Abstract
Zika virus (ZIKV) is an emerging mosquito-borne virus that can cause ZIKV congenital syndrome when a pregnant woman is infected. Sexual transmission has also been described for ZIKV, though the relationship between sexual transmission and vertical transmission has not been investigated. Here, viral dissemination to the female reproductive tract and fetuses was assessed in immunodeficient (AG129) female mice that were exposed to ZIKV by subcutaneous (s.c.) inoculation, intravaginal (ivag.) inoculation, or sexual transmission from infected male AG129 mice. Pregnant females had significantly increased ZIKV dissemination to the female reproductive tract compared to non-pregnant females when exposed by s.c. or ivag. inoculation. Sexual transmission resulted in significantly greater morbidity and mortality in females and higher ZIKV titers in the female reproductive tract than s.c. or ivag. inoculation. Ovaries from females infected sexually contained ZIKV RNA within the ovarian follicles. Furthermore, ZIKV titers were significantly higher in fetuses from dams exposed sexually compared to fetuses from dams exposed by s.c. or ivag. inoculation. These results demonstrate that sexual transmission enhances dissemination of ZIKV to the female reproductive tract and developing fetuses in a mouse model.
Similar content being viewed by others
Introduction
Zika virus (ZIKV; Flaviviridae) is a mosquito-borne virus that is also transmitted sexually and in utero. ZIKV congenital syndrome occurs in fetuses or infants from at least 5% of infected pregnant women1, and disease outcomes vary in severity but can include microcephaly and other brain abnormalities, as well as eye, muscle and joint defects2. The route of infection (mosquito bite vs. sexual transmission) of pregnant women with fetuses or infants with ZIKV congenital infection is typically unknown. Some epidemiological studies have shown an increased incidence of ZIKV in women compared to men during outbreaks3,4, which may be due to male-to-female sexual transmission. ZIKV has been cultured from the semen of infected men5,6, and ZIKV RNA can be detected in semen from 30–50% of infected men in the first month after disease onset7,8. These findings indicate that ZIKV sexual transmission has the potential to occur frequently early after infection.
Vertical transmission of ZIKV has been shown by detection of the virus in fetal tissue. ZIKV RNA was detected in the placental or fetal tissue of 12% of pregnancies with possible maternal ZIKV exposure9. There have been several reports of prolonged ZIKV RNA in the serum of infected pregnant women for more than 2 weeks post-onset of disease10,11,12 or in urine for more than 4 weeks13, suggesting that immunosuppression from pregnancy or fetal infection may prolong maternal viremia; however, viremia in pregnant and non-pregnant women have not been directly compared. In rhesus macaque studies, ZIKV RNA was detected in serum for a longer duration in pregnant females compared to non-pregnant females14,15; however, another study showed persistent ZIKV RNA in the serum of non-pregnant rhesus macaques16.
Immunodeficient mice lacking all or part of the interferon response are more susceptible to ZIKV infection than wild-type mice due to the resistance of murine STAT-2 to ZIKV antagonism17,18 and are thus used for studies of ZIKV pathogenesis. Pregnant immunodeficient mouse models of ZIKV have shown in utero transmission following intravaginal inoculation19, subcutaneous inoculation20, and sexual transmission21,22,23. These and other studies of ZIKV infection in pregnant, immunodeficient female mice have demonstrated intrauterine growth restriction, fetal demise, fetal brain infection, and decreased cranial size of infected fetuses24. Transplacental infection of the fetus has been implicated by infection of placental trophoblasts in human placentas25,26,27,28 and pregnant mice20 and monocytes from human placentas29, though other studies have shown trophoblasts from human placentas to be resistant to ZIKV infection30. Infection of the maternal decidua has also been shown in pregnant rhesus macaques and in human placental explants15,28. Thus, the route of in utero transmission is still being investigated.
In order to assess the effect of the transmission route on the dissemination of ZIKV to the female reproductive tract and developing fetuses, female AG129 mice were inoculated by three different routes: subcutaneous inoculation (s.c.), intravaginal inoculation (ivag.), and sexual exposure. Pregnancy was found to increase ZIKV dissemination specifically to the female reproductive tract when females were inoculated s.c. or ivag. Infection by sexual exposure also increased dissemination to the reproductive tract in both non-pregnant and pregnant females, and to developing fetuses in pregnant females.
Results
Sexual transmission of ZIKV leads to more rapid disease in female AG129 mice
To assess the effect of pregnancy and route of inoculation on ZIKV pathogenesis, female AG129 mice were exposed to ZIKV strain PRVABC59 by three different routes. Pregnant and non-pregnant females were inoculated by subcutaneous (s.c.) or intravaginal (ivag.) routes 3 days after mating. Additional females were inoculated by mating to an infected male (sexually), which led to pregnancy in 40% of matings. For both the pregnant and non-pregnant groups, females that were infected with ZIKV sexually experienced more rapid and more severe weight loss than females that were infected via s.c. or ivag. routes (Fig. 1A, p < 0.05 dpi 6–8, and Fig. 1B, p < 0.05 dpi 9). Additionally, the median survival time was significantly shorter for non-pregnant females infected with ZIKV sexually compared to non-pregnant females infected via s.c. or ivag. routes (Fig. 1D, p < 0.001 and p < 0.05, respectively). Although not significant, the trend was similar for pregnant females, in which females infected with ZIKV sexually had a shorter mean survival time than females infected via s.c. or ivag. routes (Fig. 1C; p = 0.49).
ZIKV disease by route of inoculation and pregnancy status. Gray symbols represent uninfected mice (n = 6 pregnant, n = 10 non-pregnant), black symbols represent mice inoculated s.c. (n = 8 pregnant, n = 13 non-pregnant), maroon symbols represent mice inoculated ivag. (n = 5 pregnant, n = 3 non-pregnant), and green symbols represent mice infected sexually (n = 4 pregnant, n = 4 non-pregnant). (A) Average weight of pregnant mice post-inoculation, represented as a percentage of initial weight. Error bars represent standard deviations. (B) Average weight of non-pregnant mice post-inoculation, represented as a percentage of initial weight. Error bars represent standard deviations. (C) Percent survival of pregnant mice post-inoculation. (C) Percent survival of non-pregnant mice post-inoculation. *p < 0.05, ***p < 0.001.
Inoculation route alters ZIKV kinetics in female AG129 mice
Viremia profiles of females infected with ZIKV were assessed by plaque assay, and viral RNA was detected by qRT-PCR. Pregnancy status did not modulate viremia profiles of ZIKV for any inoculation route (Fig. 2A,B). However, viremia was delayed for females inoculated ivag., with mean peak titers occurring 4 days later than females inoculated sexually or by the s.c. route (Fig. 2A p < 0.001 dpi 3 and Fig. 2B, p < 0.01 dpi 3). In addition, viremias were prolonged for females inoculated sexually compared to females inoculated via s.c. or ivag. routes (Fig. 2A p < 0.001 dpi 5 and Fig. 2B, p < 0.05 dpi 11).
Viremia of ZIKV by route of inoculation and pregnancy status. Average viral titer and RNA copy number in serum. Black symbols represent mice inoculated s.c. (n = 8 pregnant, n = 13 non-pregnant), maroon symbols represent mice inoculated ivag. (n = 5 pregnant, n = 3 non-pregnant), and green symbols represent mice infected sexually (n = 4 pregnant, n = 4 non-pregnant). Error bars represent standard deviations. The limit of detection is represented by a dashed gray line. (A) ZIKV viremia in pregnant mice. (B) ZIKV viremia in non-pregnant mice (C) ZIKV RNA copy numbers in pregnant mice. (D) ZIKV RNA copy numbers in non-pregnant mice. *p < 0.05, **p < 0.01, ***p < 0.001.
ZIKV RNA levels in serum reflected the patterns described above for viremia, in which the peak ZIKV RNA levels were delayed by 4 days in females inoculated by ivag. groups compared to females inoculated sexually or by the s.c. route (Fig. 2C). In addition, after infectious virus was cleared from the sera, ZIKV RNA was still detected at over 5 log10 ZIKV RNA copies/mL through the latest time points assessed for all groups. The persistent RNA in serum was found at indistinguishable levels in pregnant and non-pregnant females, indicating that prolonged ZIKV RNA in the serum was not pregnancy-dependent.
Sexual transmission of ZIKV enhances dissemination to the female reproductive tract and fetuses
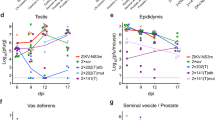
ZIKV titers were measured in maternal brain tissue, the female reproductive tract, and fetal tissues at the time of euthanasia. ZIKV titers in the brain were not significantly different between inoculation groups or by pregnancy status (Fig. 3A). For pregnant females, there were no significant differences in maternal tissue titers between any inoculation route. However, ZIKV titers in the uteri were significantly higher in non-pregnant females inoculated sexually compared to non-pregnant females inoculated s.c. or ivag. (Fig. 3B, p < 0.05) and in pregnant females compared to non-pregnant females for those inoculated s.c. (Fig. 3B, p < 0.001). Additionally, ZIKV titers in the ovaries were higher in non-pregnant females infected sexually compared to non-pregnant females infected via the s.c. route (Fig. 3C, p < 0.001) or the ivag. route (Fig. 3C, p = 0.74). The vaginal washes collected at the time of euthanasia also followed the same pattern of higher titers in non-pregnant females infected sexually compared to non-pregnant females infected via s.c. or ivag. inoculation, but titers were too low to establish significance (Fig. 3D). Thus, ZIKV disseminated to the upper and lower female reproductive tract in pregnant females irrespective of route of inoculation and in non-pregnant females only when exposed sexually.
Tissue distribution of ZIKV by route of inoculation and pregnancy status. Black symbols represent mice infected s.c. (n = 8 pregnant, n = 13 non-pregnant), maroon symbols represent mice infected ivag. (n = 5 pregnant, n = 3 non-pregnant), and green symbols represent mice infected sexually (n = 4 pregnant, n = 4 non-pregnant).Titers from individual mice are represented by circles, with means represented by solid lines. The limit of detection is represented by a dashed gray line. (A) ZIKV titer in brain tissue. (B) ZIKV titer in uterine tissue. (C) ZIKV titer in ovaries. (D) ZIKV titer in vaginal washes. (E) ZIKV titer in fetal tissue. *p < 0.05, ***p < 0.001, ****p < 0.0001.
Fetuses were collected from pregnant females at the time of euthanasia, and ZIKV titers were assessed. On average, 4.7 fetuses were collected per dam from 16 different dams. The percentage of fetuses that became infected was higher when the female was infected sexually (88%) compared to the percentage of fetuses from females infected via s.c. (50%) or ivag (53%) routes (p < 0.05). Titers were significantly higher in fetuses from females infected sexually compared to fetuses from females infected via s.c. or ivag routes. (Fig. 3E, p < 0.0001). The proportion of female mice with ZIKV-infected fetuses followed the same trend but was not significantly different between inoculation groups (100%, 63%, and 50% for sexual, s.c., and ivag. exposure routes, respectively). These data suggest ZIKV infection of fetuses occurs via both ascending and hematogenous routes.
ZIKV RNA localizes to ovarian follicles and vasculature in females infected sexually
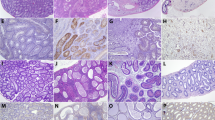
In order to further assess the route of in utero transmission and whether infection of the ovary may contribute to decreased fertility of infected females, ZIKV RNA and antigen were localized within tissues of females infected sexually using in situ hybridization (ISH) and immunohistochemistry (IHC). Tissues from females infected by the s.c. or ivag. route were not assessed. By ISH, ZIKV nucleic acid was detected within the theca interna of the perifollicular stroma and within vascular endothelium in the ovaries from 6 out of 7 infected females, and within scattered granulosa cells of the ovarian follicles from 2 out of 7 females (Fig. 4A–C). One of the two also showed ZIKV antigen in scattered granulosa cells by IHC. The two females with follicular granulosa cell staining were euthanized earliest on dpi 8, and one of the two females was pregnant. These results suggest that ZIKV infection of the ovarian follicles occurs early. Furthermore, ZIKV was detected by ISH within the uterine vasculature of both non-pregnant and pregnant females and in association with neutrophilic inflammation in the myometrium of one non-pregnant animal, and within placental sites, including trophoblasts, of pregnant females by ISH and IHC (Fig. 4D,E).These findings suggest the potential for transplacental ZIKV transmission to the fetus, as well infection from the maternal decidua.
Localization of ZIKV in ovary and uterus of AG129 mice infected by sexual transmission. ZIKV RNA localization in ovarian granulosa cells (A), thecal cells (B), and ovarian vessels (C) by ISH. ZIKV RNA localization in uterine vessels by ISH (D). ZIKV antigen localization in trophoblasts of placental sites in uterus by IHC (E). Original magnifications: 200X (A–E).
Discussion
Sexual transmission of ZIKV to female AG129 mice increased the infection rate and viral titer in developing fetuses compared to s.c. or ivag. inoculation routes (Fig. 3). In addition, dissemination of ZIKV to the female reproductive tract was enhanced for non-pregnant females following sexual transmission. While pregnancy increased ZIKV titers in the female reproductive tract following inoculation by s.c. or ivag. routes, the effect of pregnancy on viral dissemination was not evident when females were infected sexually, suggesting that an ascending infection is a more efficient route of infection in the absence of pregnancy. In this mouse model, these results indicate that sexual transmission of ZIKV alters the dissemination of the virus, leading to more frequent in utero transmission.
Intravaginal inoculation of pregnant mice and sexual transmission have previously been shown to lead to fetal infection19,21. Female macaques are also susceptible to intravaginal ZIKV inoculation31, and intravaginal ZIKV inoculation of rhesus macaques leads to altered kinetics compared to subcutaneous inoculation32, similar to the results presented here. Previous studies have shown that hormone treatment alters susceptibility to intravaginal ZIKV inoculation in macaques and mice32,33, and may thus alter dissemination of the virus in the female reproductive tract.
Prolonged ZIKV RNA in blood has been reported in a few cases of infected pregnant women10,11,12; however, the results presented here show that ZIKV RNA clearance from the blood is similar in pregnant and non-pregnant female mice and was therefore not dependent on pregnancy status in this model. The results here do show that pregnancy may increase the dissemination of ZIKV to the female reproductive tract after s.c. or ivag. inoculation, perhaps because pregnancy decreases the cellular adaptive immune response in mice infected with ZIKV34. However, we have shown here that ivag. inoculation does not accurately mimic sexual transmission, perhaps because in mice sex results in deposition of ejaculates directly or nearly directly into the uterus35,36. Components of seminal plasma or cell-associated virus, which are absent in ivag. inoculation, may also be responsible for the enhanced distribution of ZIKV after sexual transmission. The inflammation induced in the female reproductive tract by semen37,38 may impact susceptibility to viral infection, or seminal amyloids such as semen-derived enhancer of viral infection (SEVI), which have been shown to enhance HIV infection39, may play a role with ZIKV pathogenesis in the female reproductive tract as well. Some caveats to these results are that viral dose cannot be controlled during sexual transmission and that infection through the sexual route occurred simultaneously with pregnancy. However, previous studies demonstrated that titers of 103 PFU per ejaculate were common during the infectious period of seminal shedding in inoculated male AG129 mice21,40. Simultaneous pregnancy and infection through the sexual route could explain the failure to identify a significant enhancing effect of pregnancy on titers in the female reproductive tract after sexual transmission.
ZIKV RNA was detected within ovarian follicles of mice exposed to ZIKV sexually. Whether ZIKV infection can alter female fertility is unknown, though in male mice ZIKV testicular infection causes testicular atrophy41,42, and a study on male fertility using small numbers of human samples showed a modest, transient decrease in total sperm after ZIKV infection43. Further studies of the effect of ZIKV infection on ovulation and female fertility are warranted.
The results presented here suggest that sexual transmission may play an important role in ZIKV infection during pregnancy and the development of fetal ZIKV congenital syndrome. Sexual transmission of ZIKV may alter the tropism and/or dissemination of the virus throughout the female reproductive tract compared to mosquito-borne transmission in both pregnant and non-pregnant women. Understanding how sexual exposure could alter dissemination of ZIKV may help to explain the mechanisms of in utero ZIKV transmission to a developing fetus and provide a scientific foundation for assessing risk factors for sexual transmission to reduce congenital ZIKV infections.
Materials and Methods
Inoculation of AG129 mice
Interferon α/β and –γ receptor knockout AG129 mice were originally obtained from B&K Universal (Hull, United Kingdom) and bred in-house. Thirty-nine female AG129 mice were mated to uninfected male AG129 mice. Three days after a copulatory plug was identified, females were inoculated with 103 PFU of ZIKV isolate PRVABC59 either through the s.c. route in the footpad (n = 11) or through the ivag. route (n = 28), as described previously21. Twenty-six mice were not mated and were inoculated on a random day of their estrous cycle by the s.c. route (n = 10) or through the ivag. route (n = 16). Ten additional female AG129 mice were mated to male AG129 mice beginning 7 days after the males were inoculated s.c. with 103 PFU to maximize exposure40, until observation of a copulatory plug as evidence of mating, which took a maximum of 6 days. Mice were euthanized by isoflurane-induced deep anesthesia followed by cervical dislocation at gestational day 18 or when clinical evidence of disease was observed. Tissues, serum, and vaginal washes were collected at the time of euthanasia. Tissue samples were weighed, and an equal volume of BA-1 medium was added before homogenization using a pestle, followed by centrifugation to clarify samples. Viral titers were measured by Vero cell plaque assay21. All animal studies were conducted under approved IACUC protocols at the Centers for Disease Control and Prevention. All protocols and practices for the handling and manipulation of mice were in accordance with the guidelines of the American Veterinary Medical Association (AVMA) for humane treatment of laboratory animals.
ZIKV RNA quantification
RNA was extracted from serum samples using the MagMAX Viral RNA isolation kit (Ambion). ZIKV RNA was quantified using real-time RT-PCR primers and probe as described previously44. A standard curve was generated by in vitro transcription of a plasmid containing a fragment of ZIKV spanning nucleotides 859–1278 as described previously21. The detection limit for this assay was 2.3 log10 RNA copies/mL.
Histology, IHC, and ISH
Tissues were placed into 10% neutral buffered formalin for 3 days and then stored in 70% ethanol prior to processing. Sections were cut at 4 microns and stained with hematoxylin and eosin or by in situ hybridization (ISH) for ZIKV RNA. The ISH assay was performed using the ViewRNA ISH tissue assay (ThermoFisher) with a probe set targeting the ZIKV (+) strand RNA genome. As a negative control, tissue sections from an uninfected female mouse were stained using the ZIKV probe set. As a positive control, tissues were stained using probes against the housekeeping murine mRNAs GAPDH, PIPB, and β-actin. Tissue sections were de-paraffinized and underwent pre-treatment and protease treatment for ten minutes each. Positive staining (red punctate staining) was detected using an alkaline-phosphatase label probe. The slides were counterstained with Gill’s Hematoxylin I. The IHC assay was performed using a polymer-based indirect immunoalkaline phosphatase detection system with colorimetric detection of antibody/polymer complex with Fast Red chromogen. The primary antibody was a rabbit polyclonal antibody generated against ZIKV VLPs21. Negative controls for IHC comprised both uninfected tissues incubated with the primary antibody, and infected tissues incubated with normal rabbit serum in place of the primary antibody.
Statistical analyses
ZIKV RNA, viremia, and weights were compared using t-tests with a correction for multiple comparisons. ZIKV titers in tissues were compared using ANOVA with a correction for multiple comparisons. Proportions were compared using Fisher’s exact test. Survival curves were compared using a logrank test. All tests were performed in Prism7.
References
Shapiro-Mendoza, C. K. et al. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 66, 615–621, https://doi.org/10.15585/mmwr.mm6623e1 (2017).
Costello, A. et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ 94, 406–406 A, https://doi.org/10.2471/BLT.16.176990 (2016).
Duffy, M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360, 2536–2543, https://doi.org/10.1056/NEJMoa0805715 (2009).
Coelho, F. C. et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis 51, 128–132, https://doi.org/10.1016/j.ijid.2016.08.023 (2016).
Musso, D. et al. Potential sexual transmission of Zika virus. Emerg Infect Dis 21, 359–361, https://doi.org/10.3201/eid2102.141363 (2015).
D’Ortenzio, E. et al. Evidence of Sexual Transmission of Zika Virus. N Engl J Med 374, 2195–2198, https://doi.org/10.1056/NEJMc1604449 (2016).
Paz-Bailey, G. et al. Persistence of Zika Virus in Body Fluids - Preliminary Report. N Engl J Med. https://doi.org/10.1056/NEJMoa1613108 (2017).
Musso, D. et al. Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin Microbiol Infect, https://doi.org/10.1016/j.cmi.2017.07.006 (2017).
Reagan-Steiner, S. et al. Evaluation of Placental and Fetal Tissue Specimens for Zika Virus Infection - 50 States and District of Columbia, January-December, 2016. MMWR Morb Mortal Wkly Rep 66, 636–643, https://doi.org/10.15585/mmwr.mm6624a3 (2017).
Suy, A. et al. Prolonged Zika Virus Viremia during Pregnancy. N Engl J Med 375, 2611–2613, https://doi.org/10.1056/NEJMc1607580 (2016).
Driggers, R. W. et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med 374, 2142–2151, https://doi.org/10.1056/NEJMoa1601824 (2016).
Meaney-Delman, D. et al. Prolonged Detection of Zika Virus RNA in Pregnant Women. Obstet Gynecol, https://doi.org/10.1097/AOG.0000000000001625 (2016).
Terzian, A. C. B. et al. Long-Term Viruria in Zika Virus-Infected Pregnant Women, Brazil, 2016. Emerg Infect Dis 23, 1891–1893, https://doi.org/10.3201/eid2311.170078 (2017).
Dudley, D. M. et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7, 12204, https://doi.org/10.1038/ncomms12204 (2016).
Nguyen, S. M. et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13, e1006378, https://doi.org/10.1371/journal.ppat.1006378 (2017).
Hirsch, A. J. et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 13, e1006219, https://doi.org/10.1371/journal.ppat.1006219 (2017).
Grant, A. et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 19, 882–890, https://doi.org/10.1016/j.chom.2016.05.009 (2016).
Kumar, A. et al. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep 17, 1766–1775, https://doi.org/10.15252/embr.201642627 (2016).
Yockey, L. J. et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 166, 1247–1256 e1244, https://doi.org/10.1016/j.cell.2016.08.004 (2016).
Miner, J. J. et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 165, 1081–1091, https://doi.org/10.1016/j.cell.2016.05.008 (2016).
Duggal, N. K. et al. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep 18, 1751–1760, https://doi.org/10.1016/j.celrep.2017.01.056 (2017).
Uraki, R. et al. Fetal Growth Restriction Caused by Sexual Transmission of Zika Virus in Mice. J Infect Dis 215, 1720–1724, https://doi.org/10.1093/infdis/jix204 (2017).
Winkler, C. W. et al. Sexual and Vertical Transmission of Zika Virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep 7, 7176, https://doi.org/10.1038/s41598-017-07099-7 (2017).
Jagger, B. W. et al. Gestational Stage and IFN-lambda Signaling Regulate ZIKV Infection in utero. Cell Host Microbe 22, 366–376 e363, https://doi.org/10.1016/j.chom.2017.08.012 (2017).
Aagaard, K. M. et al. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci Rep 7, 41389, https://doi.org/10.1038/srep41389 (2017).
Sheridan, M. A. et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci USA 114, E1587–E1596, https://doi.org/10.1073/pnas.1616097114 (2017).
Tabata, T. et al. Zika Virus Replicates in Proliferating Cells in Explants from First-trimester Human Placentas, Potential Sites for Dissemination of Infection. J Infect Dis, https://doi.org/10.1093/infdis/jix552 (2017).
Tabata, T. et al. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 20, 155–166, https://doi.org/10.1016/j.chom.2016.07.002 (2016).
Quicke, K. M. et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 20, 83–90, https://doi.org/10.1016/j.chom.2016.05.015 (2016).
Bayer, A. et al. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 19, 705–712, https://doi.org/10.1016/j.chom.2016.03.008 (2016).
Haddow, A. D. et al. High Infection Rates for Adult Macaques after Intravaginal or Intrarectal Inoculation with Zika Virus. Emerg Infect Dis 23, 1274–1281, https://doi.org/10.3201/eid2308.170036 (2017).
Carroll, T. et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog 13, e1006537, https://doi.org/10.1371/journal.ppat.1006537 (2017).
Tang, W. W. et al. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep 17, 3091–3098, https://doi.org/10.1016/j.celrep.2016.11.070 (2016).
Winkler, C. W. et al. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol 198, 3526–3535, https://doi.org/10.4049/jimmunol.1601949 (2017).
Bedford, J. M. & Yanagimachi, R. Initiation of sperm motility after mating in the rat and hamster. J Androl 13, 444–449 (1992).
Kawano, N. et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci USA 111, 4145–4150, https://doi.org/10.1073/pnas.1320715111 (2014).
Sharkey, D. J., Macpherson, A. M., Tremellen, K. P. & Robertson, S. A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 13, 491–501, https://doi.org/10.1093/molehr/gam028 (2007).
Sharkey, D. J., Tremellen, K. P., Jasper, M. J., Gemzell-Danielsson, K. & Robertson, S. A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 188, 2445–2454, https://doi.org/10.4049/jimmunol.1102736 (2012).
Munch, J. et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131, 1059–1071, https://doi.org/10.1016/j.cell.2007.10.014 (2007).
McDonald, E. M., Duggal, N. K. & Brault, A. C. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis 11, e0005990, https://doi.org/10.1371/journal.pntd.0005990 (2017).
Govero, J. et al. Zika virus infection damages the testes in mice. Nature, https://doi.org/10.1038/nature20556 (2016).
Ma, W. et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 167, 1511–1524 e1510, https://doi.org/10.1016/j.cell.2016.11.016 (2016).
Joguet, G. et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis, https://doi.org/10.1016/S1473-3099(17)30444-9 (2017).
Lanciotti, R. S. et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14, 1232–1239, https://doi.org/10.3201/eid1408.080287 (2008).
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We thank DVBD staff members Jason Velez for cell culture support and Sean Masters for animal husbandry and animal care needs throughout this study. We thank Heather Hayes for histology and immunohistochemistry support. This research was made possible through support provided by the Office of Infectious Disease, Bureau for Global Health, U.S. Agency for International Development, under the terms of an Interagency Agreement with CDC. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development.
Author information
Authors and Affiliations
Contributions
N.K.D., E.M.M., J.M.M., and A.C.B. performed the experiments. N.K.D. wrote the manuscript and prepared Figures 1–3. J.M.M. prepared Figure 4. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duggal, N.K., McDonald, E.M., Ritter, J.M. et al. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep 8, 4510 (2018). https://doi.org/10.1038/s41598-018-22840-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22840-6
This article is cited by
-
Low expression of RNA sensors impacts Zika virus infection in the lower female reproductive tract
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.