Abstract
The hypothalamic neurohormone oxytocin decreases food intake via largely unexplored mechanisms. We investigated the central nervous mediation of oxytocin’s hypophagic effect in comparison to its impact on the processing of generalized rewards. Fifteen fasted normal-weight, young men received intranasal oxytocin (24 IU) or placebo before functional magnetic resonance imaging (fMRI) measurements of brain activity during exposure to food stimuli and a monetary incentive delay task (MID). Subsequently, ad-libitum breakfast intake was assessed. Oxytocin compared to placebo increased activity in the ventromedial prefrontal cortex, supplementary motor area, anterior cingulate, and ventrolateral prefrontal cortices in response to high- vs. low-calorie food images in the fasted state, and reduced calorie intake by 12%. During anticipation of monetary rewards, oxytocin compared to placebo augmented striatal, orbitofrontal and insular activity without altering MID performance. We conclude that during the anticipation of generalized rewards, oxytocin stimulates dopaminergic reward-processing circuits. In contrast, oxytocin restrains food intake by enhancing the activity of brain regions that exert cognitive control, while concomitantly increasing the activity of structures that process food reward value. This pattern points towards a specific role of oxytocin in the regulation of eating behaviour in humans that might be of relevance for potential clinical applications.
Similar content being viewed by others
Introduction
Oxytocin is produced in the paraventricular nucleus of the hypothalamus and released into the circulation via the posterior pituitary; in addition it is secreted directly into limbic, hind- and midbrain regions1. Oxytocin modulates psychosocial function and affective processing like social bonding, emotion regulation, childbirth, and sexual behaviour2,3,4 (for review see ref.1) by influencing the interplay between amygdalar pathways, key regulators of emotional behaviour5, and prefrontal cortical networks6. Effects on the brain’s reward circuitry, in particular on dopaminergic signalling, likely contribute to the psychosocial impact of the hormone7,8. Research in animals and humans consistently indicates that oxytocin also acts as an anorexigenic factor in the control of food intake9,10,11,12,13. Lesions of oxytocin-expressing hypothalamic nuclei result in increased food intake and body weight14, whereas the administration of oxytocin inhibits eating behaviour12,15. Of particular interest in the clinical context, oxytocin reduces food intake also in animals with diet-induced obesity16,17. More recently, the peptide has been demonstrated to improve peripheral glucose homeostasis in healthy humans18.
Studies in humans indicate that oxytocin administered to the brain via the intranasal route curbs the consumption of palatable snacks in normal-weight individuals11 and acutely reduces breakfast intake in normal-, but also overweight subjects9. The hypophagic effect of oxytocin may even be more pronounced in obese than normal-weight men13, and intranasal oxytocin administration for eight weeks has been found to reduce body weight in obese subjects19. The neuronal mechanisms underlying oxytocin’s restraining effect on ingestive behaviour are only poorly understood. In animals, food intake increases oxytocin secretion from the pituitary20, and the peptide acts as a downstream mediator of the anorexigenic effect of the adipocyte leptin by sensitizing caudal brain stem nuclei to satiety signals like cholecystokinin21,22,23. Still, eating behaviour does not merely depend on food deprivation and consumption leading to hunger and, respectively, satiety, but implies a strong reward-related component24. Respective experiments in humans available so far9,11,13 indeed suggest that oxytocin decreases food intake in part by acting on reward-processing neuronal circuits. However, it is unclear whether the contribution of oxytocin to the regulation of human eating behaviour is established via food-specific effects on distinct brain networks or, rather, is a by-product of oxytocin’s involvement in general reward processing; our study was performed to address this question. In short, healthy, fasted men received intranasal oxytocin (24 IU) or placebo before their brain activity was assessed via fMRI scans while they looked at food and non-food pictures and performed the monetary incentive delay (MID) task, which assesses responses to monetary rewards (fasted-state runs). Subsequently, subjects ate from an ad-libitum breakfast buffet and the food picture and MID tasks were repeated in the scanner for exploratory purposes (satiated-state runs). Finally, casual snack intake was covertly assessed (see Fig. 1a for experimental procedure). We expected to identify eating-related brain mechanisms that mediate the hypophagic effect of oxytocin, but differ from those that establish oxytocin’s role in the processing of generalized (monetary) rewards.
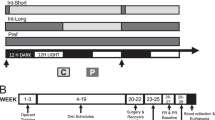
Experimental procedure, plasma oxytocin concentrations and breakfast intake. (a) Following baseline assessments of food-related and psychological variables and blood sampling, healthy young men were intranasally administered oxytocin (24 IU) and placebo, respectively, around 09.00 h (t = 0; spray symbol). After substance administration, participants were placed in the fMRI scanner to first undergo several technical scans followed by the food picture (35 min post-adminsitration) and the monetary incentive delay (MID) task (55 min), with an additional blood sampling in between. Seventy-five min after administration, subjects were allowed to eat ad libitum from a free-choice test buffet for 30 min, and the fMRI scans were repeated in the postprandial state. Around 85 min after termination of the buffet, snack intake was measured under the pretext of a taste-rating task, olfactory function was tested and appetite as well as short-term memory were assessed. (b) Mean plasma oxytocin concentrations (±SEM) assessed before and after intranasal administration (upright dotted line) of oxytocin (24 IU; grey dots and solid lines) and placebo (vehicle; white dots and dotted lines). (c) Calorie consumption from a test breakfast offered 75–105 min post-administration. N = 14–15; **p < 0.01, *p < 0.05 for comparisons between conditions (pairwise t-tests).
Results
Plasma oxytocin concentrations, food intake, appetite and auxiliary measures
Plasma oxytocin concentrations increased after intranasal administration of the peptide (F(1,116) = 8.91, p < 0.001 for treatment × time), reaching peak values of 8.87 ± 2.11 pmol/L 25 min after administration (Fig. 1b). Oxytocin compared to placebo curbed calorie intake by around 150 kcal or 12% during breakfast (F(1,13) = 8.22, p = 0.013; Fig. 1c). This effect was reflected by trend-wise decreases in the consumption of fat (F(1,13) = 4.41, p = 0.056), protein (F(1,13) = 4.16, p = 0.062) and carbohydrates (F(1,13) = 3.60, p = 0.068), but did not differ between macronutrients (p = 0.30 for treatment × macronutrient; Table 1). Oxytocin neither induced a shift in the intake of different food types, i.e., sweet, savoury and neutral items (F(1,69) = 0.02, p = 0.98 for treatment × type). Snack intake measured 10 min after the satiated-state fMRI scans, i.e., around 3 hours after substance administration, did not differ between conditions (all p > 0.46; Table 1). Calorie intake collapsed across breakfast and snacks was likewise reduced by oxytocin compared to placebo (1294 ± 112 vs. 1438 ± 113 kcal, F(1,14) = 5.51, p = 0.034). The effects of oxytocin on calorie intake remained unchanged when corrected for body weight.
Rated hunger declined after breakfast by around 85% across conditions, without differences between conditions (p = 0.43). All other measures of desire for food including the food control questionnaire-state were not affected by oxytocin (all p > 0.5). Auxiliary measures of mood, vigilance, working memory, and olfactory function were likewise comparable between conditions (see Supplementary Information). Participants could not differentiate between oxytocin and placebo treatment (p > 0.44, chi-squared test).
Food picture task
Food vs. non-food stimuli
In the fasted-state run, food pictures compared to non-food pictures evoked stronger responses in bilateral insula, lateral orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), fusiform gyrus, frontal gyrus, parahippocampus, and putamen, without modulatory effects of oxytocin. Respective effects in the satiated state focused on the insula (all whole-brain analyses, p < 0.05 family-wise error (FWE)-corrected; see Table 2 for the main food-related fMRI results).
Oxytocin effect on high- vs. low-calorie food stimuli
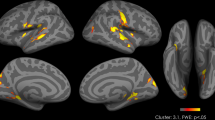
Analyses distinguishing between the responses to high- and low-calorie food pictures indicated that when subjects were fasted, oxytocin compared to placebo increased activity in response to high- vs. low-calorie food items in the vmPFC, supplementary motor area (SMA), anterior cingulate cortex (ACC), and bilateral ventrolateral prefrontal cortex (vlPFC; all region of interest (ROI) analyses, p < 0.05, FWE-corrected at cluster and peak level; Fig. 2 and Table 2). Analyses of the satiated-state runs indicated that these effects were restricted to the fasted-state, i.e., pre-breakfast measurements.
Oxytocin effects on neuronal activity during food picture presentation. Oxytocin-induced relative increases in neuronal activity (compared to placebo) in response to high- vs. low-calorie food pictures presented in the fasted state, and respective parametric estimates (±SEM). vmPFC, ventral medial prefrontal cortex, SMA, supplementary motor area, ACC, anterior cingulate cortex, vlPFC, ventrolateral prefrontal cortex; threshold of p < 0.001, uncorrected for multiple comparisons k > 25. N = 15.
Comparisons of the effects of oxytocin on brain activation in response to high- vs. low-calorie food stimuli and breakfast intake yielded a positive correlation between the oxytocin-induced increase in activation of the right vlPFC and the respective decrease in calorie intake from sweet breakfast ingredients (r = 0.57, p = 0.034). Corresponding, albeit only trend-wise results emerged for the relationship between increases in left vlPFC activation and decreases in calorie intake from savoury breakfast items (r = 0.50, p = 0.069) as well as for the respective relationships between SMA and, respectively, ACC activation and total breakfast intake when measured in grams (r = 0.49, p = 0.076; r = 0.46, p = 0.099), but not calories (n.s.). We also found a positive correlation of oxytocin plasma peak concentrations and, respectively, area under the curve (AUC) values in both conditions with activation in the ACC (r = 0.39, p ≤ 0.05; r = 0.48, p = 0.01) and the vlPFC (r = 0.43, p = 0.03; r = 0.51, p ≤ 0.01). Note that these correlative analyses were performed in an exploratory fashion.
MID task
Performance
In each trial of the MID task, the participant first saw a cue symbol announcing potential gains, losses, or no monetary outcome. The target stimulus appeared within a variable delay after the cue, and subjects, in dependence on the specific cue stimulus, had to press a button to win money or to avoid losing money, or had to abstain from responding. They were notified about their gains and losses after each trial.
In the fasted state, hit rate, i.e., proportion of successful button presses during target presentation (mean 63 ± 0.7%), and reaction times for hits (mean 197 ± 3.0 ms) did not differ between conditions (p > 0.19 for all comparisons). Reaction times were lower for responses to cues announcing comparatively higher rewards (F(1,180) = 6.00, p < 0.001). Comparable results were found in the satiated state (hit rate, 64 ± 1%; reaction times for hits, 185 ± 2.9 ms, all p > 0.30; F(1,180) = 13.5, p < 0.001 for comparisons of responses to cues announcing higher vs. lower rewards).
Effect of anticipation
Anticipating the execution of a response in comparison to no response (possibility to win or lose compared to neutral cue) in the fasted state was associated with increased activation of motor cortex, bilateral striatum, precentral gyrus, insula, midbrain, and OFC (whole-brain level, all p < 0.05 FWE-corrected; see Table 3 for main MID-related results). This effect was increased in response to high-value (5 Euro) cues (bilateral striatum and motor cortex; both p < 0.05 FWE-corrected). A comparable pattern was observed in the satiated state (see Table 3). We did not observe interactions between treatment and the incentive value of the cues.
Oxytocin effect during anticipation
In the fasted-state run, oxytocin compared to placebo elicited increased activation in the OFC, bilateral putamen, left caudate, frontal inferior operculum, and insula (all p < 0.05 FWE-corrected) during anticipation of rewards and losses (Fig. 3 and Table 3). In the satiated-state run, activation of the hypothalamus at first appeared to be increased by oxytocin (p < 0.05 FWE-corrected), but this effect was not significant when calorie intake during preceding breakfast was included as a covariate. We did not observe any differences between conditions in the neuronal activation in response to the possibility of win/high gain vs. no win/low gain. Further explorative analyses indicated positive correlations of oxytocin plasma peak concentrations and, respectively, AUC values with activation of OFC (r = 0.44, p = 0.02; r = 0.45, p = 0.02) and putamen (r = 0.61, p ≤ 0.01; r = 0.58, p = 0.02) when analysed across conditions.
Oxytocin effects on neuronal activity during anticipation of monetary rewards and losses. Oxytocin-induced relative increases in neuronal activity (compared to placebo) in anticipation of rewards and losses during the MID task measured in the fasted-state run, and respective parametric estimates (±SEM). OFC, orbitofrontal cortex, inf. operculum, inferior operculum; threshold of p < 0.001, uncorrected for multiple comparisons for left caudate, left and right putamen, and insula k > 25. N = 15.
Effect of feedback
In the fasted-state run, feedback on wins in comparison to misses was associated with increased activation of the medial OFC and caudate (both p < 0.05 FWE-corrected). Striatal activation (putamen) increased (p < 0.05 FWE-corrected) when subjects avoided losing money (Table 3). No differences between conditions were observed with regard to feedback effects.
Discussion
Our study in healthy men indicates that the brain mechanisms underlying the inhibitory effect of oxytocin on food intake can be differentiated from mediators of oxytocin-induced changes in the processing of generalized, monetary rewards. Intranasal oxytocin administration to food-deprived subjects altered fMRI-assessed neuronal activation patterns in food intake-regulatory pathways in response to food stimuli. Activity in the vmPFC as well as in SMA, vlPFC, and ACC was enhanced by oxytocin during exposure to high- vs. low-calorie visual food stimuli, indicating that the neuropeptide on the one hand increases the activity of networks that process the reward value of food and, on the other hand, enhances the function of structures that mediate cognitive control of consummatory behaviour. Fittingly, oxytocin compared to placebo also decreased subsequent ad-libitum breakfast intake by around 12%. Oxytocin did not affect behavioural performance on the MID task, although orbitofrontal, insular and striatal activation was enhanced by oxytocin during the anticipation of monetary rewards or losses. Our results indicate that the restraining effect of oxytocin on calorie intake found in previous9,11,13 and corroborated in the current study may not be a mere by-product of changes in general reward processing, but rather be driven by specific alterations in central nervous networks which process higher cognitive processes related to the acute control of food intake.
When our participants viewed high- as compared to low-calorie food stimuli in the fasted state, oxytocin in comparison to placebo increased neuronal responses in vmPFC, SMA, vlPFC, and ACC. Activity of the vmPFC is known to be positively correlated with the reward value of food stimuli25,26, suggesting that the inherent rewarding value of the high-calorie food items and associated reward expectancy were enhanced by oxytocin. This assumption is in line with previous studies showing that intranasal oxytocin increases connectivity between vmPFC/OFC, ACC and thalamus6. Moreover, frontal structures contribute to emotional evaluation and modulate activation of the amygdala27, a key emotion-processing brain structure that responds to oxytocin1,5. Importantly, oxytocin also increased activation of the SMA which receives input from the striatum and is associated with inhibitory processes in the regulation of the desire for palatable food28 and the success of self-regulation29. The oxytocin-induced increase in vlPFC activation supports the conclusion of an oxytocin-induced improvement in inhibitory control28, not least because this structure is engaged in focusing on the long-term cost of consuming unhealthy food30. Increased activity of the ACC, which contributes to emotional regulation during cognitive reappraisal31 and self-regulation of food intake32, may add to oxytocin-induced improvements of cognitive control capacities. This assumption is buttressed by the reduction in ad-libitum breakfast intake by around 150 kcal or 12% of the amount ingested in the placebo condition. Our correlational findings moreover yielded tentative evidence for a functional relationship between the stimulating effect of oxytocin on vlPFC, SMA and ACC activation and its inhibitory effect on food intake. Fittingly, a trend towards reduced food craving along with signs of increased neuronal activity in prefrontal regions was found in women after oxytocin administration33. Oxytocin-induced increases in the activity of areas that mediate inhibitory control might override the stimulating effect of the neuropeptide on reward value representation that is indicated by increased vmPFC activity, and attenuate actual food intake. This interpretation converges with our finding that actual desire for food did not differ between conditions, but should be further substantiated in fine-grained investigations into oxytocin’s role in food-related cognitive control.
Acute oxytocin-induced decreases in energy intake have been found in normal- and overweight humans9,11,13. Whereas our previous results suggested that the hypophagic effect of oxytocin in normal-weight humans focuses on reward-driven snacking in the satiated state11, in the present study oxytocin reduced hunger-driven calorie consumption during breakfast but did not affect snack intake, which took place somewhat later than in the aforementioned studies. Subtle differences in experimental timing and set-up may underlie these discrepancies that underline the need for further studies of the behavioural specifics of oxytocin’s anorexigenic impact. They will be of particular relevance when potential clinical uses are considered. In line with previous experiments11,13, self-rated hunger was not affected by oxytocin. Rather than influencing the motivation to eat, oxytocin therefore might contribute to the termination of meals by enhancing inhibitory control mechanisms. We could not confirm recent reports of oxytocin-induced decreases in hypothalamic activation in response to food cues34. Food intake in animals triggers c-Fos protein expression in hypothalamic oxytocin neurons35 and increases plasma oxytocin concentrations20. In our study, oxytocin appeared to increase hypothalamic activity during MID reward/loss anticipation in the satiated state, but this effect vanished after correction for preceding breakfast intake. It is to note that the fixed temporal sequence of fasted- and satiated fMRI runs in the present experiments may have lead to habituation effects, which could explain the scarcity of oxytocin-induced postprandial changes in brain activity and renders respective conclusions preliminary.
Performance on the MID task was not affected by oxytocin, indicating that oxytocin exerts no or only mild acute effects on the behavioural consequences of processing generalized rewarding stimuli. Oxytocin in comparison to placebo increased activation of caudate and putamen during the anticipation of rewards and losses, key areas of the dopaminergic reward network, and induced a trendwise increase in insular activity. These results accord with oxytocin effects in patients with posttraumatic stress disorder and controls who performed a social version of the MID task36, which points to a broad impact of oxytocin on reward-processing structures. Oxytocin-induced enhancements of striatal and insular responses during the anticipation of monetary rewards moreover concur with improved responses in the mesolimbic dopaminergic system found in studies on social reward and punishment8,37,38. This pattern suggests that the strong psychosocial impact of oxytocin is mediated to a large extent by its effect on the brain’s reward circuitry, including limbic prefrontal areas39.
In sum, we show that intranasal oxytocin administration to fasted normal-weight subjects exerts stimulatory effects on fronto-cortical brain regions that encode the reward value of a stimulus as well as on connected areas that establish inhibitory control of behavioural responses, which in concert may mediate the observed reduction in food intake. During the anticipation of monetary, generalized rewards, oxytocin increases activity in left caudate and bilateral putamen without immediate behavioural consequences. In some contrast to its primarily reward-related effects on psychosocial behaviour, oxytocin appears to limit food intake in humans by enhancing the activity of fronto-cortical brain areas that establish cognitive control processes, thereby overriding the hedonic drive to eat. In light of the promising results of proof-of-concept experiments in subjects with elevated body weight9,13, this mechanism of action may still be functional in obesity, which has been reported to be associated with alterations in circulating oxytocin concentrations40,41,42, and open up new avenues to normalize eating behaviour in the clinical context43,44.
Methods
Subjects
Twenty healthy male non-smokers participated in the study (mean age ± SEM, 25.7 ± 2.6 years, BMI, 22.7 ± 1.3 kg/m2) after a screening session including clinical examination. Exclusion criteria included eating disorders, excessive alcohol consumption, any diseases, medication, and contraindications for MRI. The final sample consisted of fifteen participants because one subject withdrew from participation after one session, and data sets of four subjects had to be omitted from fMRI analyses due to movement artefacts. One additional subject had to be omitted from the analyses of energy intake because of a technical failure (missing breakfast ingredients in one session). Participants gave written informed consent to the study that confirmed to the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty of the University of Tübingen. Participants received 100 Euro upon completion of the study.
Study design and procedure
Experiments were carried out in a double-blind, crossover, within-subject design. Subjects participated in two sessions (oxytocin and placebo) on two separate days at least 14 days apart. The order of conditions was balanced across participants, who were kept unaware of hypothesized treatment effects on food intake. Participants were instructed to abstain from food and caffeinated/alcoholic beverages after 20.00 h on the day preceding each session.
Upon arrival of the subject between 07.00 and 08.00 h in the morning, blood was sampled for baseline assessments of oxytocin via a venous cannula placed in the subject’s arm, and subjects underwent tests characterizing appetite, food preferences, vigilance, memory function and mood. After a practice-run of the MID task, subjects were intranasally administered oxytocin (24 IU in 0.6 ml, Syntocinon, Defiante Farmacêutica, Funchal Madeira, Portugal) or placebo (vehicle) via six 0.1-ml puffs (three per nostril) at 30-s intervals; this dose of intranasal oxytocin is known to increase blood as well as cerebrospinal fluid concentrations of the peptide45. Thirty-five min after substance administration, the 16-min food picture and the 13-min MID tasks were performed in the scanner (fasted-state runs). Seventy-five min after administration, subjects were presented with an ad-libitum breakfast buffet and subsequently again underwent the appetite/food/vigilance/mood test set. Thereafter, the food picture and MID tasks were repeated in the scanner (satiated-state runs) and an anatomical scan was performed. Eventually, casual snack intake was covertly assessed and working memory and olfactory performance were tested. Appetite and mood questionnaires were repeatedly filled in during sessions.
Food picture task
Food stimuli were standardized images of sweet and savoury food items (e.g., apples and pretzel sticks) and non-food items matched for size and shape (office supplies; e.g., pencils) provided by Charbonnier and colleagues46. Ninety food (45 low-calorie and 45 high-calorie) and 45 non-food pictures were displayed in randomized order on a screen through a computer interface using Presentation (Neurobehavioural Systems Inc., Berkeley, CA). Pictures were presented for 3 s each with a variable inter-stimulus interval of 1–1.2 s in a jittered, pseudo-random sequence. To maintain attention, subjects intermittently (after ten picture stimuli on average) had to indicate via button press within 3 s if the most recent picture displayed a food or non-food item.
Monetary incentive delay task (MID)
The MID task adopted from Knutson and co-workers47 consisted of 25 trials in the practice run and 90 trials in the scanner runs. In each trial, the participant first saw a cue symbol (circle, square or triangle) for 250 ms that announced a potential gain (circle), potential loss (square), or no monetary outcome (triangle). The number of lines in each symbol indicated how much was at stake (between € 0 and € 5). The target stimulus (solid white square) appeared within a variable delay of 2–2.5 s after the cue, and subjects had to press a button to win money (in response to the circle cue) or to avoid losing money (square cue), or had to abstain from responding (triangle cue). Feedback (1.65 s) provided immediately after the button press notified participants of whether they had won or lost money during that trial and indicated their cumulative total at that point. Difficulty (the time given to respond to the target) was individually titrated according to performance at the practice run (ranging from 150–250 ms; see ref.48) to approximate a 66% hit rate. The different trial types were pseudo-randomly ordered within each session. Subjects were informed that they would earn the monetary reward in addition to their reimbursement.
Breakfast and snack intake
Seventy-five min after substance administration, subjects were offered an ad-libitum free-choice breakfast buffet comprising a variety of food choices13 from which they could eat undisturbed in a separate room for 30 min. They were told that breakfast was scheduled as a break between scan sessions, but were not aware that intake was measured by weighing buffet components before and after. Ninety min after breakfast, reward-related eating in the relative absence of hunger was assessed with a snack test validated in previous studies11,49 (see Supplementary Information).
Auxiliary measures and analyses of plasma oxytocin
Tests were performed at baseline and after substance administration (see Fig. 1a for experimental schedule) to assess oxytocin effects on appetite, food preferences, mood, vigilance, working memory, and olfactory function. Blood samples were repeatedly collected for the determination of plasma oxytocin concentrations. See Supplementary Information for details.
Acquisition, processing and analysis of fMRI data
Scans were performed with a 3-Tesla PRISMA Siemens scanner with a 20-channel head coil. Functional images during the MID (367 volumes) and food picture task (475 volumes) were acquired with a single-shot echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR = 2000 ms), adjusted flip angle (90°), echo time (TE = 30 ms), matrix size (64 × 64), and 30 slices (thickness = 3 mm), resulting in a voxel size of 3.3 × 3.3 × 3.0 mm3. The T1-weighted anatomical scan was acquired with a TR/TE of 61/8.4 ms, a flip angle of 30°, FOV of 288 × 175 mm, 175 axial slices, and a voxel size of 1 × 1 × 1 mm3.
Functional imaging data were analysed using SPM12 (Wellcome Department of Cognitive Neurology, London, UK) run with MATLAB 2013 (Mathworks Inc, Natick, MA) and the WFU Pickatlas-tool50 using standard procedures51,52,53,54. Functional image volumes were pre-processed, starting with slice timing and realignment of the images to the mean functional image. To allow for susceptibility by movement artefacts, unwarping of time series was performed. In addition, the high-resolution T1 image was co-registered to the mean image of the EPI series for each participant. Co-registered T1-weighted MR images were segmented for gray and white matter to compute spatial transformation parameters for normalization. The registered functional scans were normalized to a standard Montreal Neurological Institute (MNI) template. Normalized images were spatially smoothed with a 9 mm full-width half-maximum Gaussian kernel. A statistical parametric map was generated by fitting a boxcar function to each time series, convolved with the canonical hemodynamic response function. Data were high-pass filtered with a cut-off of 128 s. Scans that included head movements exceeding 3 mm in any direction during task performance were excluded from further analysis.
Within-subject analyses
Each subject underwent four scan sessions including measurements during the food picture and the MID tasks, i.e., oxytocin-fasted state, oxytocin-satiated state, placebo-fasted state, and placebo-satiated state. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was modelled by a canonical hemodynamic response function51. For the food picture task, five conditions were modelled: viewing high-calorie food, low-calorie food, and non-food items, rest, and question. Responses to rest and question were neglected in further analyses. Two food-related neural activation contrast images were calculated: food vs. non-food (FvsNF) and high- vs. low-calorie food (HvsL). For the analysis of the MID task, 15 conditions were modelled: anticipation of potential gain of € 0, € 0.20, € 1 or € 5, anticipation of potential loss of € 0, € 0.20, € 1 or € 5, anticipation of no monetary outcome, rest, target, and feedback for win, no win, loss, no loss. In accordance with previous applications of the MID task36,47,48 contrast images were calculated for reward anticipation vs. neutral, loss anticipation vs. neutral, gain (€ 0.20, € 1 and € 5) vs. no gain (€ 0), and high gain € 5) vs. low gain € 0). For the analysis of feedback, four contrast images were calculated, win (reward/hit), no win (reward/miss), loss (loss avoidance/miss), and no loss (loss avoidance/hit; all vs. neutral). The motion correction parameters from the realignment procedure were added to all models as regressors to correct for motion-related variance.
Group-level analyses
Individual contrasts were entered into a repeated-measure ANCOVA including the factors ‘treatment’ (oxytocin or placebo) and ‘state’ (fasted or satiated). Order of conditions was introduced as an additional regressor. For the food picture task, two ANCOVAs were performed to compare neuronal activation in response to food vs. non-food items, and in response to high- vs. low-calorie items in dependence of treatment and state. Results of the MID task were analysed using respective repeated-measure ANCOVAs including the additional factor ‘outcome’ (gain or loss), resulting in a 2 × 2 × 2 design.
Statistical analyses
Behavioural results and oxytocin concentrations were analysed using SPSS 22.0 (IBM Corp.) and are presented as means ± SEM. Analyses relied on a linear mixed effects model with a fixed treatment effect, time effect, and treatment × time/macronutrient/food type interaction as appropriate. Subjects were added as random variables. To adjust for baseline differences, baseline values were added as covariates in models with more than two measurements. Post-hoc, paired t-tests were performed (Bonferroni-corrected for multiple comparisons) to specify treatment effects. Statistical relationships between measurements of brain activation and behavioural as well as plasma oxytocin results were investigated in an exploratory fashion by calculating two-tailed Pearson correlations between neuronal activation (beta-values of significant task-specific brain activation clusters), peak and AUC values of plasma oxytocin, and food intake parameters. For breakfast-related analyses, the included measures were the differences between conditions in vmPFC, SMA, ACC, vlPFC (L) and vlPFC (R) activation, and in total breakfast intake (in kcal and g) as well as in the intake (in kcal) from sweet and savoury items. A p value ≤ 0.05 was considered significant.
In fMRI analyses, main effects and interactions were considered significant at cluster level at p < 0.05, FWE correction, using a primary uncorrected threshold of p < 0.001 (k > 10)55. In addition to whole-brain analyses, ROI analyses with a priori defined masks were performed. Predicted regions for oxytocin effects were considered significant at p < 0.05, FWE-corrected at cluster and peak level within regions of interest defined according to previous findings. For the food picture task, they covered prefrontal areas as indicated by Striepens and colleagues33, as well as the OFC, inferior frontal and fusiform gyrus and insula whose activity increases during the presentation of food pictures54,56. With regard to the MID task, Nawijn and colleagues36 reported on oxytocin-induced changes in insula and striatum activation during the MID task; prefrontal areas and amygdala were found by Knutson and colleagues to be sensitive to MID performance57. For display, all maps are thresholded at p < 0.001 uncorrected with a cluster criterion of 25 voxels.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Meyer-Lindenberg, A., Domes, G., Kirsch, P. & Heinrichs, M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12, 524–538 (2011).
Bartz, J. A. et al. Oxytocin selectively improves empathic accuracy. Psychol. Sci. 21, 1426–1428 (2010).
Ishak, W. W., Kahloon, M. & Fakhry, H. Oxytocin role in enhancing well-being: a literature review. J. Affect. Disord. 130, 1–9 (2011).
Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U. & Fehr, E. Oxytocin increases trust in humans. Nature 435, 673–676 (2005).
Bethlehem, R. A., van Honk, J., Auyeung, B. & Baron-Cohen, S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38, 962–974 (2013).
Bos, P. A., Panksepp, J., Bluthe, R. M. & van Honk, J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front. Neuroendocrinol. 33, 17–35 (2012).
Strathearn, L., Fonagy, P., Amico, J. & Montague, P. R. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 34, 2655–2666 (2009).
Striepens, N. et al. Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 39, 74–87 (2014).
Lawson, E. A. et al. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 23, 950–956 (2015).
Maejima, Y. et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany N.Y.) 3, 1169–1177 (2011).
Ott, V. et al. Oxytocin reduces reward-driven food intake in humans. Diabetes 62, 3418–3425 (2013).
Olson, B. R. et al. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12, 113–118 (1991).
Thienel, M. et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. (Lond.) 40, 1707–1714 (2016).
Shor-Posner, G., Azar, A. P., Insinga, S. & Leibowitz, S. F. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol. Behav. 35, 883–890 (1985).
Arletti, R., Benelli, A. & Bertolini, A. Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93 (1989).
Deblon, N. et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 6, e25565 (2011).
Morton, G. J. et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 302, E134–E144 (2012).
Klement, J. et al. Oxytocin improves β-cell responsivity and glucose tolerance in healthy men. Diabetes 66, 264–271 (2017).
Zhang, H. et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 8, e61477 (2013).
Verbalis, J. G., McCann, M. J., McHale, C. M. & Stricker, E. M. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science 232, 1417–1419 (1986).
Blevins, J. E., Schwartz, M. W. & Baskin, D. G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R87–R96 (2004).
Ho, J. M. et al. Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology 155, 2845–2857 (2014).
Altirriba, J., Poher, A. L. & Rohner-Jeanrenaud, F. Chronic oxytocin administration as a treatment against impaired leptin signaling or leptin resistance in obesity. Front. Endocrinol. (Lausanne) 6, 119 (2015).
Berthoud, H. R. Metabolic and hedonic drives in the neural control of appetite, who is the boss? Curr. Opin. Neurobiol. 21, 888–896 (2011).
Gottfried, J. A., O’Doherty, J. & Dolan, R. J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301, 1104–1107 (2003).
Hare, T. A., O’Doherty, J., Camerer, C. F., Schultz, W. & Rangel, A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 28, 5623–5630 (2008).
Kringelbach, M. L. & Rolls, E. T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 72, 341–372 (2004).
Hollmann, M. et al. Neural correlates of the volitional regulation of the desire for food. Int. J. Obes. (Lond.) 36, 648–655 (2012).
van der Laan, L. N., de Ridder, D. T., Viergever, M. A. & Smeets, P. A. Activation in inhibitory brain regions during food choice correlates with temptation strength and self-regulatory success in weight-concerned women. Front. Neurosci. 8, 308 (2014).
Yokum, S. & Stice, E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int. J. Obes. (Lond.) 37, 1565–1570 (2013).
Buhle, J. T. et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990 (2014).
Giuliani, N. R., Mann, T., Tomiyama, A. J. & Berkman, E. T. Neural systems underlying the reappraisal of personally craved foods. J. Cogn. Neurosci. 26, 1390–1402 (2014).
Striepens, N. et al. Oxytocin enhances cognitive control of food craving in women. Hum. Brain. Mapp. 37, 4276–4285 (2016).
van der Klaauw, A. A. et al. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci. Rep. 7, 4266 (2017).
Johnstone, L. E., Fong, T. M. & Leng, G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 4, 313–321 (2006).
Nawijn, L. et al. Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology 66, 228–237 (2016).
Groppe, S. E. et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol. Psychiatry 74, 172–179 (2013).
Scheele, D. et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl. Acad. Sci. USA 110, 20308–20313 (2013).
Veening, J. G. & Olivier, B. Intranasal administration of oxytocin: behavioral and clinical effects, a review. Neurosci. Biobehav. Rev. 37, 1445–1465 (2013).
Qian, W. et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J. Clin. Endocrinol. Metab. 99, 4683–4689 (2014).
Stock, S., Granstrom, L., Backman, L., Matthiesen, A. S. & Uvnas-Moberg, K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes 13, 213–222 (1989).
Schorr, M. et al. Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum. J. Clin. Endocrinol. Metab. 102, 2814–2824 (2017).
Ho, J. M. & Blevins, J. E. Coming full circle: contributions of central and peripheral oxytocin actions to energy balance. Endocrinology 154, 589–596 (2013).
Lawson, E. A. The effects of oxytocin on eating behaviour and metabolism in humans. Nature Reviews Endocrinology 13(12), 700–709 (2017).
Striepens, N. et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 3, 3440 (2013).
Charbonnier, L., van Meer, F., van der Laan, L. N., Viergever, M. A. & Smeets, P. A. Standardized food images: A photographing protocol and image database. Appetite 96, 166–173 (2016).
Knutson, B., Adams, C. M., Fong, G. W. & Hommer, D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 21, RC159 (2001).
Adcock, R. A., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B. & Gabrieli, J. D. E. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50, 507–17 (2006).
Higgs, S., Williamson, A. C. & Attwood, A. S. Recall of recent lunch and its effect on subsequent snack intake. Physiol. Behav. 94, 454–462 (2008).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Friston, K. et al. Statistical parametric maps in functional imaging. A general linear approach. Hum Brain Mapp 2, 189–210 (1994).
Spetter, M. S., de Graaf, C., Viergever, M. A. & Smeets, P. A. M. Anterior cingulate taste activation predicts ad libitum intake of sweet and savory drinks in healthy, normal-weight men. J. Nutr. 142, 795–802 (2012).
Frank, S. et al. Dopamine Depletion Reduces Food-Related Reward Activity Independent of BMI. Neuropsychopharmacology 41, 1551–1559 (2016).
Frank, S. et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 1350, 159–166 (2010).
Woo, C. W., Krishnan, A. & Wager, T. D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419 (2014).
van der Laan, L. N., de Ridder, D. T., Viergever, M. A. & Smeets, P. A. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 55, 296–303 (2011).
Knutson, B., Fong, G. W., Bennett, S. M., Adams, C. M. & Hommer, D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272 (2003).
Acknowledgements
The authors thank Brian Knutson (Stanford University) and Graham Finlayson (University of Leeds) for their help in task preparation, and Sarah Ayyash, Daniel Glückman and Mario Haberstroh-Hess for technical assistance. This work was supported by a Junior grant to M.S.S. from the fortüne program of the Faculty of Medicine of the University of Tübingen, grants from the Deutsche Forschungsgemeinschaft (SFB 654), from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.; 01GI0925), and from the Helmholtz Alliance Imaging and Curing Environmental Metabolic Diseases (ICEMED), through the Initiative and Networking Fund of the Helmholtz Association. The funding sources had no input in the design and conduct of this study; in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
M.S.S. and M.H. designed the research with support from G.B.F., H.P. and M.A.H.; M.S.S. performed the research with support from M.T.; M.S.S. analyzed the data; M.S.S. and M.H. wrote the paper; G.B.F., M.T., H.P. and M.A.H. contributed to the final version of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spetter, M.S., Feld, G.B., Thienel, M. et al. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci Rep 8, 2736 (2018). https://doi.org/10.1038/s41598-018-20963-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20963-4
This article is cited by
-
A comprehensive review of genetic causes of obesity
World Journal of Pediatrics (2024)
-
Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions
Molecular Psychiatry (2023)
-
Oxytocin receptor gene polymorphism and low serum oxytocin level are associated with hyperphagia and obesity in adolescents
International Journal of Obesity (2021)
-
Neural Basis of Dysregulation of Palatability-Driven Appetite in Autism
Current Nutrition Reports (2021)
-
Investigating resting brain perfusion abnormalities and disease target-engagement by intranasal oxytocin in women with bulimia nervosa and binge-eating disorder and healthy controls
Translational Psychiatry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.