Abstract
Diabetes mellitus is a risk factor for severe dengue in adults, but few studies have examined the association between metformin use and disease severity in dengue. In addition to its effect on glucose control, metformin has been associated with pleiotropic properties in preclinical studies. Using a cohort of laboratory-confirmed adult (≥21 years) dengue patients with diabetes mellitus admitted to Tan Tock Seng Hospital, we conducted a retrospective cohort study involving 131 (58.7%) metformin users and 92 (41.3%) non-users. Dengue severity was categorized as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) in World Health Organization (WHO) 1997 criteria and severe dengue (SD) in WHO 2009 criteria. Multivariable Poisson regression with robust error variance was used to estimate risk ratio (RR). Compared with non-use, metformin use was associated with a decreased risk of developing severe dengue (adjusted risk ratio [aRR] = 0.60, 95% confidence interval [CI]: 0.37–0.98, P = 0.04). Additionally, there was an inverse dose-response relationship (aRR = 0.69, 95% CI: 0.49–0.98, P = 0.04) with dengue severity as classified by WHO 2009 criteria. Use of metformin, however, was not associated with dengue severity based on WHO 1997 criteria; and no dose-response relationship was noted. Our results suggest metformin use could attenuate disease severity in dengue-infected diabetes mellitus individuals.
Similar content being viewed by others
Introduction
Dengue is the most common arthropod-borne viral infection, and the number of reported cases are increasing in the Western Pacific, the Americas and even Europe1. It is caused by four antigenically distinct serotypes of dengue viruses (DENV-1, DENV-2, DENV-3 and DENV-4) belonging to the Flavivirus family, all of which are transmitted by the Aedes aegypti and Aedes albopictus mosquitoes2. An estimated 390 million dengue infections occur worldwide each year, including 96 million with clinical symptoms. Asia is projected to have the highest burden, with 273 million infections annually3. Dengue infection manifests from asymptomatic infection to mild undifferentiated fever to a severe life-threatening condition. The World Health Organization (WHO) 1997 and the more recent WHO 2009 dengue classification criteria are the two most common classification criteria used to define dengue severity.
Against this background of increasing dengue incidence worldwide, the non-communicable disease burden is also growing. Among this group, diabetes mellitus (DM) is one of the most important and prevalent. According to the WHO, DM currently affects 347 million individuals worldwide, and it is predicted to be the seventh leading cause of death by 20304. Of all the oral hypoglycemic agents (OHAs) to treat DM, metformin which belongs to the biguanide family is the most commonly prescribed and widely used agent. The blood glucose lowering action of metformin is achieved by suppressing gluconeogenesis5 and increasing peripheral tissue sensitivity to insulin6,7. In addition, metformin has also been demonstrated to have multiple pleiotropic actions including anti-inflammatory8, immunomodulatory9, anti-cancerous10, cardio-protective and vasculo-protective actions11 and limits the growth of micro-organisms12,13,14. Given dengue pathogenesis is a complex intertwined process involving the humoral and cellular immune systems, inflammatory cytokines and chemical mediators15, there may be a potential reduction in the risk of severe dengue manifestations among diabetic patients on metformin treatment because of immunomodulatory and anti-inflammatory properties.
Many observational studies have identified DM as an independent risk factor for severe dengue16,17,18,19. On the other hand, several observational studies in Asia and Europe have suggested a reduced risk of viral or bacterial infections and infection-related complications in metformin users12,20,21. To our knowledge, no studies have examined the role of metformin on dengue disease severity among diabetic patients independent of its effect on blood glucose.
We performed a retrospective cohort study among adult DM patients with acute dengue infection to evaluate the association between metformin use and risk of severe dengue, using both WHO 1997 and WHO 2009 dengue classification criteria.
Methods
Setting and data source
The study included patients admitted to the Department of Infectious Diseases, Tan Tock Seng Hospital (TTSH), the largest university teaching hospital in Singapore in dengue treatment. The source population was identified from three previous hospital-based dengue studies conducted in TTSH in 200422, 2005–200823 and 2012–2013. All of these studies included adult laboratory-confirmed dengue patients i.e., either positive dengue polymerase chain reaction (PCR)24 or non-structural protein 1 (NS1) antigen tests based on a single acute sample25,26. The databases of the three studies provided information on demographics, dengue laboratory test results, comorbidities and detailed clinical data for the present study.
Study design and population
We conducted a hospital-based retrospective cohort study among adult (≥21 years) diabetic patients presenting with dengue. From the described source population, the study population was selected with the following eligibility criteria:
-
a.
Clinical manifestations of acute dengue infection according to WHO 1997 or 2009 guidelines with laboratory confirmation by either positive dengue PCR24 or NS1 antigen25,26 and
-
b.
Documented history of DM in hospital electronic medical records. Gestational diabetes without progression to DM was excluded.
Exposure data
Participants were followed up from the date of presentation with dengue. The exposure of interest was current treatment with metformin assessed by tracking the most recent prescription from hospital medication prescription database records in 180 days prior to hospital presentation. Metformin usage was confirmed with medication reconciliation records performed by hospital pharmacists which listed all the medications patients were taking before hospitalization. Patients were classified as metformin users if they were on metformin treatment at the time of presentation and non-users otherwise. Metformin exposure was categorized as follows:
-
Users: When there was a record for metformin use in the 180 days prior to hospital presentation, the prescription start date and duration of drug supply were collected to calculate the prescription end date. Patients were categorized as metformin users if the prescription end date was on or after the hospitalization date for dengue.
-
Non-users: Patients with a metformin prescription ending before the hospital presentation date, or without any record of metformin use, were categorized as non-users.
-
Prescribed metformin doses: Data on metformin dose was extracted for each user and was computed by multiplying the prescribed dose by daily prescription frequency.
Outcome data
The study outcome, dengue severity was categorized as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) based on WHO 1997 criteria and severe dengue (SD) according to WHO 2009 criteria. Patients were retrospectively classified into the two WHO dengue criteria with available clinical, laboratory and radiological data in each study using the same criteria across all previous studies. Clinical variables during hospital stay were documented using a standardized dengue care path for all dengue patients and the data were extracted by medically trained research assistants from the first day of hospital presentation until discharge date for inpatients, and to the end of acute follow-up for outpatients.
WHO 1997 classification
Dengue fever (DF) was diagnosed when there was fever and two or more of: headache, eye pain, myalgia, arthralgia, rash, hemorrhagic manifestations or leucopenia. DHF was diagnosed when there was fever and all three of: hemorrhagic manifestations; thrombocytopenia <100 × 109/L; and plasma leakage evidenced by pleural effusion or ascites or change in haematocrit ≥20% or hypoproteinaemia. To classify as DSS, there must be DHF as well as additional criteria including tachycardia with narrow pulse pressure lower than 20 mmHg or hypotension for age (systolic blood pressure <90 mmHg)27.
WHO 2009 classification
Probable dengue was diagnosed when there was fever and two or more of: nausea, vomiting, rash, aches and pains, leukopenia and presence of any warning signs which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement >2 cm and increase in hematocrit concurrent with rapid decrease in platelet count. Severe dengue was defined as: (i) severe plasma leakage with respiratory distress or shock; (ii) severe bleeding defined as a minimum of WHO grade 2 bleeding scale including hematemesis, melena, hematochezia, hematuria, menorrhagia, hemoptysis or any other bleeding that required whole blood or packed red cell transfusion; (iii) severe organ involvement defined by either acute liver injury – aspartate transaminase (AST) and alanine transaminase (ALT) ≥1000 unit/L or acute kidney injury or encephalopathy or myocarditis28.
Potential confounders
Covariates likely to be associated with dengue severity and likelihood of receiving metformin treatment were identified. These covariates were measured at the same time period as metformin exposure. Potential confounders included age29, gender30, ethnicity31, obesity32,33 as defined by body mass index (BMI) ≥30 kg/m2, smoking, and concurrent medications including other OHAs, statins, angiotensin-converting-enzyme inhibitors (ACEI) and angiotensin-receptor blockers (ARB)34. Dengue serotype is known to influence disease severity in Singapore35. However, patient-specific dengue serotype data for each subject was unavailable; thus the year of presentation was used as a surrogate index to estimate dominant circulating serotype in each epidemic year. Based on the Singapore communicable diseases surveillance data published by Ministry of Health Singapore, during the year of 2004 to 2006 and 2013, DENV-1 serotype predominated (61–82% of all circulating serotypes), and DENV-2 was the predominant serotype during 2007–2008 and 2012 (66–87%)36.
Comorbidities were measured by Charlson’s comorbidity index (CCI)37, and other comorbidities included hypertension, hyperlipidemia, allergy and past dengue38. Diabetes control was determined using glycated hemoglobin (HbA1c)39 measured in the 3 months prior to the dengue presentation date and categorized into ≤7.0% (optimal), 7.1–8.0% (suboptimal) and >8.0% (unacceptable). The diabetes complications severity index (DCSI)40, a validated 13-point clinical tool from seven categories of diabetes-related complications, was also included as a potential confounder. The decision to prescribe metformin depends on the renal function of patients. Therefore, serum creatinine >2.0 mg/dL and nephropathy on admission described in previous study40 were included. The diagnostic data to compute CCI and DCSI were drawn from the TTSH healthcare intelligence (TTSH-HI) system using the validated International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) coding algorithms based on inpatient and outpatient encounters registered in TTSH-HI before the admission date. TTSH-HI is an integrated system with consolidated information from different sources to allow retrieval of structured variables such as demographic, clinical, laboratory and medication prescription data of each subject.
Data collection
Microsoft Access database 2013 was used to collect, manage and collate the data. Data extraction was performed by two trained research assistants independently following standardized data collection procedures. Rule-based data validation was performed through the entire dataset to ensure data integrity and internal consistency. Additionally, 20% of the subjects were randomly selected for repeat data entry by another research assistant, and data discrepancies were resolved by independent source document verification by a senior medically-trained study team member. All subject identifiers were anonymized for analysis.
Statistical methods
Characteristics of metformin users and non-users were described with frequencies and percentages, and median and interquartile range (IQR) for categorical and continuous variables respectively. Pearson’s chi-square test or Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables were used for bivariate inference methods. We used Poisson regression with robust error variance41 to assess the association between metformin use and severe dengue manifestations, adjusting for potential confounding variables. In order to build the model, a forward stepwise variable selection approach was used to add the a priori defined clinically important variables including age, gender, ethnicity, CCI, DCSI, nephropathy, serum creatinine >2.0 mg/dL, HbA1c, year of presentation and variables with P < 0.20 between the metformin non-users and users. The best fit model, eventually, was compared against nested models evaluating with Akaike information criterion (AIC). Univariable and multivariable Poisson regression with robust error variance analyses provided crude and adjusted risk ratio (cRR and aRR) respectively with 95% confidence interval (CI). As a secondary analysis, the linear trend of dose-response relationship was studied and prescribed metformin doses were also categorized into no use, ≤1500 mg and >1500 mg based on the median prescribed dose.
All statistical analyses were conducted with Stata/SE14.0 (College Station, TX: StataCorp LP). Statistical significance was set at P < 0.05 and all reported P values were two-tailed.
Ethics consideration and STROBE statement
This study was approved by Domain Specific Review Board of National Healthcare Group, Singapore (DSRB – 2015/01053) with waiver of informed consent from subjects. This study is reported in accordance with STROBE guidelines for cohort studies (see Supplementary Table S1).
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information files, or are available from the corresponding author upon reasonable request.
Results
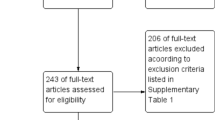
From 2004 to 2008 and 2012–2013, we screened 13,975 potential subjects from the three past studies and identified 230 diabetic subjects with laboratory-confirmed acute dengue. Of these, 223 subjects met the eligibility criteria. Among eligible patients, 131 received metformin treatment (Fig. 1).
The median age was 58 (IQR: 49–69.5) and 59 (IQR: 50–69) years for metformin non-users and users respectively. There were no significant differences between metformin users and non-users in terms of gender (P = 0.88), ethnicity (P = 0.09), proportion of overweight and obese patients (BMI ≥25–29.99 and ≥30 respectively, P = 0.57), proportion of current smokers (P = 0.48), median CCI (P = 0.47), median DCSI (P = 0.60), nephropathy (P = 0.39), HbA1c category (P = 0.27), serum creatinine >2.0 mg/dL (P = 0.20) or predominant circulating serotype (P = 0.07) (Table 1). There was a statistically significant difference in the proportion of hyperlipidemia (P = 0.01) whereas no difference was observed for other comorbidities.
Metformin users were observed to have a lower proportion of severe dengue (42.4% in non-users v. 28.2% in users, P = 0.03). Amongst the three criteria of severe dengue in WHO 2009 classification, a significantly lower proportion of metformin users experienced severe bleeding compared with non-users (20.7% v. 9.2%, P = 0.02). Table 1 displayed the difference between non-users and metformin users with standard dose (≤1500 mg) and high dose (>1500 mg). There was a higher proportion of HbA1c >8.0% in high dose users and the difference was statistically significant (P = 0.03).
Multivariable analysis based on WHO 2009 dengue classification
There were 37 (28.2%) severe dengue patients among metformin users and a significant inverse association was observed with metformin use and severe dengue on WHO 2009 classification. Compared with non-users, metformin users had a 33% reduction in a risk of severe dengue (cRR = 0.67, 95% CI: 0.46–0.96, P = 0.03) on univariable analysis. The risk reduction was 40% (aRR = 0.60, 95% CI: 0.37–0.98, P = 0.04) after controlling for age, gender, ethnicity, CCI, DCSI, nephropathy, hyperlipidemia, serum creatinine >2.0 mg/dL, HbA1c and concurrent medications including statins, ACEI, sulfonylurea and insulin, and year of presentation (Table 2).
On secondary analysis, there was an inverse dose-response relationship between metformin doses and severe dengue manifestations. Compared with non-users, the risk of severe dengue was 36% lower for patients taking ≤1500 mg (aRR = 0.64, 95% CI: 0.39–1.04, P = 0.07) and 50% lower for those taking >1500 mg (aRR = 0.50, 95% CI: 0.25–0.99, P = 0.05). In addition, there was evidence supporting an inverse linear trend in this association (aRR = 0.69, 95% CI: 0.49–0.98, P = 0.04) (Table 2).
Multivariable analysis based on WHO 1997 dengue classification
There were 39 (29.8%) of DHF/DSS patients among metformin users and use of metformin had no statistically significant association with dengue severity based on WHO 1997 classification even after adjustment of potential confounders (aRR = 0.91, 95% CI: 0.55–1.52, P = 0.73). Likewise, no significant dose-response relationship was observed (aRR = 1.12, 95% CI: 0.79–1.57, P = 0.53) (Table 3).
Discussion
This retrospective cohort study of adult diabetic patients with acute dengue infection indicated that patients on metformin treatment had a 33–40% lower risk of developing severe dengue based on WHO 2009 dengue criteria. Risk of severe dengue was also significantly reduced with increasing metformin dose intensity. Based on WHO 1997 dengue severity criteria, the association was not evident and neither was the dose-response relationship.
As the study suggested significant inverse association only for the analysis based on WHO 2009 classification, the relationship between metformin and severe dengue may be specific only to pathophysiology documented in the WHO 2009 dengue criteria. There are three severity markers in WHO 2009 dengue criteria – severe plasma leakage, severe bleeding and severe organ involvement. The pathogenesis of dengue virus infection leading to severe bleeding is complex but the major mechanisms involved can be summarized as platelet consumption, endothelial cell damage, imbalance profile of cytokines and chemical mediators, and suppression of hematopoiesis15. Investigations from in-vitro studies have found that metformin had direct vascular anti-inflammatory effect by attenuation of the inflammatory cytokine response8 while in-vivo studies have shown an effect of metformin on increasing the size of hematopoietic stem cell compartment and enhancing the quiescent state in hematopoietic stem cells and progenitor cells42. The effect of metformin on endothelial cell function has been well-studied and many clinical and pre-clinical studies suggest that metformin improves endothelial cell function in animal models43,44, and also among chronic diabetic subjects45,46. Furthermore, metformin exerts antioxidant effects on platelets47, strengthening and stabilizing the thrombocyte colonies48. Therefore, under a causal assumption, the most plausible hypothesis of a decrease risk of severe bleeding in metformin-exposed diabetic dengue individuals can be collectively explained by these pleiotropic actions of metformin, which could interact and inhibit the pathogenic mechanisms of dengue infection that lead to severe bleeding manifestations.
Our study has several strengths. Firstly, to our knowledge, this is the first observational study to explore the relationship between metformin exposure and dengue severity in adults. Secondly, severe dengue was analyzed with two different dengue severity classifications. Thirdly, finding of a dose-response relationship provides strong, biologically plausible support for the link between metformin use and reduced risk of developing severe dengue49. Fourthly, since the standardized dengue care path was used for clinical documentation in TTSH during the whole study period, the clinical and laboratory data for the dengue outcome severity was consistent with minimal outcome information bias. Lastly, evidence of significant reduction in dengue severity associated with metformin from this study gives insights to advance further studies.
The current study had several limitations with the first being the retrospective nature with missing data being unavoidable. Other limitations included the lack of data to adjust for the effect of different dengue serotypes on disease severity as patient-specific dengue serotype data was not available for the whole study population. However, the year of presentation to hospital for each patient was used as a surrogate indicator to estimate the different circulating dengue serotypes in each epidemic year. Similarly, potential effect of prior serostatus on disease severity was not described as serostatus is not routinely investigated in clinical practice. Although compliance of metformin was unknown, metformin usage was ascertained by using not only medication prescription database system but also medication reconciliation records performed on the first day of hospital admission before clinical outcomes developed by the trained pharmacists; as such risk of metformin misclassification is low. Additionally, dengue severity classification was performed retrospectively with available clinical and laboratory data with a risk of outcome misclassification bias although it could be minimal as standardized dengue care path was used in the whole study period. Furthermore, as with any observational studies, there is potential residual confounding with respect to unknown confounding variables that were not collected for the present study. Finally, there is a risk of channelling bias i.e. prescription of metformin is dependent on patient’s characteristics such as age, gender, ethnicity, presence of comorbidities and diabetes related complications but was reduced by adjusting these potential confounding variables.
The study included only adult DM patients presenting with dengue to a single hospital. Dengue cases in this study may not be representative of dengue-infected individuals in the general population, because there may be mild or asymptomatic dengue patients with DM. Hence, the study results may not be generalizable to other populations of adult dengue patients with DM. Finally, the study was not sufficiently powered to examine the interactions and different effects metformin might exert on different subgroups of patients, for instance, based on gender, age, ethnic groups and DM complications.
Metformin is an essential drug to manage diabetic patients. This study provides preliminary evidence of a reduced risk of severe dengue among metformin users, and also on an inverse dose-response relationship. As dengue burden is rising1 and prevalence of diabetes mellitus is growing worldwide, the study findings are of major importance to the public health.
To date, no curative measure has been shown to be effective in treating dengue infection and supportive management is still the mainstay treatment option. Observational and interventional studies have also proved that prophylactic platelet transfusion is ineffective in preventing severe bleeding while adverse events are more commonly observed in transfused patients23,50,51,52. Our study suggested the potential protective effect of metformin on dengue severity particularly on severe bleeding. For future research, preclinical studies could be employed to evaluate the possible biological mechanism link between metformin use and risk of severe dengue.
In summary, our study findings showed that usage of metformin at presentation was associated with lower risk of developing severe dengue in hospitalized adult dengue diabetic patients, compared with non-users, in the absence of prior serostatus knowledge. In addition, a significant inverse dose-response relationship was noted. The results also suggest the difference in severe bleeding may be a factor in explaining the lower risk of severe dengue among metformin users.
References
Murray, N. E., Quam, M. B. & Wilder-Smith, A. Epidemiology of dengue: past, present and future prospects. Clinical epidemiology 5, 299–309, https://doi.org/10.2147/CLEP.S34440 (2013).
Simmons, C. P., Farrar, J. J., van Vinh Chau, N. & Wills, B. Dengue. New England Journal of Medicine 366, 1423–1432 (2012).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507, https://doi.org/10.1038/nature12060 (2013).
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40, https://doi.org/10.1016/S0140-6736(11)60679-X (2011).
Pernicova, I. & Korbonits, M. Metformin–mode of action and clinical implications for diabetes and cancer. Nature reviews. Endocrinology 10, 143–156, https://doi.org/10.1038/nrendo.2013.256 (2014).
McIntyre, H. D. et al. Metformin increases insulin sensitivity and basal glucose clearance in type 2 (non-insulin dependent) diabetes mellitus. Australian and New Zealand journal of medicine 21, 714–719 (1991).
Bailey, C. J. & Turner, R. C. Metformin. New England Journal of Medicine 334, 574–579 (1996).
Isoda, K. et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arteriosclerosis, thrombosis, and vascular biology 26, 611–617, https://doi.org/10.1161/01.ATV.0000201938.78044.75 (2006).
Choi, Y. K. & Park, K. G. Metabolic roles of AMPK and metformin in cancer cells. Molecules and cells 36, 279–287, https://doi.org/10.1007/s10059-013-0169-8 (2013).
Currie, C. J., Poole, C. D. & Gale, E. A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52, 1766–1777, https://doi.org/10.1007/s00125-009-1440-6 (2009).
Forouzandeh, F. et al. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. Journal of the American Heart Association 3, e001202, https://doi.org/10.1161/JAHA.114.001202 (2014).
Singhal, A. et al. Metformin as adjunct antituberculosis therapy. Science translational medicine 6, 263ra159–263ra159 (2014).
Shirazi, F. et al. Diet modification and metformin have a beneficial effect in a fly model of obesity and mucormycosis. PloS one 9, e108635 (2014).
Xun, Y. H. et al. Metformin inhibits hepatitis B virus protein production and replication in human hepatoma cells. Journal of viral hepatitis 21, 597–603, https://doi.org/10.1111/jvh.12187 (2014).
Martina, B. E., Koraka, P. & Osterhaus, A. D. Dengue virus pathogenesis: an integrated view. Clinical microbiology reviews 22, 564–581, https://doi.org/10.1128/CMR.00035-09 (2009).
Mahmood, S., Hafeez, S., Nabeel, H., Zahra, U. & Nazeer, H. Does comorbidity increase the risk of dengue hemorrhagic fever and dengue shock syndrome? ISRN Tropical Medicine 2013 (2013).
Figueiredo, M. A. A. et al. Allergies and diabetes as risk factors for dengue hemorrhagic fever: results of a case control study. PLoS Negl Trop Dis 4, e699 (2010).
Pang, J. et al. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS Negl Trop Dis 6, e1641 (2012).
Pang, J., Hsu, J. P., Yeo, T. W., Leo, Y. S. & Lye, D. C. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: A matched case-control study. Scientific reports 7 (2017).
Shih, C.-J. et al. Association between use of oral anti-diabetic drugs and the risk of sepsis: A nested case-control study. Scientific reports 5, 15260 (2015).
Ekstrom, N. et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ open 2, https://doi.org/10.1136/bmjopen-2012-001076 (2012).
Lee, V. J. et al. Predictive value of simple clinical and laboratory variables for dengue hemorrhagic fever in adults. Journal of Clinical Virology 42, 34–39 (2008).
Lee, T. H. et al. Potential Harm of Prophylactic Platelet Transfusion in Adult Dengue Patients. PLoS Negl Trop Dis 10, e0004576, https://doi.org/10.1371/journal.pntd.0004576 (2016).
Barkham, T. M., Chung, Y. K., Tang, K. F. & Ooi, E. E. The performance of RT-PCR compared with a rapid serological assay for acute dengue fever in a diagnostic laboratory. Transactions of the Royal Society of Tropical Medicine and Hygiene 100, 142–148, https://doi.org/10.1016/j.trstmh.2005.05.015 (2006).
Gan, V. C. et al. Diagnosing dengue at the point-of-care: utility of a rapid combined diagnostic kit in Singapore. PLoS One 9, e90037, https://doi.org/10.1371/journal.pone.0090037 (2014).
Blacksell, S. D. et al. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 42, 1127–1134, https://doi.org/10.1086/501358 (2006).
World Health Organization. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. Geneva: World Health Organization (1997).
World Health Organization et al. Dengue: guidelines for diagnosis, treatment, prevention and control. (World Health Organization, 2009).
Wichmann, O. et al. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Tropical medicine & international health: TM & IH 9, 1022–1029, https://doi.org/10.1111/j.1365-3156.2004.01295.x (2004).
Hanafusa, S. et al. Clinical features and differences between child and adult dengue infections in Rayong Province, southeast Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 39, 252 (2008).
Sierra, BdlC., Kouri, G. & Guzmán, M. Race: a risk factor for dengue hemorrhagic fever. Archives of virology 152, 533–542 (2007).
Levri, K. M. et al. Metformin as treatment for overweight and obese adults: a systematic review. The Annals of Family Medicine 3, 457–461 (2005).
Pichainarong, N., Mongkalangoon, N., Kalayanarooj, S. & Chaveepojnkamjorn, W. Relationship between body size and severity of dengue hemorrhagic fever among children aged 0-14 years. Southeast Asian journal of tropical medicine and public health 37, 283 (2006).
Sehdev, A. et al. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer 121, 1071–1078, https://doi.org/10.1002/cncr.29165 (2015).
Yung, C. F. et al. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, singapore. The American journal of tropical medicine and hygiene 92, 999–1005, https://doi.org/10.4269/ajtmh.14-0628 (2015).
Ministry of Health, S. Communicable Diseases Surveillance in Singapore 2013, https://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2014/communicable-diseases-surveillance-in-singapore-2013.html (2013).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 40, 373–383 (1987).
Toledo, J. et al. Relevance of non-communicable comorbidities for the development of the severe forms of dengue: a systematic literature review. PLoS neglected tropical diseases 10, e0004284 (2016).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj 321, 405–412 (2000).
Young, B. A. et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. The American journal of managed care 14, 15–23 (2008).
Zou, G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology 159, 702–706 (2004).
Zhang, Q. S. et al. Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood 128, 2774–2784, https://doi.org/10.1182/blood-2015-11-683490 (2016).
Matsumoto, T. et al. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. American journal of physiology. Heart and circulatory physiology 295, H1165–H1176, https://doi.org/10.1152/ajpheart.00486.2008 (2008).
Cheang, W. S. et al. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arteriosclerosis, thrombosis, and vascular biology 34, 830–836, https://doi.org/10.1161/ATVBAHA.113.301938 (2014).
Mather, K. J., Verma, S. & Anderson, T. J. Improved endothelial function with metformin in type 2 diabetes mellitus. Journal of the American College of Cardiology 37, 1344–1350 (2001).
De Jager, J. et al. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. Journal of internal medicine 257, 100–109, https://doi.org/10.1111/j.1365-2796.2004.01420.x (2005).
Gargiulo, P. et al. Metformin decreases platelet superoxide anion production in diabetic patients. Diabetes/metabolism research and reviews 18, 156–159, https://doi.org/10.1002/dmrr.282 (2002).
Gregorio, F. et al. Poorly controlled elderly Type 2 diabetic patients: the effects of increasing sulphonylurea dosages or adding metformin. Diabetic medicine: a journal of the British Diabetic Association 16, 1016–1024 (1999).
Swaen, G. & van Amelsvoort, L. A weight of evidence approach to causal inference. Journal of clinical epidemiology 62, 270–277, https://doi.org/10.1016/j.jclinepi.2008.06.013 (2009).
Lye, D. C., Lee, V. J., Sun, Y. & Leo, Y. S. Lack of efficacy of prophylactic platelet transfusion for severe thrombocytopenia in adults with acute uncomplicated dengue infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 48, 1262–1265, https://doi.org/10.1086/597773 (2009).
Khan Assir, M. Z. et al. Effectiveness of platelet transfusion in dengue Fever: a randomized controlled trial. Transfusion medicine and hemotherapy: offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 40, 362–368, https://doi.org/10.1159/000354837 (2013).
Lye, D. C. et al. Prophylactic platelet transfusion plus supportive care versus supportive care alone in adults with dengue and thrombocytopenia: a multicentre, open-label, randomised, superiority trial. Lancet, https://doi.org/10.1016/S0140-6736(17)30269-6 (2017).
Acknowledgements
The authors acknowledge Ms Ling Weiping and Ms Adriana Tan for data retrieval and Ms Hsu Jung Pu for data management.
Author information
Authors and Affiliations
Contributions
H.L.H., T.W.Y. and D.C.L. conceptualised and designed the study. J.P. and Y.S.L. partly helped in study design. H.L.H. collected the data, supported by J.P., Y.S.L. and D.C.L. H.L.H. and C.C.T. analyzed the data. H.L.H., T.W.Y., C.C.T. and D.C.L wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Htun, H.L., Yeo, T.W., Tam, C.C. et al. Metformin Use and Severe Dengue in Diabetic Adults. Sci Rep 8, 3344 (2018). https://doi.org/10.1038/s41598-018-21612-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21612-6
This article is cited by
-
Glycolysis is reduced in dengue virus 2 infected liver cells
Scientific Reports (2024)
-
Dengue: Update on Clinically Relevant Therapeutic Strategies and Vaccines
Current Treatment Options in Infectious Diseases (2023)
-
Blood glucose promotes dengue virus infection in the mosquito Aedes aegypti
Parasites & Vectors (2021)
-
The antiviral effect of metformin on zika and dengue virus infection
Scientific Reports (2021)
-
Hyperlipidemia, statin use and dengue severity
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.