Abstract
Phyllodes tumor (PT) of the breast is a rare but clinically important fibroepithelial tumor with potential risks of recurrence and metastasis. Recent studies identified recurrent TERT promoter mutations in PTs. However, the clinical significance of this alteration has not been fully examined. Two hundred and seven PTs from two intuitions were included. All cases were subjected to immunohistochemical analysis for TERT expression. Analysis of TERT promoter mutations was further performed by Sanger sequencing targeting the hotspot mutation region on cases from one of the involved institutions. The expression of TERT was correlated with clinicopathologic features, mutation status and recurrence. There was an association of TERT expression and its promoter mutation. Both stromal TERT expression and its promoter mutation correlated with PT grading and older patient age. Recurrence free survival (RFS) of PT patients with high stromal TERT expression was shorter if the excision margin was positive. Our findings suggested a possible pathogenic role of TERT alteration in PT malignancy. Currently there is no consensus for re-excision for PT patients with positive surgical margin, particularly for low grade cases. Stromal TERT expression could be potentially useful to guide management patients with benign PTs.
Similar content being viewed by others
Introduction
Phyllodes tumor (PT) of the breast is a rare but clinically important fibroepithelial tumor, accounting for 0.3–0.5% of breast tumors1. Histologically, PTs are graded as benign, borderline or malignant basing on a combination of histologic criteria2. Accurate grading of PT is clinically relevant. While local recurrence of PTs may occur in all grades, metastasis is mostly limited to malignant and few borderline cases3. Treatment is mainly surgical as PTs do not response well to systemic therapy4.
Recently, studies using massive parallel sequencing identified a number of driver alterations with therapeutic relevance and alterations with diagnostic and prognostic significance in different cancers5. For PTs, high frequency of MED12 mutations affecting exon 2 was reported6,7,8. In addition, recurrent somatic mutations, albeit in lower frequency, have been described for other genes including RARA, FLNA2, SETD2 and KMT2D8. This mutational landscape was also associated with PT grading. Common somatic mutations affecting cancer associated genes, including TP53, RB1 and EGFR occur exclusively in high grade PTs6,7,8,9. In addition, frequent mutations in non-coding region of telomerase reverse transcriptase (TERT) promoter has also been described7,10,11.
Telomerase activation is a known hallmark of cancer12. The transcriptional regulation of TERT is a decisive factor for controlling telomerase activity. TERT promoter mutations are the most common non-coding mutations in cancers13. The mutations in the promoter occurred mainly at −124 and −146 bp positions from ATG start site, conferring enhanced promoter activity by putatively generating a consensus binding site (GGAA) for ETS transcription factors14,15,16. TERT reactivation/ promoter mutation has been reported to be associated with tumor aggressiveness and poor patient outcome in thyroid17 and urothelial18 cancers.
TERT promoter mutation was described recently in PT7,10,11, but its relationship with TERT expression has not been evaluated. Moreover, these studies included small number of cases with limited analysis on their clinicopathologic association. In the present study, the expression of TERT and its promoter mutation in PTs was investigated. Their potential association with each other, clinicopathologic features and patient outcome was evaluated.
Results
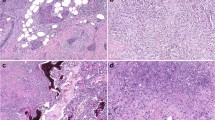
Two hundred and seven PTs were included in this cohort. Patients’ age ranged from 16 to 86 years (mean 43 years, median 44 years). Tumor size ranged from 12 to 250 mm (mean 59.6 mm, median 45 mm). Histologically, there were 134 benign (64.7%), 49 borderline (23.7%) and 24 malignant (11.6%) PTs. Post-surgical adjuvant therapy information was available in 178 patients, among them, 30 patients received radiotherapy and three were treated with chemotherapy. Details of the clinico-pathologic features were summarized in Table 1. Overall, TERT staining could be assessed in the stromal component in all 207 cases and in the epithelial component in 197 cases. The mean TERT expression scores in the stromal and epithelial components in the entire cohort were 54.9 ± 41.9 (median = 50; interquartile range (IQR) = 25–70) and 144.3 ± 61.3 (median = 150; IQR = 95–190) respectively. For benign, borderline and malignant PTs, the mean TERT scores in the stromal component were 52.1 ± 41.9 (median = 50; IQR = 18.1–70.6), 56.6 ± 40.0 (median = 52.5; IQR = 33.8–71.3) and 67.2 ± 46.4 (median = 70.0; IQR = 30.0–101.3) respectively. The cases were classified into groups of low, intermediate and high expression of roughly equal frequency for both stromal and epithelial TERT. There were 65 (31.4%), 67 (32.4%) and 75 (36.2%) cases of low, intermediate and high stromal TERT groups; and 58 (34.5%), 65 (33.0%) and 64 (32.5%) cases of low, intermediate and high epithelial TERT staining. Representative staining was shown in Fig. 1.
Clinico-pathologic correlation of stromal TERT expression
Stromal TERT expression was associated with increased patients’ age (p = 0.022), PT grade (p = 0.044), mitotic count (p = 0.004) and stromal overgrowth (p = 0.037). Higher TERT expression also showed a trend of association with infiltrative border (p = 0.086) and increased pleomorphism (p = 0.068). No significant correlation of stromal TERT was found with tumor size, increased cellularity and post-surgical adjuvant therapy (Table 1). Additionally, there were seven cases with necrosis and no association was found with TERT alterations. Due to the limited case number, a conclusive result cannot be reached. Multivariate analysis demonstrated that age (p = 0.009) and stromal overgrowth (p = 0.018) were the independent factors associated with stromal TERT expression (Supplementary Table S1).
Clinico-pathological correlation of TERT promoter mutation
One hundred and four cases from one of the involved institutions were available for TERT promoter mutation analysis. Among them, 96 cases had interpretable results from PCR and Sanger sequencing. Overall, 70 cases (72.9%) were wild type and 26 cases (27.1%) showed TERT promoter mutation. Except in one case with C > T mutation at −75 bp upstream of start codon, all other cases had hotspot C228T mutation (Fig. 2). The presence of TERT promoter mutations was related to older age (p = 0.053); but statistical significance was not reached. The mutation status was not associated with tumor size. Borderline case showed that highest mutation frequency (40%; 10/25 cases), followed by malignant (28.5%; 4/14 cases) and benign (21.1%; 12/57) tumors. For other histologic features, TERT promoter mutation was associated with stromal overgrowth (p = 0.032), but not with infiltrative border, increased mitotic count, cellularity or pleomorphism (Table 2). The mutational status correlated with stromal but not epithelial TERT expression. PTs with higher number of mutant correlated showed high stromal TERT expression compared to those with low/intermediate mutant level (p = 0.042). High stromal TERT remained independently associated with promoter mutation in multivariate analysis (Supplementary Table S2). Such association was mainly observed in borderline cases (p = 0.024), but not in benign and malignant cases (Table 2).
Correlation of TERT expression with recurrence
Among the 207 cases, 192 cases were primary PTs and outcome data were available for 181 cases. The mean follow-up period was 48 months (median = 38 months, ranged 1–193 months). There were 24 cases with relapse (five cases with distant metastases, 19 cases with local recurrences and one case with both) in which 17 recurrences occurred in the first five years (early relapse). Four cases of death recorded and all of them were preceded by metastatic events. Given the association of high stromal TERT with higher PT grading, its relationship with PT recurrence was examined. Kaplan Meier analysis demonstrated no association between high stromal TERT expression and RFS in PTs (Fig. 3). For the confounding factors, except surgical margin (p = 0.046, Fig. 3), all others factors such as age, size, grade and other histologic factors (border, mitosis, cellularity, pleomorphism, and stromal overgrowth) were not significantly associated with RFS. More intriguingly, those cases with positive margin and high stromal TERT expression demonstrated the worst RFS (Fig. 3) and recurrences from benign PTs. The combined status of high stromal TERT expression and positive margins remained to be an independent prognostic factor for RFS in multivariate analysis (Supplementary Table S3). Of note, cases with high stromal TERT had a trend of early relapse (p = 0.071) in the overall series and were significantly associated with early relapse in cases with positive surgical margin (p = 0.025) (Table 3). However, it did not reach any statistical significance in multivariate analysis for early relapse in cases with clear margin status.
Discussion
Telomerase reactivation is associated with the acquisition of immortalisation and malignancy in human somatic cells. Its reactivation has been reported in many tumors, including PTs18. Telomerase activation has been attributed to several mechanisms. Mutation within the TERT promoter region, particularly at two hotspot locations, was suggested to be a common mechanism for telomerase reactivation. In this study, we showed stromal TERT expression in PTs was associated with higher grade, increased stromal mitosis, stromal overgrowth and higher patients’ age. A similar trend was also found with TERT promoter mutation. In addition, TERT promoter mutation could lead to increased TERT expression – a higher frequency of mutation was associated with higher stromal TERT expression, when compared to those with low/intermediate frequency, particularly in borderline PTs. In contrast, there was no correlation between epithelial TERT expression and promoter mutation.
A few studies demonstrated TERT promoter mutation in PT7,10,11, however, TERT protein expression in PT has not been studied. We observed TERT promoter mutation in 27% of the cases, which was lower than some reported studies (35–65%)7,11. It is interesting to note the TERT promoter mutation pattern in the current analysis was similar to that reported for Asian population11, with a higher prevalence found in borderline cases and the presence of mutations in non-hotspot locations, compared to those from Western populations7. Given also the higher prevalence of PT in Asia, the results might possibly reflect the difference in the repertoire of somatic mutations in PTs affecting different ethnicity and differences in PT pathogenesis. The observation of a higher rate of TERT mutation in borderline but not malignant PTs may imply the role played by TERT mutation occurs early in the malignant progression19. TERT mutation status showed a significant correlation with stromal TERT expression in borderline cases supporting the potential role of promotor mutation in driving telomerase activation in those cases. In malignant PTs, there was also high stromal TERT expression even though there was a lack of correlation with TERT promoter mutation. It is possible that the acquisition of other molecular abnormalities, independent of promoter mutation, in malignant PTs could be sufficient to drive the increased TERT expression. Several factors, including cellular transcriptional factor, hormonal receptors and cytokines, are involved in TERT transcription20. Some of these factors were shown to be altered in PTs. For instance, c-Myc which was considered to be a major regulator in TERT promoter activity21, was described to be over-expressed in malignant PTs22. On the other hand, p53 which has been shown to repress TERT transcription in a Sp-1 dependent manner23, was found to be mutated in malignant PT8,9. Recently, a role of histone methylation of TERT regulation has been demonstrated24. Of interest, stromal EZH2, an epigenetic modifier, was significantly associated with PT malignancy25. Additionally, TERT gene amplification was only reported in malignant PTs7. Thus, TERT upregulation could be part of the molecular mechanism in PT malignant transformation. Chromosomal changes could be found in both epithelial and stromal components of PTs26. However, alterations between the two components appear to be distinct. Earlier studies demonstrated that microsatellite instabilities were exclusively found in epithelial components26. On the other hand, gene mutations in PT revealed by recent massively parallel sequencing analysis were harboured only in the stromal components7,8. In line, TERT promoter mutations were reported only in the stromal, not the epithelial components7,11. This finding was reflected here with the lack of correlation between its promoter mutation status and epithelial protein expression.
We also observed that stromal TERT expression and its promoter mutation was closely associated with older age. A similar association has been reported in other cancers27,28,29 and PTs11. The presence of TERT promoter mutations have been detected both in early and late stage of diseases30. However, the critical effects of TERT on malignancy may occur at a later time point when the telomeres have become critically short. In fact, recent data showed that TERT reactivation may occur at a relatively late stage in molecular carcinogenesis, secondary to the activation of other oncogenic signalling pathways such as MAP kinase signalling in melanoma31 or Wnt signalling in hepatocellular carcinoma15. With increased age, telomere shortening continued. TERT reactivation could play major roles in preventing crisis when other oncogenic events in PTs took place in aged cells for its malignant progression.
Management of PTs is challenging because of the difficulties in PT diagnosis on core needle biopsy and predicting outcome. Given the correlation of PT grading with its clinical behaviour, it would be helpful in management of patients if PT can be graded in the core needle biopsy. So far, there were no reliable markers that aid diagnosis. On basis of its association with TERT alterations, TERT analysis could be developed as a potential ancillary diagnostic tool, particularly in distinguishing between borderline and benign cases. Surgical margin status is known to impact significantly on the likelihood of PT recurrence1. This is confirmed in the current series. About one-fourth of our cases showed positive margin, which was the main significant factor associated with RFS. Interestingly, our results showed that PTs with positive margin and high stromal TERT expression had the worst RFS. It can be explained by the more aggressive nature of tumors with high TERT expression. In routine clinical practice, the management of positive margins after primary excision of PTs varies, with some authors recommending immediate surgery whereas others suggested active monitoring, particularly for low grade lesions32. These results suggest the potentials of stromal TERT expression evaluation in the decision making of PT re-excision, and warrant further investigations.
In summary, our data showed an association of TERT expression and its promoter mutation in PT of the breast. Such association mainly occurred in borderline PTs. However, due to the limited cases in subgroup analysis, further examination with a larger cohort will be required. Both stromal TERT expression and its promoter mutation correlated with PT grading and older patient age. Our findings have possible implications in the pathogenic role of TERT alteration in PT malignancy. Despite the relatively low relapse rate of PTs and limited number of cases, we found the RFS of PT patients was worst when the PT showed positive margin AND high stromal TERT expression. As currently there is no consensus for re-excision in PT patients with positive surgical margin, particularly for low grade cases, assessment of stromal TERT expression could be of potential utility to aid surgical management decision making.
Materials and Methods
PT cases were collected from the archives of the two participating institutions in Hong Kong. Four-micron slides from the formalin fixed paraffin embedded (FFPE) tissue were prepared routinely and stained with hematoxylin and eosin (H&E) stain. All the slides were reviewed first by one of the co-authors (YN or SC) followed by an experienced breast pathologist with expertise in phyllodes tumor (GT) for the following histologic parameters: 1. stromal cellularity; 2. nuclear pleomorphism; 3. stromal overgrowth; 4. mitotic rate; and 5. margin of the tumor, whether infiltrative or rounded. Stromal cellularity and nuclear pleomorphism were graded as 1, 2 and 3 representing low/mild, moderate or high/ severe respectively; stromal overgrowth was graded as absent [presence of epithelial elements within a low power field (x40, Nikon Labophot, field area 1.9 mm2)] or present (absence of epithelial element within a low power field). Mitotic count was denoted as the number of mitotic figures per 10 high power fields (hpf) (x400, Nikon Labophot, field area 0.19 mm2. The PTs were graded into benign, borderline or malignant using WHO criteria2. Patients’ age, tumor size, and outcome data were retrieved from the medical records. Recurrence free survival (RFS) was defined as the duration from the date of initial diagnosis to the first detection of relapse (including both local recurrence and metastatic recurrence). Research was approved by the Joint CUHK-NTEC Research Ethics Committee. All experiments were performed in accordance with relevant guidelines and regulations. All archival samples from pathology tissue bank were retrieved after its use for diagnosis retrospectively. The specimens were obtained in ethical manner and no potential harm will be caused to the patients. For patient confidentiality, all samples were coded by laboratory accession number and were non-identifiable. Therefore, the research could be permissible without consent.
Tissue microarray (TMA) and immunohistochemistry (IHC)
TMA blocks containing representative epithelial and stromal regions were constructed with quadruple 0.6 mm tissue cores. One section from each TMA was stained with H&E and reviewed to confirm the presence of representative PT.
Immunohistochemical staining of TERT (Alpha Diagnostic International, polyclonal, 1:100, antigen retrieval with Ventana CC1; Incubation room temperature one hour) was performed on the TMA using Optiview Universal DAB Detection Kit (Ventana, Arizona, USA) after deparaffinization, rehydration and antigen retrieval and counterstained with haematoxylin. The validity of the antibody has been reported for western blotting and immunostaining33. The sections were scored for the presence and intensity of nuclear staining in the stromal and epithelial cells separately. An immunoscore was obtained by multiplying the staining intensity (graded from 0 to 3, with 0 being no staining, 1 being weak staining, 2 being moderate staining and 3 being strong staining) and the proportion of stained cells (expressed as actual percentage). The immunoscore ranged from 0–300. TERT was classified into three equal groups according to the frequency of expression. Low stromal TERT was scored if immunoscore was ≤30, intermediate expression was scored for immunoscore 31–60 and high level for immunoscore >60. For its epithelial expression, cases with immunoscore ≤120 were classified as low expression while cases with immunoscore 121–169 and ≥170 were classified as intermediate and high expression respectively.
Extraction of DNA and sequencing of TERT promoter
Two 5 micron thick unstained slides were obtained from representative blocks of FFPE samples. Tumor areas were dissected from the section. Adjacent non-tumor tissues were removed using a sterile blade. Genomic DNA was isolated using QIAamp DNA FFPE Tissue Kit (Qiagen) according to manufacturer’s instruction. The concentration of DNA was determined using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies).
PCR was performed in 10-μl reactions containing 100 ng template DNA, 0.3 mM of each dNTP, 2.5 mM MgCl2, 0.3 μM of each primer, and 0.2 U of KAPA HiFi HotStart DNA Polymerase (Kapa Biosystems Wilmington, DE, USA), and was initiated at 95 °C for 5 min, followed by 40–45 cycles of 98 °C for 20s, 68 °C for 15s and 72 °C for 30s, and a final extension of 72 °C for 1 min. Primer pairs used to amplify the 163 bp fragment spanning the two mutational hotspots (chr5, 1 295 228 (C228T, −124 bp from ATG) and 1 295 250 (C250T, −146 bp from ATG)) in TERT promoter region were as follows: TERT-F (5′-GTCCTGCCCCTTCACCTT-3′) and reverse primer TERT-R (5′-CAGCGCTGCCTGAAACTC-3′). PCR products were subjected to electrophoresis on 1.5% agarose gel and visualised by Molecular Imager Gel Dic XR+ System with Image Lab 4.1 software (Bio-Rad). The PCR products with single band of correct size were sent to BGI-HK for sequencing. The samples were regarded as mutation positive if a mutation was detected in both forward and reverse directions, together with a manual review of chromatograms.
Statistical Analysis
Statistical analysis was performed using SPSS 23.0 software (SPSS Chicago, IL). Correlation analysis between TERT expression/mutation and clinicopathological parameters were performed using Chi-square test and Fisher’s exact test (when cell size >5). Mann-Whitney U test was used to examine the differences in age and tumor sizes between different stromal TERT expression and promoter mutation status. RFS was estimated with the Kaplan-Meier analysis and two-sides log rank test. Statistical significance was established at p < 0.05.
References
Tan, P. H. et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol 123, 529–540, https://doi.org/10.1309/U6DV-BFM8-1MLJ-C1FN (2005).
Lakhani, S. R., Ellis, I. O., Schnitt, S. J., Tan, P. H. & van de Vijver, M. J. WHO Classification of Tumors of the Breast (IARC Press, Lyon, 2012).
Tan, B. Y. et al. Phyllodes tumours of the breast: a consensus review. Histopathology 68, 5–21, https://doi.org/10.1111/his.12876 (2016).
Mishra, S. P., Tiwary, S. K., Mishra, M. & Khanna, A. K. Phyllodes tumor of breast: a review article. ISRN Surg 2013, 361469, https://doi.org/10.1155/2013/361469 (2013).
Gagan, J. & Van Allen, E. M. Next-generation sequencing to guide cancer therapy. Genome Med 7, 80, https://doi.org/10.1186/s13073-015-0203-x (2015).
Cani, A. K. et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol Cancer Res 13, 613–619, https://doi.org/10.1158/1541-7786.MCR-14-0578 (2015).
Piscuoglio, S. et al. Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and TERT promoter hotspot mutations and TERT gene amplification as likely drivers of progression. J Pathol 238, 508–518, https://doi.org/10.1002/path.4672 (2016).
Tan, J. et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet 47, 1341–1345, https://doi.org/10.1038/ng.3409 (2015).
Tsang, J. Y., Go, E. M. & Tse, G. M. Identification of clinically relevant alterations in phyllodes tumor of the breast by amplicon-based next-generation sequencing. Breast Cancer Res Treat 151, 717–719, https://doi.org/10.1007/s10549-015-3396-1 (2015).
Geyer, F. C. et al. Genetic analysis of uterine adenosarcomas and phyllodes tumors of the breast. Mol Oncol 11, 913–926, https://doi.org/10.1002/1878-0261.12049 (2017).
Yoshida, M. et al. TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br J Cancer 113, 1244–1248, https://doi.org/10.1038/bjc.2015.326 (2015).
Low, K. C. & Tergaonkar, V. Telomerase: central regulator of all of the hallmarks of cancer. Trends Biochem Sci 38, 426–434, https://doi.org/10.1016/j.tibs.2013.07.001 (2013).
Bell, R. J. et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol Cancer Res 14, 315–323, https://doi.org/10.1158/1541-7786.MCR-16-0003 (2016).
Huang, F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science 339, 957–959, https://doi.org/10.1126/science.1229259 (2013).
Nault, J. C. et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 4, 2218, https://doi.org/10.1038/ncomms3218 (2013).
Horn, S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961, https://doi.org/10.1126/science.1230062 (2013).
Kim, T. H. et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer 23, 813–823, https://doi.org/10.1530/ERC-16-0219 (2016).
Mokbel, K., Ghilchik, M., Parris, C. N. & Newbold, R. F. Telomerase activity in phyllodes tumours. Eur J Surg Oncol 25, 352–355, https://doi.org/10.1053/ejso.1999.0656 (1999).
Tan, E. Y. et al. Recurrent phyllodes tumours of the breast: pathological features and clinical implications. ANZ J Surg 76, 476–480, https://doi.org/10.1111/j.1445-2197.2006.03754.x (2006).
Kyo, S., Takakura, M., Fujiwara, T. & Inoue, M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci 99, 1528–1538, https://doi.org/10.1111/j.1349-7006.2008.00878.x (2008).
Kyo, S. et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res 28, 669–677 (2000).
Sawyer, E. J. et al. Malignant phyllodes tumours show stromal overexpression of c-myc and c-kit. J Pathol 200, 59–64, https://doi.org/10.1002/path.1318 (2003).
Kanaya, T. et al. Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin Cancer Res 6, 1239–1247 (2000).
Atkinson, S. P., Hoare, S. F., Glasspool, R. M. & Keith, W. N. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res 65, 7585–7590, https://doi.org/10.1158/0008-5472.CAN-05-1715 (2005).
Zhang, Y., Liss, A. L., Chung, E., Pierce, L. J. & Kleer, C. G. Stromal cells in phyllodes tumors of the breast are enriched for EZH2 and stem cell marker expression. Breast Cancer Res Treat 158, 21–28, https://doi.org/10.1007/s10549-016-3853-5 (2016).
Sawyer, E. J. et al. Molecular analysis of phyllodes tumors reveals distinct changes in the epithelial and stromal components. Am J Pathol 156, 1093–1098, https://doi.org/10.1016/S0002-9440(10)64977-2 (2000).
Liu, T. et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr Relat Cancer 21, 427–434, https://doi.org/10.1530/ERC-14-0016 (2014).
Killela, P. J. et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 110, 6021–6026, https://doi.org/10.1073/pnas.1303607110 (2013).
Vinagre, J. et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 4, 2185, https://doi.org/10.1038/ncomms3185 (2013).
Jafri, M. A., Ansari, S. A., Alqahtani, M. H. & Shay, J. W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med 8, 69, https://doi.org/10.1186/s13073-016-0324-x (2016).
Heidenreich, B. et al. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat Commun 5, 3401, https://doi.org/10.1038/ncomms4401 (2014).
Moo, T. A. et al. Association Between Recurrence and Re-Excision for Close and Positive Margins Versus Observation in Patients with Benign Phyllodes Tumors. Ann Surg Oncol, https://doi.org/10.1245/s10434-017-5955-7 (2017).
Hiyama, E., Hiyama, K., Yokoyama, T. & Shay, J. W. Immunohistochemical detection of telomerase (hTERT) protein in human cancer tissues and a subset of cells in normal tissues. Neoplasia 3, 17–26, https://doi.org/10.1038/sj/neo/7900134 (2001).
Acknowledgements
We thank Y. Shao for her technical support in the study.
Author information
Authors and Affiliations
Contributions
J.T. collected data, analysed data and wrote the manuscript; Y.H., M.-A.L., M.L. and Y.N. collected and assembled data; S.C., C.W. and A.K. provided the study materials and patients samples; G.T. conceived the idea for the paper, provided guidance and critically revised the paper. All authors read and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsang, J.Y.S., Hui, YK., Lee, M.A. et al. Association of clinicopathological features and prognosis of TERT alterations in phyllodes tumor of breast. Sci Rep 8, 3881 (2018). https://doi.org/10.1038/s41598-018-22232-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22232-w
This article is cited by
-
Droplet-digital PCR reveals frequent mutations in TERT promoter region in breast fibroadenomas and phyllodes tumours, irrespective of the presence of MED12 mutations
British Journal of Cancer (2021)
-
SETD2 alterations and histone H3K36 trimethylation in phyllodes tumor of breast
Breast Cancer Research and Treatment (2021)
-
Morphologic and genetic heterogeneity in breast fibroepithelial lesions—a comprehensive mapping study
Modern Pathology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.