Abstract
Nearly pure N2 fluid inclusions (Th (L) = −151~−168 °C; Th (V) = ~150.3 °C) were identified in W-mineralized quartz veins from the Yangjingou scheelite deposit, in the eastern Yanbian area, NE China. Other fluid inclusion populations include N2-CO2, NaCl-H2O ± N2 and CO2 ± N2-NaCl-H2O, but no hydrocarbons were detected. The host rocks are part of the Wudaogou Group metamorphic series, which mainly consist of Ca-rich mica schist. Subhedral sulfide minerals occur in early disseminated W-mineralized quartz veins, or have partially replaced early scheelite. ThN2 and ThN2-H2O indicate N2 fluid-trapping from 315 °C to 410 °C and from 80 MPa to 350 MPa. Oxygen and hydrogen isotopic data (δD = −74.9‰~−77‰, δ18O = 9.6‰~12‰, V-SMOW) suggest that the mineralizing fluids were composed of mixed magmatic and metamorphic water, N2-rich inclusions (δ15N = −0.5‰ to 1.4‰) indicate fluid-rock interaction with metamorphic rocks. The N2-rich fluid was closely associated with scheelite precipitation. During thermal decomposition under high oxygen fugacity conditions, which occurred synchronously with metamorphism and magmatic activity, large amounts of N2 were liberated from NH4+-micas, which then accumulated in the parent fluid of the quartz scheelite veins.
Similar content being viewed by others
Introduction
Nitrogen is the dominant constituent of the atmosphere and a key component in the biosphere. High-density CO2-N2 inclusions have been detected in high-grade metamorphic rocks from the upper mantle and lower crust (i.e., granulites and eclogites)1,2,3. In silicates, under conditions of elevated temperatures, low water activity, and high oxygen fugacity, N2 is favorably released from minerals containing ammonium (e.g., feldspar and mica)4,5.
Scheelite containing nitrogen is most commonly recognized to be traced from sedimentary rocks6. In sedimentary-type deposits, tungsten (W) is liberated as a result of exhalative processes, forming stratiform scheelite6, in which mineralizing fluids containing CH4 and N2 are generally attributed to in situ organic matter6. Several examples of scheelite deposits related to metamorphic hydrothermal fluids are reported to contain small amounts of N27,8. These types of scheelite deposits are of interest to the geologist, because they are related to Au deposits9. Furthermore, Gibert8 found that the addition of only 5% N2 would decrease the solubility of scheelite in micaschists from 40 ppm to less than 3 ppm W, indicating that N2 may drive scheelite participation.
Nearly pure N2 (>88 mol%) was identified in fluid inclusions in W-mineralized quartz veins, which is absent of hydrocarbons. Previous studies have shown that NaCl-H2O and CO2 fluid inclusions are common in ore-forming fluids; however, the presence of N2-rich fluids in ore deposits is rarely reported. This study aims to identify the origin of the W-mineralizing fluid and the formation of the N2-rich fluid inclusions by combining petrography, Raman microspectroscopy, H, O and N isotope geochemistry, and microthermometry on fluid inclusions. Results from this study will improve our understanding of how N2 modulate scheelite mineralization.
Geology of the Yangjingou scheelite deposit
The Yangjingou scheelite is the largest identified scheelite deposit in the Yanbian belt, NE China. It is located between the Cuihongshan and Sanjiazi scheelite deposits in the Heilongjiang-Jilin metallogenic belt, 8 km south of the Xiaoxinancha Au deposit, approximately 25 km east of the Russian border (Fig. 1c). In this deposit, the main economic mineral is scheelite, accompanied by minor Au, Cu, Mo, Fe, and P mineralization. The area is dominated by low-grade metamorphic rocks of the Wudaogou Group, which formed during a regional episode of low-grade epidote to low-grade amphibolite metamorphism. Regional stratigraphic correlations, metamorphic studies, and U–Pb geochronology from metamorphic detrital zircons indicate that sedimentation and regional metamorphism dated 323 ± 23 Ma10 and 266–249 Ma, respectively11. The Wudaogou Group is subdivided into the lower Madida Formation, the middle Yangjingou Formation, and the upper Xiangfangzi Formation12. The rocks from these formations are composed of muscovite, K-feldspar, amphibolite, plagioclase, quartz, and small amounts of cordierite, andalusite, rutile and kaolinite12,13.
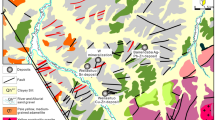
Geological map showing the main units in the Jilin and Heilongjiang metallogenic belt as well as ore deposit locations in the Yanbian area. (A) Location of the Central Asian Orogenic Belt and NE China; (B) Location of the Jilin and Heilongjiang metallogenic belt; (C) Location of the major tectonic units in the Jilin and Heilongjiang metallogenic belt (After Wu55; https://www.researchgate.net/publication/229317465_Geochronology_of_the_Phanerozoic_granitoids_in_Northeastern_China).
The Yangjingou scheelite deposit (Fig. 2) is located within a N-S-oriented asymmetrical syncline on the western limb of the Wudaogou synclinorium. The eastern limb dips 45–50°NE, and the western limb dips 55–60°NW (and locally up to 75–80°). The mine is situated in a NW-SE-oriented secondary syncline with an E-W-striking fold axis, crosscut by a N-S- to NW-SE-striking fault. The eastern limb of the synclinorium has been extensively modified by magmatic intrusions, whereas the western limb remains relatively unaltered. The scheelite ore bodies are structurally controlled by 3 secondary N-S-, NW-SE-, and NNW-SSE-striking normal faults. The N-S-striking fault is 2 km in length, dips 60–80°NW and lies parallel to the principle compressional fault in the region. The 0.8–1.0 km NW-NNW-oriented fault, which dips 40–70°SW and lies oblique to the major compressional fault and is extensively mineralized
Several generations of magmatic intrusions, including late Hercynian granodiorite (267 ± 1 Ma)14 and early Indosinian Yangjingou granodiorite (249.4 ± 2.7 Ma)15 have locally deformed rocks of the Wudaogou Group. Based on the Yangjingou granodiorite chemical signature, a transition from I- to S-type magmatism is observed, interpreted as a result of subduction16. Yanshanian intrusives, including monzonitic granodiorite (178.5–197 Ma)17 and dioritic porphyry dykes (120.73–157.27 Ma)17 were later emplaced within zones of pre-existing structural weakness. Previous studies have revealed a close association between Indosinian magmatism and W mineralization in the Yangjingou deposit16,18. Furthermore, Shan19 and Hu17 suggest that the dikes present in our study area are genetically related to an unexposed granite intrusion.
The scheelite ore bodies are mainly hosted in Ca-rich mica schists. The deposit can be subdivided into a southern and a northern ore block, separated by a N-W-trending fault (Fig. 2). In the northern ore block (Fig. 3b), mineralization is structurally controlled by normal faults striking 295–315° and dipping 65–75°. Mineralization occurs in quartz stringers and large quartz veins that have been emplaced along intraformational faults, which partially crosscut the upper Hercynian granodiorite. Individual veins are typically sub-parallel, appear as lenticular or spindle-shaped bodies, exhibit pinching and swelling, and measure approximately 10 cm in width. The ore bodies exhibit a total length of more than 900 m, a width from 60 to 425 m, and a stacking thickness of approximately 95 m.
(a) Cross section of the southern ore block in the Yangjingou scheelite deposit; (b) Cross section of the northern ore block in the Yangjingou scheelite deposit (After Shan19; http://kckc.org.cn/ch/reader/create_pdf.aspx?file_no=2010Z106&year_id=2010&quarter_id=Z1&falg=1).
Mineralization in the southern ore block is structurally controlled by normal faults striking 300–350° and dipping 60–75° (Fig. 3a). The width of individual veins ranges from 2 to 10 cm (locally reaching up to 65 cm). The total length of the ore bodies is approximately 800–1400 m, with a maximum width of 450 m. The scheelite-bearing veins in both the northern and southern ore blocks share similar characteristics in terms of their orientation and geometry.
The average W oxide (WO3) grade of the mine is 0.22% to 1.5%, reaching up to 5.25%, with accessory Au (0.49–0.79 g/t), Cu, Mo, Fe, and P19. Scheelite mineralization predominantly occurred via micro-vein dissemination and thin vein mineralization (Fig. 4a,b). The ore minerals include scheelite, pyrrhotite, pyrite, arsenopyrite, and sphalerite, while the gangue assemblage is comprised of quartz, muscovite, hornblende, andalusite, epidote, and actinolite. Sulfide minerals typically exhibit euhedral to subhedral crystal habits with granular embayments and metasomatic relict textures. Scheelite grains are subhedral (Fig. 4f) and disseminated in microfractures within quartz veins with a low sulfide content (Fig. 4c). Arsenopyrite has replaced early scheelite minerals and occurs as euhedral-subhedral disseminations in W-mineralized quartz veins (Fig. 4c). Pyrite postdates pyrrhotite and together with chalcopyrite, replaces pyrrhotite. Based on the mineral assemblages, micro-textures and associated alteration types, two stages of mineralization are distinguished: (1) the quartz-scheelite stage (oxide stage) and (2) the later pyrrhotite-arsenopyrite-pyrite stage (sulfide stage), with the quartz-scheelite stage being the major metallogenic stage. The alteration of the ore bodies mainly includes sericitization, actinolitization, albitization, carbonatation, and epidotization (Fig. 4h,I,k).
(a and b) Scheelite paragenetic quartz veins (scheelite showing blue-fluorescence under the ultraviolet irradiation); (c) arsenopyrite replaced early scheelite minerals and existed in early disseminated W-mineralizationquartz veins; (d) sphalerite and copper pyrites replacing pyrrhotite; (e) pyrrhotites replaced early scheelite; (f) subhedral scheelite; (g and h) actinolitization, epidotization; (i) muscovite quartz schist.
Analysis and Methods
Fluid inclusions
Twenty-six highly transparent 100–300-μm doubly polished thick quartz plates of paragenetic scheelite quartz veins from the southern and northern ore blocks of the Yangjingou deposit were analysed. Fluid petrography and microthermometry analyses were performed at the Geological Fluid Analysis Center of Jilin University, Changchun, China. The microthermometric analysis of fluid inclusions was conducted with a Linkam THMSG 600 stage mounted on a Carl Zeiss Axiolab microscope (10 × 50) capable of temperature measurements from −196 to 600 °C. The accuracy between 31 °C and −100 °C is better than ±0.2 °C and better than 5 °C at >300 °C. The heating/freezing rate is generally 0.2–1 °C/min but is reduced to 0.1 °C/min close to the phase transformation point. The FLUIDS20,21 software package used for the calculation of the isochors and the phase transition temperature of N2-CO2 were plotted in the diagram of Thiery22 and Van den Kerkhof & Thiery23 in order to determine vX properties of the fluid.
Raman microspectroscopy analysis
Raman analysis was performed at the Geological Fluid Analysis Center of Jilin University. The Raman instrument is a Renishaw RM-1000 and was mounted on an Olympus BX40 microscope. A 532 nm green diode-pumped solid state laser was used as an excitation source. The spectrum counting time was 30–60 s, the spectral resolution 1–2 cm−1, and the laser beam diameter 1–2 μm. A detailed description of the measurement procedure was provided by Wopenka24. The measurements reveal that the major gaseous fluid inclusion components are N2 and CO2. A large proportion of the inclusions show only a single strong band at 2328.2–2328.3 cm−1, indicating pure N2, which is the first time pure N2 inclusions have been observed in the W deposit (Fig. 5). Some of the inclusions feature peaks at 3399–3500 cm−1, indicating the presence of water. The CO2 component corresponds to the Fermi diad at 2328–2330 and 1280–1386 cm−1.
Photomicrographs of representative fluid inclusion types at room temperature in the quartz–scheelite stage. Type I: pure N2 fluid inclusions (a–c,g,u); Type II: CO2–N2 inclusions (o,m,n), occasionally solid CO2 phase below −100 °C (n,r) and three phases under the −160 °C (o); Type IIIa: inclusions containing 10%–30% gas (e,f); Type IIIb, inclusions in which the proportion of the gaseous phase ranges from 70% to 95% (d,j,i), and the liquid phase of N2 partially exists at temperatures below −150 °C (p). One phase (k) at room temperature and two phases below −160 °C (l); Type IV: NaCl–H2O–CO2 fluid inclusions (e). Representative Raman spectra of fluid inclusions related to the mineralizing fluid of the Yangjingou scheelite. Panel A shows that the vapour bubbles are mainly composed of N2; panel B shows that the bubbles contain mainly N2 and some CO2; and panel C shows that the fluid inclusions are mainly composed of N2 and H2O.
Stable isotope analysis
The oxygen and hydrogen isotopic compositions of primary fluid inclusions in the main stage (oxide stage) of the disseminated scheelite quartz veins were analysed at the analytical laboratory of Beijing Research Institute of Uranium Geology (BRIUG), China, using a MAT253-type mass spectrometer. Grains (40–60 nm) were handpicked to ensure the absence of mineral impurities. Oxygen was liberated from quartz for isotopic analyses via quantitative reaction with BrF5 using a CO2 laser as a heat source. H2O was first released by heating the samples to temperatures above 550 °C then reacted with zinc at 800 °C to produce hydrogen. The results are reported in per mil (‰) relative to the Vienna Standard Mean Ocean Water (VSMOW) standard, with precisions of ±2‰ for δD and ±0.2‰ for δ18O at the 1σ level. δ18Oquartz was measured in order to calculate the δ18OH2O values of hydrothermal fluids using the following equation:
where T represents the homogenization temperature of the fluid inclusions25.
Seven quartz samples from scheelite quartz veins were selected for N isotopic analysis. In order to exclude atmospheric interference, samples were degassed for 15 minutes under vacuum (<10−3 Pa). N2 was then directly released from fluid inclusions by heating the quartz samples in a high-purity quartz cell to temperatures above 600 °C. The released N2 was then separated using a chromatographic column cold trap26. Nitrogen isotopic compositions of the samples are reported using δ notation, where δ15Nsample (‰) = [(15N/14N) sample/(15N/14N) standard −1] ×1000. The results are reported in per mil (‰) relative to atmospheric N227, and the precision is ±1‰ at the 1σ level.
Results
Fluid inclusions
The criteria by Roedder28 for defining primary, secondary and pesudosecondary inclusions were applied. However, microtheromology measurements were conducted on primary fluid inclusions, because only primary inclusions represent W-mineralized fluid. Primary inclusions occur in isolation or as random clusters within intragranular quartz crystals28. Our study focuses on the main W-mineralizing stage, i.e., the scheelite-quartz stage. Based on composition, phase proportion at room temperature (21 °C) and phase transition during total homogenization, four types of fluid inclusions have been identified in quartz crystals from the scheelite paragenetic quartz veins: pure N2 fluid inclusions (Type I), CO2-N2 fluid inclusions (Type II), CO2 ± N2-NCl-H2O inclusions (Type III) and aqueous two-phase VL inclusions (Type IV; Table 1).
The Type I (pure N2) fluid inclusions (Fig. 5a,b,c,g,u) occur as a single phase at room temperature and are 6–30 μm in size. The inclusions are mostly oval in shape or exhibit a negative crystal shape and are randomly distributed in quartz grains. Type I inclusions are identified as N2 homogenizes to liquid or gas (L + V- > L(G)) and are closely related to scheelite deposits, but have been rarely reported. At low temperatures, bubbles nucleate at −162 °C~−182 °C. The final homogenization of the liquid phase Th(L) occurs between −151 °C~−168 °C, although a few Type I fluid inclusions homogenize to the gas phase at −150.3 °C (Fig. 6a,c). Raman analysis of the Type I inclusions yields compositions of >88 mol% N2, with minor amounts of CO2 exhibiting molar volumes of 42–150 cm3/mol22,23 (Fig. 6c). No other gases were identified.
(a) The homogenization temperature of the fluid inclusions (Type I, II, III and IV). (b) Salinity of type IIIa and IV fluid inclusions. (c) The P–T conditions of the fluid inclusions in the scheelite quartz veins in the Yangjingou scheelite deposit (molar volums between 30 and 500 cm3/mole) (After Kerkhof amd Thiéry23 and Winter52). Numbers denote molar volumes in cm3/mole; numbers in brackets are the homogenization temperature of N2.
The Type II (CO2–N2) inclusions (Fig. 5m,s) are 5–25 μm in size, and most are between 8–15 μm. These inclusions exhibit euhedral and negative crystal habits and are randomly distributed in the quartz grains. During cooling, the inclusions show bubble and solid CO2 nucleation. The phase transitions on warming follow one of two sequences: S+L+G->S+L(G)>S+L+G->L+G->L(G) (H-type, i.e. Th > Tm), showing homogenization as the final phase transition or S+L+G->S+L(G)->L(G) (S-type, i.e. Th < Tm) showing sublimation as the final phase transition29,30. Bubbles of nitrogen nucleate between −178 and −159 °C (Fig. 5o); solid CO2 is infrequently observed below −100 °C (Fig. 5n,r). In S-type inclusions the partial homogenization (ThN2) to a liquid (gas) of is between −163.2 and −147.5 °C. Critical homogenization can be observed around −147 °C and the total homogenization (ThCO2) is characterized by sublimation to liquid (gas) phase from −86~−69 °C. The ThN2 of H-type inclusions to the liquid (gas) phase coincided with critical homogenization and occurred between −149 and −148 °C. The total homogenization (ThCO2) to liquid (gas) varied from −60.8 to −45.6 °C with the TmCO2 between −60.9 and −60.7 °C. Raman spectroscopy shows that the inclusions are composed of 42–79 mol% N2, and 21–58 mol% CO2 with molar volumes between 30 and 65 cm3/mole, in agreement with microthermometry results (Figs 6a and 7).
vX diagrams calculated for the CO2-N2 system of the Yangjingou deposit showing low molar volumes (v < 100 cm3/mole). The thick red line G (SL) defines the difference between S-and H-typeII inclusions. Inclusions plotting below the L = G homogenize to gas, above the critical curve to liquid. Thin read solid line = homogenization (ThCO2). Dashed dotted red line = partial homogenization (ThN2). Thin black line = initial melting of CO2 (TmCO2) (After Kerkhof and Thiéry23; Thiéry et al.22).
The Type III (NaCl–H2O) inclusions contain liquid and gas at room temperature and have been subdivided into Type IIIa and Type IIIb populations. Type IIIa inclusions contain gas volume fractions of <50%, where most are between 10 and 30% (Fig. 5f). N2 less than 10% were infrequently detected in these inclusions. Fluid inclusions are generally 4–16 μm in size, clustering between approximately 6 and 10 μm and appear as elongated and spindle shapes, which are randomly distributed in quartz grain. These inclusions yield Tmice of −6.7 °C to −3.5 °C and homogenize to liquid between 250 and 400 °C, predominantly between 300 and 375 °C (Fig. 6a). Therefore, the salinity of these inclusions is 6–10 equiv. wt% NaCl20 (Fig. 6b). In Type IIIb inclusions, the gas volume fractions are >50%, where most are between 70–95% (Fig. 5d,j,i). The total homogenization temperature to the gas phase occurs between 314 and 409 °C, predominantly between 334 and 381 °C (Fig. 6a). The liquid phase of N2 can be locally observed at temperatures below −150 °C (Fig. 5p).
The Type IV (NaCl–H2O–CO2 ± N2) inclusions contain CO2 gas, CO2 liquid, and a saline solution at room temperature (Fig. 5e). The volume fraction of CO2 bubbles in the inclusions exceed 40%, with the majority of the inclusions featuring volume fractions of 50–80%. The gas phase comprises 75–90% of this volume fraction. These primary inclusions show euhedral and irregular polygonal shapes. They occur in randomly distributed clusters in quartz grains and individual inclusions are small, measuring 4–20 μm. Solid CO2 melts at −60.8~−58.1 °C, indicate the presence of minor N2. CO2 clathrate melting occurs at 5.3–8.1 °C and the salinity of the inclusions ranges from 4 to 9 equiv. wt% NaCl (Fig. 6b). Partial homogenization of CO2 to liquid occurs at 9.8–20.5 °C, and total homogenization to liquid occurs at 283–423 °C, predominantly at 320–402 °C (Fig. 6a).
Stable isotopes
The δD and δ18O values of the W-mineralized quartz veins exhibit relatively narrow ranges (−74.9~−77‰ and 9.6~12‰, respectively; Table 2). Wood and Samson31 demonstrated that scheelite precipitation in W deposits hosted by siliceous rocks occurs at temperatures of 200–500 °C. Consequently, the mode homogenization temperature of 375 °C in the Type IIIb fluid inclusions was chosen to calculate the δ18O value of the water in the ore-forming fluid. In the δ18O–δD diagram, the calculated δ18OH2O values (4.96−7.36‰; Fig. 8a) fall within or close to the fields of metamorphic water and primary magmatic water. The δ15N values of the N2 extracted from the fluid inclusions vary from −0.5‰ to 1.4‰ (Table 2; Fig. 8b). In the δ15N graph, all the samples from the W-mineralized quartz veins fall in the range characterized by sub-greenschist-facies metamorphism.
(a) Plot of δD values versus δ18O values for the ore-forming fluids related to the Yangjingou scheelite deposit (modified after Taylor56). The data are from the following sources: Naozhi Au deposit (Huang et al.41), Xiaoxinancha Au deposit (Wang42), Baishilazi W deposit (Zhao16) in Yanbian area and Nyakabingo W deposit, central Africa (Dewaele et al.7). (b) Nitrogen isotopic compositions of the fluid inclusions in the Yangjingou scheelite deposit and (After Bebout et al.27).
Discussion
Nitrogen in ore-forming fluids
The presence of N in W-bearing mineralizing fluids has previously been reported7,8,32; however, fluids with >50 mol% N2 are rare. Lin33 assumed that the N2-rich fluid inclusions identified at the Dongchuan Cu deposit resulted from either mantle devolatization during the break-up of Rodinia or the trapping of gas derived from decomposing organic materials. In contrast, the Yangjingou deposit formed in a compressional environment during the closure of the Paleo-Asian Ocean. There is no evidence for mantle degassing during regional orogenic activity in this area. Therefore, the presence of N2 in the deposit may be associated with plate collision, ocean closure, regional Barrovian metamorphism, and eclogite-facies metapelites34,35.
Under alkaline conditions, W is transported in the form of H2WO4, M2WO4 (M = Na, K), and WO4−2 complexes in W-mineralizing fluid. The presence of NH4+ in the solution stabilizes the W polyacid, thereby increasing its compatibility in migrating fluids36. The stability of NH4+ (substituting for K+) widely depends on the metamorphic and oxygen fugacity conditions27,37. Nevertheless, under metamorphic conditions, NH4+-bearing minerals may release N2 (350–600 °C) or NH3 (650–700 °C) at higher oxygen fugacity and concentrate in inclusions. The following equation illustrates the production of N2 under oxidizing conditions38:
The main cause for scheelite precipitation is an increase in the activity of Ca2+, which results in the chemical equilibration of the mineralizing fluid with the host rock and has been recorded at a range of pressures and temperatures32. The addition of the non-polar volatile N2 via water–rock interactions in mica may decrease scheelite solubility in common metamorphic assemblages. N2 has been demonstrated to dominate changes in the distribution of W aqueous species and increase coefficient activity8. In other words, an increase in the N2 content in the mineralizing fluid may promote scheelite precipitation.
N2-rich fluids in the Yangjingou scheelite deposit
Nature of the ore-forming fluids
The ore must have precipitated from H2O-CO2-N2-NaCl-bearing fluids with low to moderate salinity at moderate to high temperatures. The only volatile components in this fluid are CO2 and N2; hydrocarbons (i.e., CH4) have not been detected. From the vx plot (Figs 6, 7c) we can observe that the fluid inclusions richer in CO2 (30–65 cm3/mol) have lower molar volumes than those richer in N2(42-150 cm3/mol). This has been observed in many metamorphic areas3,29 indicating that CO2 and N2 may not have the same origin38. The homogenization temperatures of N2 (ThN2) and H2O ± N2-NaCl (ThH2O) constrain the pressure-temperature (P–T) conditions of trapping to 315–410 °C at 80–350 MPa (Fig. 6c), suggesting that the W mineralization occurred during late stage greenschist-facies metamorphism at temperature of ~300–600 °C39.
Stable isotopes
The stable isotopic composition of a vein provides direct information regarding the transport processes during vein formation25. Several sources have been suggested for mineralizing fluids in hydrothermal systems. These include magmatic origin, in which fluids evolve in relatively closed systems. Such mineralizing fluids are only observed in association with vein-type wolframite deposits40. Several models that result in mineral precipitation from magmatic fluids of mixed origin are as follows (Fig. 8a): 1) a dominantly magmatic fluid mixing with meteoric waters (e.g., the Xiaoxinancha porphyry Cu–Au deposit, Naozhi alteration Au deposit and Bashilazi skarn-type scheelite deposits)16,41,42 and 2) the interaction of magmatic fluids with NH4+-rich host rocks, and/or mixing with metamorphic fluids in equilibrium with metasedimentary lithologies (e.g., the vein-type Nyakabingo W deposit)7.
Low δ18O and δD of the Yangjingou scheelite deposit indicate that the magmatic component of the fluid played a more dominant role in this deposit compared to the Nyakabingo deposit, in which the mineralizing fluid was metamorphic (Fig. 8a). Earlier C and S isotopic studies yielded δ13CV-PDB values of −3.4‰ to −6.5‰ for CO2 and δ34SV-CDT values of −0.4‰ to 4.7‰ for pyrrhotite, pyrite, and arsenopyrite in scheelite-mineralized veins15, consistent with mantle values (δ13CV-PDB = −5‰ and δ34SV-CDT = ±5‰), demonstrating that the fluid was derived from the mantle. However, compared with magmatic hydrothermal deposits in this area (Fig. 8a), the Yangjingou scheelite deposit shows different δD values which may be interpreted in one of two ways: 1) the original mineralizing magmatic hydrothermal fluid interacted with NH4+-rich metamorphic minerals and/or mixed with metamorphic fluids during host rock equilibration or 2) the mineralizing fluid of magmatic origin, mixed with low-δD meteoric waters. Because W-mineralized in the presence of Ca-mica schist host rock coupled with N2-rich fluid inclusions in the Yangjingou deposit, the first explanation is preferred.
The N isotopic system may potentially provide a detailed record of fluid-rock interaction characteristics and other mixing processes in the crust and mantle37. N2 has been shown to be released during metamorphism and the breakdown of NH4+-bearing minerals, such as biotite, cordierite, and white mica43. During metamorphism, isotopically “light” N is preferentially fractionated into metamorphic fluids39,44,45,46, and the δ15N values of fluid inclusions in quartz veins in low-grade metamorphic rocks yield values ranging from −3 to 5‰31. Similarly, the W-mineralized quartz veins in the Yangjingou deposit feature low δ15N values (Fig. 8b), which may be attributed to fluid–rock interaction and the continuous liberation of N2 in a metamorphic environment. The “light” N is preferentially fractioned into the fluid and is then trapped in the fluid inclusions.
The origin of the Yangjingou N2-rich fluid
The Wudaogou Group host rock was deposited at 323 ± 23 Ma10. From 269-228 Ma, the tectonic regime of the region was dominated by the subduction of the Paleo-Asian oceanic plate beneath the North China plate47. Regional greenschist- to epidote-amphibolite-facies metamorphism occurred between 269 ± 4 Ma and 249 ± 4 Ma13. The age of muscovite crystallization in the W-mineralized quartz veins is 230.79 ± 1.19 Ma16, indicating that W mineralization occurred in the earliest Late Triassic and during late-stage Wudaogou Group metamorphism. Therefore, the fluid system containing N2 ± CO2–H2O–NaCl was intimately associated with complex multiphase tectonics and magmatic activity.
There are three mechanisms by which N2 ± CO2 may have entered the mineralizing system: 1) trapping of a volatile phase separated from its parent H2O–NaCl–CO2–N2-rich fluid, 2) mixing of a magmatic–hydrothermal CO2-H2O-rich fluid with N2 released via the thermal decomposition of NH4+-bearing minerals, or 3) CO2–N2 degassing of the mantle.
The stable isotope data in this study indicate that the mineralizing fluid was of a mixed magmatic and metamorphic origin. Magmatic melt inclusion studies have shown that CO2 is released during magmatic differentiation48,49, producing a distinct δ13C signature17. Meanwhile, the Wudaogou Group contributed Ca2+ to the system, facilitating the precipitation of Ca(WO)4. Within this metamorphic environment, N occurs as NH4+, which substitutes for K+ in detrital phases such as K-feldspar, authigenic clays and illite. With increasing pressure and temperature, both feldspar and clays participate in low-grade metamorphic reactions, and illite gradually transforms into micas50. During this transformation, a large amount of carbon is released in the form of CO2 due to decarbonization during progressive metamorphism. Moreover, the reported content of CO2 in schist (<0.25%) is notably lower than that in clay and shale (2.5–5%)36. Thus, the contribution of organic matter to the mineralizing fluid was negligible. No CH4 was identified in the W-mineralizing fluid, which may be due to the high oxygen fugacity51. Subsequently, Ca2+ from the Ca-rich mica schist country rocks was incorporated into the mineralizing fluid. The P–T conditions of the Yangjingou deposit (315–410 °C at 110–350 MPa) are lower than greenschist-facies P–T conditions (350–550 °C at 150–1100 MPa)52, indicating that scheelite mineralization likely occurred in the later stages of Wudaogou Group metamorphism. Additionally, the δ15N values of the W-mineralized quartz veins show that water–rock interaction occurred under sub-greenschist-facies conditions. However, minor mantle N2 participation cannot be completely ruled out.
Yangjingou scheelite mineralization is related to the intrusion of granodiorites at 249 ± 2.7 Ma16, synchronous with Wudaogou Group metamorphism. A/CNK (1.1–1.18) and Na2O/K2O (2.45–7.06)16 major element values are characteristic of alkali series I- to S-type transient magmatism. The volatile component of the melts was influenced by pressure, which affected the volatile solubility in the magma during ascent. With decreasing pressure, gas was liberated from the magma53, resulting in an increasing volume of CO2. CO2 dissolves relatively readily in intermediate-alkaline magmas, leading to more widespread and more rapid emplacement of mineralizing fluids46. Under these conditions, the diffusion of the W-rich mineralizing fluid was accelerated along interformational structures, causing the fluid to migrate to and rapidly expand into the host rocks of the Wudaogou Group. Only minor N2 (<0.25 mol%)54 was identified in fluid inclusions in the Xiaoxinancha Cu-Au porphyry deposit (123.35 ± 0.8 Ma)42, which is younger than the deposits in the Wudaogou Group and other stratigraphic units. The high concentration of N2 observed in the scheelite deposits cannot simply be attributed to organic-rich sedimentary rocks. Findings from this study demonstrate that N2-rich fluids are closely related to the degree of metamorphism and magmatic activity.
Thus, we assume that the N2 ± CO2 fluid was a product of late-stage Wudaogou Group metamorphism, which caused H2O–NaCl–CO2-bearing magmatic–hydrothermal fluids containing HWO4− and MWO4− to migrate through fractures and pre-existing structural weaknesses via fluid circulation, thereby promoting interaction with metamorphic wall rocks (Fig. 9). An initial increase in P-T conditions at ~269 Ma resulted in early low grade metamorphic reactions that transformed feldspar and clays within illite into micas. During this process, NH4+ substituted for K+, resulting in an increase in NH4+ within the constituent minerals of the Ca-rich mica schists50. During late-stage metamorphism (~249 Ma), particularly under greenschist-facies conditions, NH4+ was released via continuous metamorphism or thermal decomposition (i.e., complete breakdown of host minerals, such as mica)27,50. Simultaneously (~249 Ma), the circulation of a magmatic–hydrothermal W-mineralizing fluid under high oxygen fugacity conditions transferred heat (~400 °C) to the surrounding metamorphic rock and broke down micas in the host rock37,51. Within an oxidizing environment at this temperature range, large amounts of N2 were released from the NH4+-bearing host rocks and stabilized into fluid43. The addition of sufficient amounts of N2 to the mineralizing fluid increased activity coefficients and catalysed the saturation of Ca2+ and WO42–, greatly decreasing the scheelite solubility and accelerating scheelite precipitation. Moreover, saturated fluids, driven by advection, ascended into the surrounding country rock, resulting in alteration (albitization, chloritization, epidotization, and sericitization) and the incorporation of H2O into new minerals, leaving N2 ± CO2 trapped in quartz inclusions. Thus, the N2-rich fluid represents W-mineralizing fluid evolution and is indicative of W fluid saturation and scheelite precipitation.
Conclusions
-
1.
Nearly pure N2 (>88 mol%) fluid inclusions have been identified in W-bearing quartz veins in the Yangjingou deposit.
-
2.
Four fluid inclusion populations have been identified in the deposit:
-
•Type I: N2-rich inclusions (N2 >88 mol%, Th(L) N2 = −151 to −168 °C, Th(V)N2 = −150.3 °C, 42–150 cm3/mole).
-
•Type II: N2 ± CO2 inclusions (N2 = 42–79 mol%, ThN2 = −150 to −163 °C, ThCO2 = −45.6 to −86.1 °C, CO2 = 21–58 mol%, 30–65 cm3/mole).
-
•Type III: NaCl–H2O inclusions (a: Th = 250–400 °C, 6–10 equiv. wt% NaCl; b: Th = 315–410 °C).
-
•Type IV: CO2 ± N2–NaCl–H2O inclusions (CO2 = 76–92 mol%, Th = 283–423 °C, 4–9 equiv. wt% NaCl).
-
3.
The fluid inclusions were trapped at 315–410 °C and 80–350 MPa. The NaCl–H2O–CO2 fluids were magmatic–hydrothermal in origin, and the N2 was derived from the breakdown of NH4+-bearing minerals during alteration.
-
4.
Hydrogen and oxygen isotopic data indicate that the mineralizing fluids were of a mixed magmatic and metamorphic origin, and the nitrogen isotopic data from the W-mineralized quartz veins suggest that water–rock interaction occurred under low greenschist-facies conditions.
-
5.
The N2-rich fluid was intimately associated with the scheelite precipitation. During alteration under high oxygen fugacity conditions, N2 was released from NH4+-bearing minerals. The N2-rich fluid represents the W-mineralizing fluid evolution, following W saturation and scheelite precipitation. Therefore, areas that have experienced regional metamorphism (orogenesis) and exhibit similar geologic histories to the Yanbian area in NE China are likely candidates for scheelite precipitation as observed in the Yangjingou deposit. In this study, we demonstrate that scheelite precipitation can be genetically related to almost pure N2 fluid inclusions. The presence of the almost pure N2 fluid inclusions in these types of localities therefore can be used as a proxy for scheelite precipitation and thus, the presence of W ± Au deposits.
References
Lehmann, M. F. Modelling nitrogen and oxygen isotope fractionation during denitrification in a lacustrine redox-transition zone. Geochim. Cosmochim. Acta. 67(14), 2529–2542 (2003).
Vigouroux, N. Volatiles in high-K magmas from the western trans-Mexican volcanic belt: evidence for fluid fluxing and extreme enrichment of the mantle wedge by subduction processes. J. Petrol. 49(9), 1589–1618 (2008).
Kooi, M. E., Schouten, J. A. & Kerkhoff, A. M. V. D. et al. The system co2-n2 at high pressures and applications to fluid inclusions. Geochiml. Cosmochim. Acta 62–16, 2837–2843 (1998).
Andersen, T., Burke, E. A. J. & Neumann, E. R. Nitrogen-rich fluid in the uppermantle: fluid inclusions in spinel dunite from Lanzarote, Canary Islands. Contrib. Mineral. Petrol. 120, 20–28 (1995).
Bohlke, J. K., Ericksen, G. E. & Revesz, K. Stable isotope evidence for an atmospheric origin of desert nitrate deposits in northern chile and southern California, USA. Chem. Geol. 136(1–2), 135–152 (1997).
Cheilletz, A. Stratiform tungsten deposit: a review. Geol Mijinbounw 67, 293–311 (1988).
Dewaele, S. et al. Genesis of the vein-type tungsten mineralization at nyakabingo (rwanda) in the karagwe–ankole belt, central Africa. Mineral. Depos. 51(2), 283–307 (2016).
Gibert, F., Moine, B., Schott, J. & Dandurand, J. L. Modeling of the transport and deposition of tungsten in the scheelite-bearing calc-silicate gneisses of the montagne noire, France. Contrib. Mineral. Petrol. 112(2–3), 371–384 (1992).
Uspensky, E., Brugger, J. & Gräser, S. REE geochemistry systematics of scheelite from the Alps using luminescence spectroscopy: from global regularities to facies control. Schweiz. Mineral. Petrogr. Mitt 78(1), 33–56 (1998).
Wei, P. et al. Single grain zircon shrimp u-pb chronology the geological significance of the wudaogou rock group, yanbian. Geology in China. 06, 597–600 (2008).
Chen, C. et al. Permian age of the Wudaogougroup in eastern Yanbian: detrital zircon U–Pb constraints on the closure of the Palaeo-Asian Ocean in Northeast China. Int. Geol. Rev. 56, 1754–1768 (2014).
Li, D. J. Stratigraphy (Lithostratic) of Jilin Province. China. Univ. Geosci. Press, Wuhan. 12–300 (1997).
Chen, C. et al. The whole-rock geochemical composition of the Wudaogou group in eastern Yanbian, NE China–new clues to its relationship with the gold and tungsten mineralization and the evolution of the Paleo-Asian ocean. Resour. Geol. 65(3), 232–248 (2015).
Ren, Y. S., Lei, E., Zhao, H. L. & Wang, H. Characteristics of fluid inclusions and ore genesis of Yangjingou large scheelite deposit in Yanbian area, NE China. J. Jilin. Univ. 40(4), 764–772 (2010).
Zhang, W. B. Geological characteristics of the Yangjingou scheelite deposit of Jilin and its ore genesis. Mineral. Resour. Geol. 21(2), 122–125 (2007).
Zhao, H. L. Ore Genesis and Geodynamic Settings of Tungsten Deposits in Eastern Jilin and Heilongjiang Province (Eds), 150, 12–135 (2013).
Hu, C. T. et al. Geological features and prospecting direction of Yangjingou scheelite deposit, Jilin. Mineral. Explor. 1(Z1), 25–30 (2010).
Ren, Y. S. Trace element and rare earth element geochemistry of the scheelite and ore genesis of the Yangjingou large scheelite deposit in Yanbian area, northeastern China. Acta. Petrol. Sin. 26, 3720–3726 (2010).
Shan, C. H., Feng, L. I., Shi, J. F. & Zhao, M. R. Geological and geochemical characteristics and ore indicator of the Yangjingou scheelite deposit. Mineral. Resour. Geol 18(5), 440–445 (2004).
Bakker, R. J. & Jansen, J. B. H. Calculated fluid evolution path versus fluid inclusion data in the COHN system as exemplified by metamorphic rocks from Rogaland, south‐west Norway. J Metamor Geol 11(3), 357–370 (1993).
Burke, E. A. J. Raman microspectrometry of fluid inclusions. Lithos 55, 139–158 (2001).
Thiéry, R., Kerkhof, A. M. V. D. & Dubessy, J. vX properties of CH4-CO2 and CO2-N2 fluid inclusions: modelling for T < 31 °C and P < 400 bars. Eur. J. Mineral. 6, 753–771 (1994).
Kerkhof, A. M. V. D. & Thiery, R. Carbonic inclusions. Lithos. 55.1, 49–68 (2001).
Wopenka, B., Pasteris, J. D. & Freeman, J. J. Analysis of individual fluid inclusions by fourier transform infrared and Raman microspectroscopy. Geochim. Cosmochim. Acta. 54(3), 519–533 (1990).
Clayton, R. N., O’Neil, J. R. & Mayeda, T. K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. Atmos. 77(17), 3057–3067 (1972).
Wen, Q. Method of measurement of nitrogen isotope ratio in natural gas. Acta Sedimentologica Sinica. 4, 015 (1990).
Bebout, G. E. & Fogel, M. L. Nitrogen-isotope compositions of metasedimentary rocks in the catalina schist, california: implications for metamorphic devolatilization history. Geochimi. Cosmochim. Acta 56(7), 2839–2849 (1992).
Roedder, E. Volume 12: Fluid inclusions. Mineralogical (1984).
Kerkhof, A. M. V. F. Isochoric phase diagrams in the systems CO2-CH4 and CO2-N2: Application to fluid inclusions. Geochimi et Cosmochim Acta 54(3), 621–629 (1990).
Kerkhof, A. M. V. D. Phase transitions and molar volumes of CO2-CH4-N2 inclusions. Bulletin de minéralogie 111(3–4), 257–266 (1988).
Wood, S. A. & Samson, I. M. The hydrothermal geochemistry of tungsten in granitoid environments: I. relative solubilities of ferberite and scheelite as a function of T, P, pH, and m Nacl. Econ. Geol. 95(1), 143–182 (2000).
Dubessy, J. et al. Physical and chemical controls (fO2, T, pH) of the opposite behaviour of U and Sn-W as exemplified by hydrothermal deposits in France and Great Britain, and solubility data. Bull. Mineral. 10, 261–281 (1987).
Lin, Y. E., Liu, Y., Yang, Y. & Wei, G. The characteristics and significance of pure nitrogen fluid inclusions in Xikuangshan copper deposit, Dongchuan, Yunnan of China. Chin. J. Chem. 31(1), 78–84 (2012).
Ague, J. J. Models of permeability contrasts in subduction zone mélange: implications for gradients in fluid fluxes, Syros and Tinos Islands, Greece. Chem Geol 239(3), 217–227 (2007).
Klemd, R., Kerkhof, A. M. V. D. & Horn, E. E. High-density CO2-N2 inclusions in eclogite-facies metasediments of the Münchberg gneiss complex, SE Germany. Contri. Mineral. Petrol. 111, 409–419 (1992).
Dong S. M. & Ying, J. L. Geochemistry of tungsten. Sci. Press. Beijing (1987).
Bebout, G. E. et al. Nitrogen isotope tracers of high-temperaturefluid–rock interactions: case study of the Catalina Schist, California. Earth. Planet. Sci. Lett. 151, 77–91 (1997).
Kerkhof, A. M. V. D., Touret, J. L. R. & Kreulen, R. Juvenile co2 in enderbites of tromøy near arendal, southern norway: a fluid inclusion and stable isotope study. J. Metamorphic Geol, 12(3), 301–310 (2010).
Watenphul, A., Wunder, B., Wirth, R. & Heinrich, W. Ammonium-bearing clinopyroxene: a potential nitrogen reservoir in the earth’s mantle. Chem. Geol. 270(1), 240–248 (2010).
Bebout, G. E., Cooper, D. C., Bradley, A. D. & Sadofsky, S. J. Nitrogen-isotope record of fluid–rock interactions in theSkiddaw Aureole and granite, English Lake District. Am. Mineral. 84, 1495–1505 (1999).
Zhu, X. Y. et al. The differences of the ore-forming fluid between the vein-type and skarn type tungsten deposits. Acta. Petrol. Sin. 31(4), 941–953 (2015).
Huang, G. Discussion on genetic relation between the Naozhi gold deposit and the mesozoic volcanic rock series in Naozhi, Jilin province. Mineral. Resour. Geol.1 (1997).
Wang, K. Y., Qing, M. & Sun, F. Y. Study on the geochemical characteristics of ore-forming fluids and genesis of Xiaoxinancha gold-copper deposit, Jilin Province. Acta. Petrol. Sin. 26(12), 3727–3734 (2010).
Duit., W., Jansen, J. B. H., Van, Breemen, A. & Bos, A. Ammonium micas in metamorphic rocks as exemplified by Dome de l’Agout (France). Am. J. Sci. 286, 702–732 (1986).
Jia, Y. Nitrogen isotope fractionations during progressive metamorphism: a case study from the Paleozoic coomameta sedimentary complex, southeastern Australia. Geochim. Cosmochim. Acta. 70(20), 5201–5214 (2006).
Svensen, H. et al. Nitrogen geochemistry as a tracer of fluid flow in a hydrothermal vent complex in the karoo basin, south Africa. Geochim. Cosmochim. Acta. 72(20), 4929–4947 (2008).
Peter, A. & Cawood., C. Buchan. Erratum to “linking accretionary orogenesis with supercontinent assembly”. Earth-Sci. Rev. 85(1–2), 82–83 (2007).
Holloway, J. R. The system pargasite-H2O-CO2: a model for melting of a hydrous mineral with a mixed-volatile fluid—experimental results to 8 kbar. Geochimi. Cosmochim. Acta. 37(3), 651–666 (1973).
Rafal’skiy, R. P., Bryzgalin, O. V. & Fedorov, P. L. Tungsten migration and scheelite deposition under hydrothermal conditions. Geochemistry International 21, 1–13 (1984).
Busigny, V. & Bebout, G. E. Nitrogen in the silicate earth: speciation and isotopic behavior during mineral-fluid interactions. Elements 9(5), 353–358 (2013).
Connolly, J. A. D. & Cesare, B. C‐O‐H‐S fluid composition and oxygen fugacity in graphitic metapelites. Journal of metamorphic geology 11(3), 379–388 (1993).
Winter, J. D. An introduction to igneous and metamorphic petrology. Prentice Hall, New Jersey (2010).
Luo, Z. H., Lu, X. X. & Guo, S. F. Metallogenic systems on the transmagmatic fluid theory. Acta. Petrol. Sin. 24(12), 2669–2678 (2008).
Sun, J. G. et al. Ore-forming mechanism for the xiaoxinancha au-rich cu deposit in yanbian, jilin province, china: evidence from noble gas isotope geochemistry of fluid inclusions in minerals. 51(2), 216–228 (2008).
Wu, F. Y. et al. Geochronology of the phanerozoicgranitoids in northeastern China. J.Asian.Earth.Sci. 41(1), 1–30 (2011).
Taylor, H. P. Jr H.P. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposits. Econ.Geol. 69(6), 843–883 (1974).
Acknowledgements
We gratefully acknowledge Alfons van den Kerkhof and an anonymous reviewer for their insightful comments, which greatly improved this manuscript. We particularly thank Editorial Board Member Dorrit Jacob for handing the manuscript. We thank Yunsheng Ren and Dacheng Jia for our samples. We thank Robert Goldstein for fluid inclusion instruction and Clay Campbell from the University of Kansas for kindly helping with the English writing. This study was sponsored by China Geological Survey, Chinese Ministry of Science and Technology (2017YFC0601304) and the Geological Fluid Analysis Center, Jilin University, China. O and H stable isotope analyses were conducted at the analytical laboratory of Beijing Research Institute of Uranium Geology (BRIUG), China.
Author information
Authors and Affiliations
Contributions
First author: Yicun Wang contributed to the analysis of fluid inclusions, paper writing and figure drawing. Corresponding author: Keyong Wang contributed to the sample collection and provided advice during the analysis and paper writing. Third author: Yassa Konare contributed to language polishing. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Wang, K. & Konare, Y. N2-rich fluid in the vein-type Yangjingou scheelite deposit, Yanbian, NE China. Sci Rep 8, 5662 (2018). https://doi.org/10.1038/s41598-018-22227-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22227-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.