems to counteract the worsening effect of advanced age on clinical outcome. Another study investigated the prognostic factors for spontaneous ICH using inclusion criteria similar to those used in this study, and likewise showed a weak correlation between age and clinical outcome in univariate analysis (P = 0.282)15.
The admission GCS scores and midline shift showed significant differences between the atrophy and non-atrophy groups. The initial GCS score is a powerful and independent prognostic factor for clinical outcome22. The GCS scores and proportion of patients with a GCS score ≥13 were significantly higher in the atrophy group than in the non-atrophy group. Midline shift resulting from brain edema is a major determinant of cerebral herniation and a malignant outcome23,24. In this study, the midline shift was smaller in the atrophy group than in the non-atrophy group. Moreover, the increase in midline shift, in proportion to the hemorrhage volume, was smaller in the atrophy group. Therefore, preexisting brain atrophy may provide protection against an unfavorable outcome by decreasing ICP and preventing midline shift in the acute phase, despite possible neurodegeneration unrelated to injury. In the atrophy group, however, involvement of the dominant hemisphere was less frequent (33.9% vs. 55.2%; P = 0.003) and hematoma volumes were relatively small (median, 29.5, IQR, 23.4–36.8 vs. 35.9, 24.9–44.4 mL; P = 0.141) compared with those in the non-atrophy group. This may have influenced the disparity in other variables between the two groups.
In the present study, only patients with a hematoma volume of 20–50 mL were included. While the treatment and prognosis of moderate-volume hemorrhage have the greatest uncertainty4, a hematoma volume <20 mL manifests in little mass effect, and bleeding >50 mL correlates with high mortality, even with adequate treatment18. Hence, the compensatory effect of brain atrophy would not be obvious in either of these groups. Additionally, patients with hemorrhage extending to the ventricles were excluded because this may influence the outflow of cerebrospinal fluid (CSF) and measurement of the ICD.
We used the ICD and SFR as linear brain atrophy parameters. However, in some patients, hematoma obscured measurements in the contralateral hemisphere. Although 7 patients were excluded from atrophy measurement because the contralateral ventricle and/or sylvian fissure were already collapsed, mild deformation was seen in a small number. This subtle change may have caused inaccuracy in measurements. Several studies have used a semiautomatic method to perform volumetric analysis of the intracranial and CSF spaces11,13. While this process improves measurement accuracy, it requires a specialized assessment technique and is not easily applicable in clinical practice. Therefore, we used linear measurements that allow simple and reliable assessment of brain atrophy, with considerable interobserver agreement16,17.
This study has the following limitations: firstly, since the study was retrospective, selection bias cannot be excluded, and some data were unobtainable; secondly, the number of enrolled patients was relatively small, which can lower the statistical power, because inclusion was limited to patients with a precise hemorrhage size and location; thirdly, treatment modalities were varied; this heterogeneity may have affected the results; finally, as mentioned earlier, the measurement of brain atrophy may not be precise in some patients due to severe brain edema affecting the contralateral hemisphere.
In conclusion, preexisting brain atrophy exhibits a protective effect in patients with basal ganglia hemorrhage. This suggests that brain atrophy, along with age, hematoma volume, and initial neurological state, may provide prognostic information regarding the functional outcome following moderate-volume basal ganglia hemorrhage.
References
Qureshi, A. I., Mendelow, A. D. & Hanley, D. F. Intracerebral haemorrhage. Lancet Lond. Engl. 373, 1632–1644 (2009).
Talacchi, A., Ricci, U. M., Caramia, G. & Massimo, G. Basal ganglia haemorrhages: efficacy and limits of different surgical strategies. Br. J. Neurosurg. 25, 235–242 (2011).
Steiner, T. et al. Recommendations for the management of intracranial haemorrhage -part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc. Dis. 22, 294–316 (2006).
Zan, X. et al. Endoscopic surgery versus conservative treatment for the moderate-volume hematoma in spontaneous basal ganglia hemorrhage (ECMOH): study protocol for a randomized controlled trial. BMC Neurol. 12, 34 (2012).
Christensen, M. C., Mayer, S. & Ferran, J.-M. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 40, 1677–1682 (2009).
Daverat, P., Castel, J. P., Dartigues, J. F. & Orgogozo, J. M. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke 22, 1–6 (1991).
Delcourt, C. et al. Associations with health-related quality of life after intracerebral haemorrhage: pooled analysis of INTERACT studies. J Neurol Neurosurg Psychiatry 88, 70–75 (2017).
Lampl, Y., Gilad, R., Eshel, Y. & Sarova-Pinhas, I. Neurological and functional outcome in patients with supratentorial hemorrhages. A prospective study. Stroke 26, 2249–2253 (1995).
Beck, C. et al. A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volume. J. Neurol. 261, 1097–1103 (2014).
Lee, S. H. et al. The effect of brain atrophy on outcome after a large cerebral infarction. J. Neurol. Neurosurg. Psychiatry 81, 1316–1321 (2010).
Minnerup, J. et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke 42, 3403–3409 (2011).
Park, J. et al. Timely assessment of infarct volume and brain atrophy in acute hemispheric infarction for early surgical decompression: strict cutoff criteria with high specificity. Acta Neurochir. (Wien) 154, 79–85 (2012).
Herweh, C., Prager, E., Sykora, M. & Bendszus, M. Cerebral atrophy is an independent risk factor for unfavorable outcome after spontaneous supratentorial intracerebral hemorrhage. Stroke 44, 968–971 (2013).
Kothari, R. U. et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27, 1304–1305 (1996).
Choo, Y. S., Chung, J., Joo, J.-Y., Kim, Y. B. & Hong, C.-K. Borderline basal ganglia hemorrhage volume: patient selection for good clinical outcome after stereotactic catheter drainage. J. Neurosurg. 125, 1242–1248 (2016).
Gomori, J. M., Steiner, I., Melamed, E. & Cooper, G. The assessment of changes in brain volume using combined linear measurements. A CT-scan study. Neuroradiology 26, 21–24 (1984).
van Zagten, M., Kessels, F., Boiten, J. & Lodder, J. Interobserver agreement in the assessment of cerebral atrophy on CT using bicaudate and sylvian-fissure ratios. Neuroradiology 41, 261–264 (1999).
Broderick, J. P., Brott, T. G., Duldner, J. E., Tomsick, T. & Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24, 987–993 (1993).
Rådholm, K. et al. Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing 44, 422–427 (2015).
LeMay, M. Radiologic changes of the aging brain and skull. AJR Am. J. Roentgenol. 143, 383–389 (1984).
Matsubayashi, K., Shimada, K., Kawamoto, A. & Ozawa, T. Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke 23, 175–180 (1992).
Wang, C.-W. et al. Hematoma shape, hematoma size, Glasgow coma scale score and ICH score: which predicts the 30-day mortality better for intracerebral hematoma? PloS One 9, e102326 (2014).
Gerriets, T. et al. Sonographic monitoring of midline shift in space-occupying stroke: an early outcome predictor. Stroke 32, 442–447 (2001).
Pullicino, P. M. et al. Mass effect and death from severe acute stroke. Neurology 49, 1090–1095 (1997).
Acknowledgements
We thank Jinsoo Rhu (Samsung Medical Center, Korea) for providing medical illustration.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. S.M.K., K.-S.C., J.Y.L. collected data and analyzed images. H.-J.Y. and Y.-J.L. interpreted data, and S.M.K. performed statistical analysis. S.M.K. and K.-S.C. drafted the manuscript. Y.K., Y.-S.K., K.-H.B., H.-J.C. critically revised the manuscript for important intellectual content. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwon, S.M., Choi, KS., Yi, HJ. et al. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Sci Rep 8, 4819 (2018). https://doi.org/10.1038/s41598-018-22916-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22916-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.