Abstract
We assessed whether a measure of more distal corneal nerve fibre loss at the inferior whorl(IW) region is better than proximal measures of central corneal nerve damage in relation to the diagnosis of diabetic peripheral neuropathy(DPN), painful DPN and quality of life(QoL). Participants underwent detailed assessment of neuropathy, QoL using the SF36 questionnaire, pain visual analogue score(VAS), and corneal confocal microscopy(CCM). Corneal nerve fibre density (CNFD), branch density (CNBD) and length (CNFL) at the central cornea and inferior whorl length (IWL) and average(ANFL) and total(TNFL) nerve fibre length were compared in patients with and without DPN and between patients with and without painful DPN and in relation to QoL. All CCM parameters were significantly reduced, but IWL was reduced ~three-fold greater than CNFL in patients with and without DPN compared to controls. IWL(p = 0.001), ANFL(p = 0.01) and TNFL(p = 0.02) were significantly lower in patients with painful compared to painless DPN. The VAS score correlated with IWL(r = −0.36, P = 0.004), ANFL(r = −0.32, P = 0.01) and TNFL(r = −0.32, P = 0.01) and QoL correlated with CNFL(r = 0.35, P = 0.01) and IWL(r = 0.4, P = 0.004). Corneal nerve fibre damage is more prominent at the IW, lower in patients with painful compared to painless neuropathy and relates to their QoL. IWL may provide additional clinical utility for CCM in patients with DPN.
Similar content being viewed by others
Introduction
Diabetic peripheral neuropathy (DPN) affects up to 50% of all patients with diabetes1. It is the dominant cause of increased morbidity through painful neuropathy and foot ulceration and is also associated with increased mortality2,3,4. In 2003, we demonstrated the potential of corneal confocal microscopy (CCM) as a rapid and non-invasive means to quantify central corneal nerve morphology in early and more advanced DPN5. Subsequently, many studies have confirmed the utility of CCM in quantifying nerve fibre damage in diabetic neuropathy3,6,7,8,9.
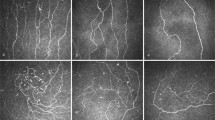
We and others have also reported corneal nerve fibre loss at the more distal inferior whorl region of the cornea in patients with DPN10,11 and progressively greater nerve fibre damage when moving from the central cornea to the inferior whorl and corneal epithelium in animal models of DPN12. This is consistent with a dying back neuropathy affecting the more distal nerve fibres in DPN. Additionally, the inferior whorl (IW) is a distinctive spiralled, clockwise sub-basal nerve fibre pattern located in the inferonasal cornea13,14. Because of its unique pattern, it has been suggested that it may be a more reliable landmark for longitudinal and interventional assessment of the corneal sub-basal nerve plexus15. Two previous reports showed that corneal nerve fibre length at the IW and centre has comparable sensitivity and reliability for detecting DPN11,16. We have also previously suggested that a combination of corneal nerve fibre length at the centre and inferior whorl may increase the sensitivity of CCM in detecting DPN11.
Painful neuropathy affects approximately 21% of patients with diabetes17,18 and can significantly reduce the quality of life19,20. A small study has demonstrated a greater reduction in central corneal nerve fibre length in patients with painful diabetic neuropathy3. In the present study, we have compared corneal nerve pathology in the central and inferior whorl regions in relation to the severity and presence of DPN and painful DPN and quality of life.
Methodology
Study subjects
116 patients with type 1 or type 2 diabetes and 22 age-matched healthy controls underwent assessment of peripheral neuropathy and corneal confocal microscopy (CCM). Patients were excluded if they had a history of malignancy, neuropathy of non-diabetic cause, current or active diabetic foot ulceration, deficiency of B12 or folate, chronic renal impairment or liver failure, connective tissue or systemic disease, corneal trauma, systemic disease that involves the cornea and cystic corneal disorders. Before participation informed consent was obtained from each participant. This research adhered to the tenets of Declaration of Helsinki and was approved by Greater Manchester east Research Ethics Committee.
Clinical and peripheral neuropathy assessment
All participants underwent assessment of body mass index (BMI) and blood pressure. Neurological deficits were assessed using the Neuropathy Disability Score (NDS), which includes an assessment of vibration perception, pinprick, temperature sensation and presence or absence of ankle reflexes21. Based on the NDS score patients were divided into two groups: without neuropathy (DN−) (NDS < 3) (n = 47) and with neuropathy (DN+) (NDS ≥ 3) (n = 69). VPT was assessed using a Horwell Neurothesiometer (Scientific Laboratory Supplies, Wilfrod, Nottingham, UK). Cold (CT) and warm (WT) perception thresholds, were assessed on the dorsolateral aspect of the non-dominant foot (S1) using a TSA-II NeuroSensory Analyser (Medoc, Ltd., Ramat-Yishai, Israel). Electrodiagnostic studies were undertaken using a Dantec ‘’Keypoint” system (Dantec Dynamics Ltd, Bristol, UK), equipped with a DISA temperature regulator to keep the limb temperature constantly at 32–35 °C. Sural sensory nerve amplitude (SSNA), sural sensory nerve conduction velocity (SSNCV), peroneal motor nerve amplitude (PMNA), and peroneal motor nerve conduction velocity (PMNCV) were assessed by a consultant neurophysiologist.
Quality of life was assessed using The Short Form 36 Health Survey (SF-36) questionnaire [15]. This survey consists of 36 questions, measures eight different dimensions, which are scored from 0 to 100. We used SF-36 total score, an average of eight dimensions as a single measure of quality of life.
A diagnosis of painful diabetic neuropathy was based on the presence of typical symmetrical neuropathic symptoms such as burning, aching pain in the feet and a visual analogue scale (VAS) > 4 mm in patients with abnormal distal sensation or decreased/absent distal reflexes (NDS ≥ 3)22.
Corneal Confocal Microscopy
All participants underwent CCM examination using a laser scanning corneal confocal microscope HRT III (Heidelberg Retinal Tomograph III Rostock Cornea Module, Heidelberg Engineering, Heidelberg, Germany) for both eyes using our established protocol for the central and inferior whorl area of the cornea11,23. Six images from the central cornea and four images from inferior whorl at the level of sub-basal nerve plexus were selected and manually quantified using CCMetrics (The University of Manchester, Manchester, UK). Images were selected based on their quality and variability using our established protocol11,24. Four corneal parameters were quantified: corneal nerve fibre density (CNFD – total number of main nerves per square millimetre (no./mm2), corneal nerve branch density (CNBD – number of nerve branches per square millimetre), corneal nerve fibre length (CNFL – total length of main nerves and nerve branches per square millimetre) (mm/mm2), inferior whorl length (IWL – total length of nerves per square millimetre at the IW region) (mm/mm2), average nerve fibre length (ANFL) − (CNFL + IWL/2 (mm/mm2)) and total nerve fibre length (TNFL) − (CNFL + IWL (mm/mm2)).
Statistical analysis
Analysis was carried out using SPSS (Version 22.0 for Macintosh, IBM Corporation, New York, NY, USA). Independent T-test (Mann Witney U test for nonparametric variables) was used to assess the estimates between two groups. The One-Way Anova (post hoc LSD) was used to compare means among groups. The analysis of covariance (Ancova) (post hoc LSD) was used to compare variables between groups, while statistically controlling for the effects of age. Pearson’s correlation coefficient (Spearman’s for non-parametric) was calculated to assess the associations among different variables. All the data are expressed as mean ± standard error (SE). P < 0.05 was considered as significant. Graphpad Prism (Version 7.0c for Macintosh, Graphpad Software, La Jolla California, USA) was used for plotting the graphs.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Clinical assessment
There was no significant difference in age between control subjects and patients with diabetes (50.32 ± 2.92 vs. 55.97 ± 1.32, P = 0.08), but patients with DN+ were older (50.32 ± 2.92 vs. 62.08 ± 1.4, P = 0.001). Therefore, the values were adjusted for age using analysis of covariance (ANCOVA). BMI (kg/m2) (26.47 ± 1.15 vs. 29.58 ± 0.73, P = 0.029) and HbA1c (mmol/mol) (36.4 ± 1.02 (5.48%) vs. 57.49 ± 1.66 (7.41%), P < 0.0001) were higher and total cholesterol (mmol/L) (5.27 ± 0.17 vs. 4.26 ± 0.11, P < 0.0001) was lower in diabetic patients compared to control subjects (Table 1). Systolic BP (mmHg) (135.60 ± 2.08 vs. 126.58 ± 2.23, P = 0.02) and BMI (kg/m2) (31.29 ± 1.04 vs. 27.49 ± 0.93, P = 0.02) were significantly higher in DN+ compared to DN−.
Neuropathy assessment
PMNCV (m/s) (43.42 ± 0.78 vs. 47.52 ± 1.05, P = 0.004) and SSNCV (m/s) (44.39 ± 1.36 vs. 50.41 ± 1.24, P = 0.003) were significantly lower and CT (°C) (19.71 ± 1.46 vs. 12.30 ± 2.13, P = 0.016) was significantly higher in DN− compared to control subjects. PMNCV (m/s) (41.48 ± 0.54 vs. 47.52 ± 1.05, P < 0.0001), SSNCV (m/s) (42.36 ± 0.60 vs. 50.41 ± 1.24, P < 0.0001), PMNA (mV) (3.14 ± 0.23 vs. 5.14 ± 0.36, P = 0.014) and SSNA (μV) (8.47 ± 0.90 vs. 17.61 ± 1.74, P < 0.0001) were significantly lower and VPT (V) (19.09 ± 1.22 vs. 6.53 ± 1.1, P < 0.0001) and CT (°C) (21.21 ± 1.03 vs. 12.30 ± 2.13, P = 0.001) were significantly higher in patients with DN+ compared to controls. PMNA (mV) (4.76 ± 0.59 vs. 3.14 ± 0.23, P = 0.009) and SSNA (μV) (12.86 ± 1.11 vs. 8.47 ± 0.90, P = 0.01) were lower and VPT (V) was higher (19.09 ± 1.22 vs. 8.29 ± 0.69, P < 0.0001) in DN+ compared to DN− (Table 2).
The total SF-36 score was reduced in DN− (69.12 ± 6.4) and DN+ (58.43 ± 3.7) but did not differ between the two (P = 0.1) (Table 2).
Corneal Confocal Microscopy
Corneal nerve fibre density (no./mm2) (25.14 ± 0.77 vs. 36.33 ± 1.49, p < 0.0001), branch density (no./mm2) (60.14 ± 2.99 vs. 90.84 ± 7.41, P = 0.001), length (mm/mm2) (22.39 ± 0.65 vs. 26.76 ± 1.04, p = 0.001, −16.3%), inferior whorl length (mm/mm2) (22.73 ± 0.88 vs. 35.31 ± 2.11, p < 0.0001, −35.6%), average (mm/mm2) (22.56 ± 0.68 vs. 31.03 ± 1.22, P < 0.0001, −27.3%) and total (mm/mm2) (45.12 ± 1.36 vs. 62.07 ± 2.45, P < 0.0001, −27.3%) nerve fibre length were significantly lower in patients with diabetes compared to controls (Table 1). Corneal nerve fibre density (no./mm2) (26.31 ± 1.19 vs. 35.42 ± 1.6, P < 0.0001), branch density (no./mm2) (64.29 ± 5.12 vs. 89.64 ± 7.25, P = 0.004), length (mm/mm2) (23.16 ± 1.06 vs. 26.57 ± 1.45, P = 0.05, −12.8%), inferior whorl length (mm/mm2) (24.08 ± 1.4 vs. 35.18 ± 2.02, P < 0.0001, −31.6%), average (mm/mm2) (23.62 ± 1.1 vs. 30.88 ± 1.51, P < 0.0001, −23.5%) and total (mm/mm2) (47.24 ± 2.21 vs. 61.75 ± 3.02, P < 0.0001, −23.5%) nerve fibre length were significantly lower in DN− compared to controls, after adjustment for age. Corneal nerve fibre density (no./mm2) (24.5 ± 0.9 vs. 35.42 ± 1.6, P < 0.0001), branch density (no./mm2) (56.94 ± 4.11 vs. 89.64 ± 7.25, P < 0.0001), length (mm/mm2) (21.84 ± 0.87 vs. 26.57 ± 1.45, P = 0.007, −17.8%), inferior whorl length (mm/mm2) (21.56 ± 1.2 vs. 35.18 ± 2.02, P < 0.0001, −38.7%), average (mm/mm2) (21.70 ± 0.9 vs. 30.88 ± 1.51, P < 0.0001, −29.7%) and total (mm/mm2) (43.4 ± 1.8 vs. 61.75 ± 3.02, P < 0.0001, −29.7%) nerve fibre length were significantly reduced in patients with DN+ compared to controls, after adjustment for age (Figs 1 and 2). There was no significant difference in any CCM parameter between diabetic patients with and without DPN (Table 2).
Error bar plots for corneal nerve fibre density (CNFD) (a), corneal nerve branch density (CNBD) (b), corneal nerve fibre length (CNFL) (c), Inferior whorl length (IWL) (d), average nerve fibre length (ANFL) (e) and total nerve fibre length (TNFL) (f) in controls and diabetic patients without (DN−) and with (DN+) neuropathy.
The Mean-2SD of controls was used to define an abnormal corneal nerve fibre length and IWL. 30% of patients with diabetes had damage in the inferior whorl but normal corneal nerve fibre length, whereas 13.5% of patients had damage in corneal nerve fibre length but a normal inferior whorl length. The scatterplot (Fig. 3) shows the relationship between inferior whorl length and corneal nerve fibre length. Inferior whorl length correlated significantly with corneal nerve fibre length in patients without (ICC = 0.75, P < 0.0001) and with (ICC = 0.66, P < 0.0001) neuropathy.
Corneal nerve branch density (r = 0.3, P = 0.008), length (r = 0.35, P = 0.01), inferior whorl length (r = 0.4, P = 0.004), average (r = 0.5, P = 0.001) and total (r = 0.5, P = 0.001) nerve fibre length correlated significantly with the total SF-36 score. There was no significant difference in corneal nerve fibre density, branch density or length, but inferior whorl length (mm/mm2) (17.25 ± 1.49 vs. 24.59 ± 1.57, P = 0.001), average (mm/mm2) (18.86 ± 1.16 vs. 23.38 ± 1.24, P = 0.01) and total (mm/mm2) (38.28 ± 2.5 vs. 46.54 ± 2.34, P = 0.02) nerve fibre length, were significantly lower in patients with painful compared to painless diabetic neuropathy (Table 3). There was a significant correlation between the severity of pain (VAS score) with inferior whorl length (r = −0.36, P = 0.004), average (r = −0.32, P = 0.01) and total (r = −0.32, P = 0.01) nerve fibre length. There was no significant difference for any CCM parameter in patients with Type 1 and Type 2 diabetes after adjusting for age, duration of diabetes and HbA1c (Supplementary Table S1).
Discussion
CCM is a rapid, reproducible ophthalmic technique that can quantify corneal nerve fibre abnormalities in diabetic and a number of other peripheral neuropathies3,25,26,27,28. Two recent studies have shown that CCM has a diagnostic ability, which is comparable to the current gold standard technique of skin biopsy9,29. We confirm that CCM can detect sub-clinical corneal nerve damage in patients without evidence of DPN11.
DPN is length-dependent neuropathy and will therefore affect the most distal nerve fibres first30 i.e. the nerves at the inferior whorl which are more distal to the nerves in the central cornea. Corneal nerve loss has been reported to be comparable at the inferior whorl and central cornea in diabetic patients with and without neuropathy11,15,16. However, in the present study, we demonstrate a ~2–3-fold greater reduction in corneal nerve fibre length at the IW compared to the central cornea in diabetic patients with and without DPN compared to controls. We also show that whilst 30% of patients with a reduction in inferior whorl length had a normal corneal nerve fibre length, only 13.5% of patients with a normal inferior whorl length had an abnormal corneal nerve fibre length; confirming more prominent distal corneal nerve loss. Given this difference we postulated that a combination of both corneal nerve fibre length and inferior whorl length, either as a total or average length may be more useful than either measure alone. Indeed, inferior whorl length, average and total nerve fibre length rather than the standard parameters of corneal nerve fibre density, branch density and length correlate with the quality of life and the presence and severity of painful diabetic neuropathy. Given that new therapies for diabetic neuropathy should induce nerve repair in the most distal part of the nerve, we propose that quantification of inferior whorl length and the average and total nerve fibre length may provide a more powerful means to assess a therapeutic response than has already been demonstrated by quantifying central corneal nerve morphology31,32,33. An additional advantage is the unique pattern of the IW, which facilitates its use as a landmark in longitudinal and interventional studies.
To our knowledge the association between CCM parameters and QoL and presence and severity of neuropathic pain has not been previously reported. QoL is an important patient outcome and we used the validated SF-36 questionnaire34 to assess the QoL in patients in this study. We show a more significant correlation between QoL and IWL compared to corneal nerve fibre length. Furthermore, inferior whorl length, average and total nerve fibre length differentiate patients with and without painful neuropathy and correlate with the severity of painful neuropathy, whereas corneal nerve fibre density, branch density and length, do not.
A potential limitation of our study is that the diagnosis of painful diabetic neuropathy was principally based on the VAS and NDS scores, but this is in accord with the IASP definition for neuropathic pain as adopted by a recent large painful neuropathy phenotyping study20. We believe future studies should better stratify cases in relation to the severity and sub-types of diabetic neuropathy.
In conclusion we have shown that there is more prominent distal corneal nerve fibre damage at the inferior whorl in patients with diabetic neuropathy. It also provides an objective measure of small nerve fibre damage, which relates to the presence of painful diabetic neuropathy and the quality of life. Further studies deploying these novel measures of more distal corneal nerve damage and repair are required in longitudinal studies and in clinical trials of new therapies to define their clinical utility.
References
Boulton, A. J. et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes care 28, 956–962 (2005).
Tesfaye, S. Epidemiology and etiology of diabetic peripheral neuropathies. Advanced Studies in Medicine 4, S1014–S1021 (2004).
Quattrini, C. et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56, 2148–2154, https://doi.org/10.2337/db07-0285 (2007).
Tesfaye, S. & Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes/metabolism research and reviews 28(Suppl 1), 8–14, https://doi.org/10.1002/dmrr.2239 (2012).
Malik, R. A. et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 46, 683–688, https://doi.org/10.1007/s00125-003-1086-8 (2003).
Ahmed, A. et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes care 35, 821–828, https://doi.org/10.2337/dc11-1396 (2012).
Petropoulos, I. N. et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes care 36, 3646–3651, https://doi.org/10.2337/dc13-0193 (2013).
Maddaloni, E. et al. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabetic Medicine 32, 262–266, https://doi.org/10.1111/dme.12583 (2014).
Ziegler, D. et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 63, 2454–2463, https://doi.org/10.2337/db13-1819 (2014).
Edwards, K. et al. Wide-field assessment of the human corneal subbasal nerve plexus in diabetic neuropathy using a novel mapping technique. Cornea 31, 1078–1082 (2012).
Petropoulos, I. N. et al. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investigative ophthalmology & visual science 56, 2498–2504, https://doi.org/10.1167/iovs.14-15919 (2015).
Davidson, E. P., Coppey, L. J., Kardon, R. H. & Yorek, M. A. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Investigative ophthalmology & visual science 55, 1222–1230, https://doi.org/10.1167/iovs.13-13794 (2014).
Marfurt, C. F., Cox, J., Deek, S. & Dvorscak, L. Anatomy of the human corneal innervation. Experimental eye research 90, 478–492, https://doi.org/10.1016/j.exer.2009.12.010 (2010).
Patel, D. V. & McGhee, C. N. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investigative ophthalmology & visual science 46, 4485–4488, https://doi.org/10.1167/iovs.05-0794 (2005).
Utsunomiya, T. et al. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investigative ophthalmology & visual science 56, 5417–5423, https://doi.org/10.1167/iovs.15-16609 (2015).
Pritchard, N. et al. Utility of Assessing Nerve Morphology in Central Cornea Versus Whorl Area for Diagnosing Diabetic Peripheral Neuropathy. Cornea 34, 756–761, https://doi.org/10.1097/ICO.0000000000000447 (2015).
Huizinga, M. M. P. A. Painful Diabetic Neuropathy: A Management-Centered Review. Clinical Diabetes 25, 6–15 (2007).
Abbott, C. A., Malik, R. A., van Ross, E. R., Kulkarni, J. & Boulton, A. J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes care 34, 2220–2224, https://doi.org/10.2337/dc11-1108 (2011).
Quattrini, C. & Tesfaye, S. Understanding the impact of painful diabetic neuropathy. Diabetes/metabolism research and reviews 19(Suppl 1), S2–8, https://doi.org/10.1002/dmrr.360 (2003).
Themistocleous, A. C. et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 157, 1132–1145, https://doi.org/10.1097/j.pain.0000000000000491 (2016).
Young, M. J., Boulton, A. J., MacLeod, A. F., Williams, D. R. & Sonksen, P. H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 36, 150–154 (1993).
Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63(Suppl 11), S240–252, https://doi.org/10.1002/acr.20543 (2011).
Tavakoli, M. & Malik, R. A. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp, https://doi.org/10.3791/2194 (2011).
Kalteniece, A. et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PloS one 12, e0183040, https://doi.org/10.1371/journal.pone.0183040 (2017).
Ferdousi, M. et al. Corneal Confocal Microscopy Detects Small Fibre Neuropathy in Patients with Upper Gastrointestinal Cancer and Nerve Regeneration in Chemotherapy Induced Peripheral Neuropathy. PloS one 10, e0139394, https://doi.org/10.1371/journal.pone.0139394 (2015).
Azmi, S. et al. Corneal Confocal Microscopy Identifies Small-Fiber Neuropathy in Subjects With Impaired Glucose Tolerance Who Develop Type 2 Diabetes. Diabetes care 38, 1502–1508, https://doi.org/10.2337/dc14-2733 (2015).
Stettner, M. et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol 3, 88–100, https://doi.org/10.1002/acn3.275 (2016).
Kemp, H. I. et al. Use of Corneal Confocal Microscopy to Evaluate Small Nerve Fibers in Patients With Human Immunodeficiency Virus. JAMA Ophthalmol 135, 795–800, https://doi.org/10.1001/jamaophthalmol.2017.1703 (2017).
Alam, U. et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PloS one 12, e0180175, https://doi.org/10.1371/journal.pone.0180175 (2017).
Tesfaye, S. et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes care 33, 2285–2293 (2010).
Tavakoli, M. et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 62, 254–260, https://doi.org/10.2337/db12-0574 (2013).
Culver, D. A. et al. Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Investigative ophthalmology & visual science 58, BIO52–BIO60, https://doi.org/10.1167/iovs.16-21291 (2017).
Lewis, E. J. H. et al. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology 88, 2294–2301, https://doi.org/10.1212/WNL.0000000000004033 (2017).
Ware, J. E. Jr. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care 30, 473–483 (1992).
Acknowledgements
We acknowledge Dr Mitra Tavakoli for undertaking CCM in a small number of participants. Supported by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network. The authors alone are responsible for the content and writing of the paper. The research received funding from the European Union Seventh Framework Programme FP7/2007–2013 (n°602273), National Institutes of Health (R01DK077903-0101; Bethesda, MD, USA) and Juvenile Diabetes Research Foundation International (27-2008-362).
Author information
Authors and Affiliations
Contributions
A.K. researched data, performed statistical analysis and wrote the manuscript; M.F. researched data, performed statistical analysis and wrote the manuscript; I.P. researched data; S.A. researched data; S.A. researched data; H.F. researched data; A.M. researched data; A.J.B. reviewed and revised the manuscript; N.E. reviewed and revised the manuscript; C.G.F reviewed and revised the manuscript; G.L. reviewed and revised the manuscript, H.S. reviewed and revised the manuscript; R.A.M. designed the study and reviewed and revised the manuscript. R.A.M. Is the guarantor of this work and, as such, had full access of the data of the study and takes the responsibility of the integrity of the data and the accuracy of the data analysis
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalteniece, A., Ferdousi, M., Petropoulos, I. et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep 8, 3283 (2018). https://doi.org/10.1038/s41598-018-21643-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21643-z
This article is cited by
-
In-vivo corneal confocal microscopy: Imaging analysis, biological insights and future directions
Communications Biology (2023)
-
Occurrence of corneal sub-epithelial microneuromas and axonal swelling in people with diabetes with and without (painful) diabetic neuropathy
Diabetologia (2023)
-
Corneal confocal microscopy in the evaluation of immune-related motor neuron disease syndrome
BMC Neurology (2022)
-
Mosaic vs. Single Image Analysis with Confocal Microscopy of the Corneal Nerve Plexus for Diagnosis of Early Diabetic Peripheral Neuropathy
Ophthalmology and Therapy (2022)
-
Could contact lens dryness discomfort symptoms sometimes have a neuropathic basis?
Eye and Vision (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.