Abstract
Kalanchoe (K.) daigremontiana is important for studying asexual reproduction under different environmental conditions. Here, we describe a novel KdNOVEL41 (KdN41) gene that may confer drought resistance and could thereby affect K. daigremontiana development. The detected subcellular localization of a KdN41/Yellow Fluorescent Protein (YFP) fusion protein was in the nucleus and cell membrane. Drought, salt, and heat stress treatment in tobacco plants containing the KdN41 gene promoter driving β-glucuronidase (GUS) gene transcription revealed that only drought stress triggered strong GUS staining in the vascular tissues. Overexpression (OE) of the KdN41 gene conferred improved drought resistance in tobacco plants compared to wild-type and transformed with empty vector plants by inducing higher antioxidant enzyme activities, decreasing cell membrane damage, increasing abscisic acid (ABA) content, causing reinforced drought resistance related gene expression profiles. The 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining results also showed less relative oxygen species (ROS) content in KdN41-overexpressing tobacco leaf during drought stress. Surprisingly, by re-watering after drought stress, KdN41-overexpressing tobacco showed earlier flowering. Overall, the KdN41 gene plays roles in ROS scavenging and osmotic damage reduction to improve tobacco drought resistance, which may increase our understanding of the molecular network involved in developmental manipulation under drought stress in K. daigremontiana.
Similar content being viewed by others
Introduction
Plant growth relies on balancing propagation and survival in a rapidly changing habitat since the plant is a stationary system that cannot escape an inhospitable environment, unlike animals1. Thus, having a flexible growth system to adapt to a rapidly changing environment is the key in a variety of plant species2,3,4. Characterization of the regulatory mechanisms of plants under abiotic stresses (drought, salt, and temperature stress) increases our understanding by considering abiotic resistance in plants as a specific event during the developmental process5,6,7,8. Many transcription factor (TF) family genes and microRNA (miR) genes such as miR398 and miR393 have been shown to improve plant abiotic resistance by manipulating downstream stress resistance gene expression via hormone signaling9,10. In addition, many studies have also indicated that cross-talk between growth and stress hormone signaling can result in developmental arrest and enhancement of plant survival, allowing propagation of the species11,12.
Kalanchoe (K.) daigremontiana is a model plant for studying photosynthetic activity in crassulacean acid metabolism (CAM) species and plantlet morphogenesis along the dentate leaf margin13,14. As a CAM species, the K. daigremontiana succulent leaf structure shows impressive tolerance in drought and high-temperature environments15. It is of great interest to understand how plantlet morphogenesis is maintained while environmental stress continues to apply pressure. Previously, we observed an interesting scenario regarding an increased number of plantlets along the K. daigremontiana leaf margin under light drought stress compared to well-watered conditions, which allowed us to study the regulatory mechanisms between development and drought resistance. Different gene expression patterns in leaves between light drought stress and well-watered plants were identified by Suppression Subtractive Hybridization (SSH) technology16. Among genes with modulated expression, the KdN41 gene, without a known function, was identified.
Here, we hypothesized that this gene may participate in drought stress signal perception, which could affect plant development in K. daigremontiana. In this study, the full cDNA and promoter sequences of KdN41 were sequenced and the characteristics of putatively encoded proteins were analyzed using bioinformatics tools. The expression patterns of the KdN41 gene were also examined in K. daigremontiana leaves under different hormone and drought stress treatments. The spatial expression pattern of the KdN41 gene was monitored by β-glucuronidase (GUS) expression driven by the KdN41 gene promoter during different abiotic stress conditions (drought, salt, and heat stress). Finally, tobacco plants (Nicotiana tabacum, NT) overexpressing KdN41 were studied to evaluate the role of this gene in drought resistance.
Results
Antioxidant genes enrich in K. daigremontiana leaf under drought stress
Previously, SSH cDNA library were constructed among well watered K. daigremontiana leaves and leaves under light stress16. The library revealed different expressed genes induced by drought stress. Lacking a complete genome sequence in K. daigremontiana, 361 ESTs were annotated by employing four public databases, non-redundant protein database (Nr), non-redundant nucleotide database (Nt), Swiss-Prot protein database (Swiss-Prot), and Kyoto encyclopedia of genes and genomes database (KEGG). (Supplementary Fig. S1).
When compared with the COG database, all annotated ESTs were divided into 19 different functional classifications, excluding those poorly characterized or functional unknown ESTs (Supplementary Fig. S2a). Among these types, the first five categories were Translation, Ribosomal structure and biogenesis, Posttranslational modification, protein turnover, chaperones, Carbohydrate transport and metabolism, Energy production and conversion, Lipid transport and metabolism.
The go ontology (GO) databases were used to further classify the functions of the predicted genes. Approximately 315 ESTs were classified into three main categories: ‘biological process’, ‘cellular component’ and ‘molecular function’ (Supplementary Fig. S2b). Within the ‘cellular component’ category, a large number of ESTs were annotated as ‘integral component of membrane’, while the major groups within the ‘molecular function’ category were ‘ATP binding’ and ‘metal ion binding’. It’s worth to mention that in the ‘Biological process’ category, ‘oxidation-reduction process’ (27 ESTs) was the most abundant subcategories, among which antioxidant enzymes like peroxidase (POD), catalase (CAT), as well as NADP related genes were included (Supplementary Table S1).
Based on these data, KdN41 was predicted to participate in the pathways of antioxidant activities to increase plant drought resistance.
Clone and bioinformatics analysis of the KdN41 gene
Based on the partial cDNA sequence of KdN41, the full cDNA sequence of KdN41 was determined to be 884 bp after splicing the products of 3′ and 5′ RACE (rapid-amplification of cDNA ends) (Supplementary Fig. S3). The full sequence of the KdN41 promoter was found to be 194 bp (from gDNA 5′ beginning to the start of ATG initiation codon), which was determined by the genome walking method.
According to NCBI ORF Finder analysis, the possible open-reading frame (ORF) sequence length for KdN41 protein is 450 bp, encoding 149 amino acids. Nucleotide BLAST and protein BLAST analyses revealed no highly similar match with other known genes in other plant species. The protein characteristics of KdN41 examined by the ProtParam tool showed that the putative protein contains 20 types of amino acids (chemical formula: C713H1112N196O250S5) with a 16.59-kD molecular mass and an isoelectric point of 4.60. According to the SWISS-Model prediction of KdN41 protein structure, the structure showed 3.50 and 11.31% similarity, respectively, to Arabidopsis thaliana Polyadenylate-binding protein 2 and Schizosaccharomyces pombe Polyadenylate-binding protein 2. Therefore, KdN41 appears to be a novel gene based on both nucleotide and amino acid sequence BLAST analyses.
The key cis-acting elements of the KdN41 gene promoter were predicted to be MBS (MYB TF binding site previously implicated in drought stress response), TC-rich repeats (stress response element), and P-box (a gibberellin-responding element) based on PlantCare analysis (Supplementary Fig. S4).
KdN41 gene expression patterns under different hormone and drought stress treatments
Based on the multiple hormone response elements presented in the KdN41 gene promoter, we hypothesized that its expression may fluctuate under different hormone signaling and drought stress conditions. Therefore, KdN41 expression profiles in K. daigremontiana leaves treated with 4 kinds of hormones [gibberellins (GA3), salicylic acid (SA), abscisic acid (ABA), or methyl jasmonate (MeJa)] and 4 different (16%, 12%, 8%, 4%) soil water contents respectively were analyzed using qRT-PCR.
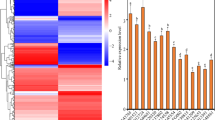
The KdN41 gene expression showed no significant difference among leaves of K. daigremontiana plants under 16%, 12% and 40% (control group, CK) soil water contents (Fig. 1a). However, a slight increase in KdN41 expression was observed in leaves of K. daigremontiana plants under 8% soil water content compared to CK (P < 0.05). Under 4% water content, the KdN41 expression boosted almost 1 time more than CK (P < 0.05) (Fig. 1). Thus, the KdN41 expression is sensitive to decreasing soil water content.
KdN41 expression patterns in response to drought and different hormone treatments. (a) KdN41expression patterns in response to increasing drought stress. The horizontal axis represents relative soil water content, the water content of 40% is CK; (b) KdN41expression patterns in response to different hormone treatments. Abscisic acid (ABA), salicylic acid (SA) gibberellins (GA), methyl jasmonate (MeJa), and H2O (CK). *Indicated a significant difference when compared with CK (p < 0.05); Errors bars represented ± SD (standard deviations) of nine independent replications. The expression level of CK at 2 h was treated as reference and calculated as 1.
An extended effect of SA in upregulating the expression of the KdN41 gene was more obvious than with other hormone treatments, suggesting that SA may be a key player in regulating this gene. After 6 h KdN41 gene expression was upregulated significantly by ABA or GA treatment, but its expression was downregulated between 4 and 6 h in the MeJa treatment. KdN41 upregulation was affected mainly by SA signaling and its downregulation was possibly affected by ABA, GA, or MeJa signaling (Fig. 1b).
Sub-cellular localization of KdN41 gene
We also used Yellow Fluorescent Protein (YFP) as a marker to monitor the sub-cellular localization of KdN41 (using its ORF sequence) in tobacco leaf epidermal cells.
A strong signal of the KdN41-YFP fusion protein was primarily detected in the nucleus (Fig. 2a) and a weak signal was observed in the cell membrane (Fig. 2a), compared to the average signal in control samples (Fig. 2d).
Therefore, the KdN41 gene expressed in the cell nucleus and membrane.
KdN41 gene expression pattern under drought stress signals
Since KdN41 gene expression may be induced under stress conditions, according to its promoter sequence, we utilized a construct of the KdN41 promoter driving GUS gene expression to determine the locations and conditions that induce KdN41 gene expression. Next, three stress conditions (drought, salt, and heat) were used to test KdN41 gene expression patterns.
Interestingly, KdN41 gene expression could not be detected in any tissue when no stress condition was applied, similar to wild-type (WT) plants (Fig. 3a and b). However, only 10 h after the onset of 20% (w/v) PEG6000 treatment (drought stress), KdN41 gene expression detected by GUS staining was mainly observed in the leaf vein (Fig. 3d) compared to WT (Fig. 3e) and positive control (PC, transformed with 35S::GUS) plants (Fig. 3f). Weak GUS staining in the main root part (Fig. 3j) indicated faint KdN41 gene expression compared to PC (Fig. 3l) and WT plants (Fig. 3k). After salt stress (Supplementary Fig. S5) or heat stress treatment (Supplementary Fig. S6), no GUS staining could be detected within the leaf vein or any root area compared to WT plants.
KdN41 GUS staining analyses of Promoter KdN41 ::GUS, wild type (WT), and 35S::GUS (PC) tobacco plants after drought stress using 20% PEG. (a–c) leaf staining results with no drought treatment; (d–f) leaf staining results under drought treatment; (g–i) root staining results with no drought treatment; (j–l) root staining results under drought treatment; Black arrows mark GUS staining area in leaf vein of Promoter KdN41 ::GUS plants.
To summarize, KdN41 gene expression was mostly silent during normal growth conditions, and only drought stress induced its high expression in the leaf vein.
Over-expression of the KdN41 gene confers drought stress resistance in tobacco
Given that the specific KdN41 gene expression pattern under drought stress, this gene could play a role in tobacco drought stress tolerance. Therefore, the ability to resist drought stress (light drought, LD; medium drought, MD; severe drought, SD) was tested among KdN41 over-expression (OE), WT, and negative control (NC, transformed with empty vector) plants.
The phenotypes of OE, WT, and NC tobacco plants were compared under increasing drought treatment. OE plants (less leaf wilting) showed better drought resistance than WT, and NC plants, especially under SD stress (less than 1% soil water content) (Fig. 4).
Different phenotypes in 35S::N41 (OE), wild type (WT) and NC (negative control) tobacco plants under increasing drought stress levels. (a) Plants with non-treatment; (b) plants under light drought (soil water content: 8–10%); (c) plants under medium drought (soil water content: 3–5%); (d) NC, OE and WT plants under severe drought (soil water content: <1%).
Therefore, over-expression of KdN41 gene improves drought stress resistance in tobacco.
Over-expression of KdN41 gene reduced reactive oxygen species (ROS) in tobacco leaves under drought stress
Drought is commonly considered as an induction of oxidative stress. The limited water availability often results in over-accumulation of ROS content such as hydrogen peroxide (H2O2) and super-oxygen ion (O2−), which is essential for plant to maintain homeostasis. Thus, we asked whether over-expression of KdN41 gene could improve the elimination of excessive ROS content in tobacco leaves during drought stress. The 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining methods were used to detect ROS localization in OE, WT, and NC tobacco leaves.
Both DAB and NBT staining results showed small staining area of ROS in leaves of three genotypes under well watered condition (Fig. 5a,b). A slight increase in staining area of ROS was observed in WT and NC tobacco leaves (Fig. 5c,d) under LD stress condition (8–10% soil water content), however, no significant expansion in staining area of ROS was shown in OE tobacco leaves (Fig. 5c,d) under LD stress condition. Under MD stress (3–5% soil water content), a large staining area of ROS was showed in WT and NC tobacco leaves (Fig. 5e,f), while only limited increase could be observed in OE tobacco leaves (Fig. 5e,f). Under SD stress (less than 1% soil water content), almost the whole area of WT and NC tobacco leaves were stained (Fig. 5g,h), however, the staining area of ROS site in OE tobacco leaves was much smaller (Fig. 5g,h).
ROS staining of 35S::N41 (OE), wild type (WT) and NC (negative control) tobacco plants under increasing drought stress levels. (a-b) Plants with non-treatment; (b-c) plants under light drought (soil water content: 8–10%); (e-f) plants under medium drought (soil water content: 3–5%); (g-h) NC, OE and WT plants under severe drought (soil water content: <1%).
The well-maintained homeostasis of ROS content in OE tobacco leaves under drought stress suggested that the activities of antioxidant enzymes such as peroxidase (POD), catalase (CAT) were more robust in OE leaves than those of in WT and NC leaves. Thus, POD, CAT activities and H2O2, O2− contents were also measured in OE, WT, and NC tobacco leaves.
The POD and CAT activities showed no significant difference between three genotypes during well water condition (Fig. 6a,b), which was consistent with H2O2 and O2− contents (Fig. 6c,d). A remarkable increase of both POD and CAT activities were found in OE tobacco leaves (P < 0.05) under LD and MD stress (Fig. 6a,b), leading to fewer contents of H2O2 and O2− than those of in WT and NC under MD stress (P < 0.05) (Fig. 6c,d). Under SD stress, though POD and CAT activities went down in OE tobacco leaves, but was still higher than those of in WT and NC (P < 0.05), which was consistent with the lower contents of H2O2 and O2− in OE tobacco leaves than those of in WT and NC leaves (P < 0.05) (Fig. 6c,d).
Changes of peroxidase (POD), catalase (CAT), H2O2 and O2− content in 35S::N41 (OE), wild type (WT) and NC (negative control) tobacco plants during increasing levels of drought stress, NT (non-treatment), LD (light drought), MD (medium drought), and SD (severe drought). Errors bars represented ± SD (standard deviations). *Indicated a significant difference compared to WT (Duncan’s multiple range test p < 0.05).
In summary, over-expression of KdN41 gene increased antioxidant enzymes activities and eliminated excessive H2O2, O2− contents in tobacco leaves.
Over-expression of KdN41 gene reduced osmotic damage in tobacco leaves under drought stress
The improved drought resistance of OE tobacco might also perform well in controlling osmotic damage and ABA signaling. Therefore, the electrolyte leakage (EL), Proline, MDA (Malondialdehyde) and ABA contents were tested in OE, WT and NC tobacco leaves.
In OE plants, EL, Proline and MDA results indicated better drought resistance and lower leaf cell membrane damage than WT and NC plants (Fig. 7a,c and d). The leaf ABA contents of OE plants slightly increased after drought treatment and was higher than those of WT and NC under LD and MD (Fig. 7b), which was consistent with the drought resistance phenotypes.
Electrolyte leakage (EL) activities, abscisic acid (ABA), Proline and malondialdehyde (MDA) content in 35S::N41 (OE), wild type (WT) and NC (negative control) tobacco plants during increasing levels of drought stress, NT (non-treatment), LD (light drought), MD (medium drought), and SD (severe drought). Errors bars represented ± SD (standard deviations). *Indicated a significant difference when compared to WT (Duncan’s multiple range test p < 0.05).
To summarize, over-expression of KdN41 gene reduced osmotic damage in tobacco leaves under drought stress.
Drought resistance related genes expression profiles in OE KdN41 gene tobacco leaves under drought stress
Given that the improved drought resistance of KdN41 gene OE plants, we also asked whether drought resistance related genes expression profiles were consistent with the phenotype. The relative expression of KdN41, respiratory burst oxidase homolog D (RbohD), POD1, CAT, Superoxide Dismutase (SOD) and dehydration-responsive element binding like (DREB-like) gene profiles in OE, WT, and NC tobacco leaves during drought stress were determined by RT-qPCR.
The KdN41 gene in OE leaves showed constitutive expression during well watered condition and drought stress (Fig. 8a). Mainly under LD stress, expressions of NtRbohD, NtSOD or NtDREB-like were found to be higher in OE than those of in WT and NC leaves (P < 0.05) (Fig. 8b,e and f). The POD1 and CAT gene expressions were both strongly up-regulated in OE leaves during drought stress, and became significant higher than those of in WT and NC leaves under LD, MD, and SD (P < 0.05) (Fig. 8c,d).
Relative expressions of drought resistance related genes in 35S::N41 (OE), wild type (WT) and NC (negative control) tobacco plants in response to increasing levels of drought stress, NT (non-treatment), LD (light drought), MD (medium drought), and SD (severe drought). Errors bars represented ± SD (standard deviations). *Indicated a significant difference compared to WT (Duncan’s multiple range test p < 0.05). The expression level of WT group under NT was treated as reference and calculated as 1.
In summary, over-expression of KdN41 gene in tobacco resulted in reinforced antioxidant enzymes encoding genes expression and lower ROS synthesis gene (RbohD) expression during drought stress, which confers reduced oxidative damage in OE leaves.
KdN41 gene over-expression causes early flowering in tobacco
Surprisingly, the early-flowering phenomenon was observed in KdN41-expressing transgenic tobacco plants compared to WT and NC tobacco plants, after watering was restored post-drought treatment (Supplementary Fig. S7). In KdN41-expressing tobacco plants, the first flower bud formed at week 20. In the following week, half of the total plants under evaluation exhibited flowering. All plants under evaluation flowered by week 22. For WT and NC plants, no flower was observed until week 21. Moreover, it was not until week 24 that all plants of these two kinds of transgenic plants were observed with flower buds. Thus, the flowering time for OE plants was earlier by almost 20 days compared to WT and NC tobacco plants (Supplementary Table S2).
This early flowering phenotype of OE plants might indicate that this gene also plays a role in strengthening the developmental phase change post-drought stress.
Discussion
The expression profiles of KdN41 gene revealed by GUS staining under drought, salt, and heat stresses showed that this gene specifically responds to drought stress signaling, which is consistent with its appearance in our previous drought stress SSH cDNA library. In other studies, ROS have been repeatedly shown to be a key signal cascade mediating stress signaling17. We explored whether ROS could accompany the expression of KdN41 gene, using DAB staining to visualize H2O2 localization in leaves under drought, salt, and heat stress (Supplementary Fig. S8 and S9). Under drought stress, the location of KdN41 gene expression by GUS staining was observed (Fig. 3) to coincide with the localization of H2O2 by DAB staining (Supplementary Fig. S9). Under salt stress, the presence of H2O2 did not trigger KdN41 gene expression. Therefore, KdN41 gene expression may be independent of ROS signaling, and instead may be a specific drought stress signaling responder.
The short promoter of KdN41 gene harboring multiple stress response elements suggests that this gene is tightly regulated by other gene networks. Thus, during normal growth conditions, the low expression of KdN41 gene could play an important regulatory role in growth. Considering the KdN41 expression patterns in response to drought signal, this gene may also function in tobacco salt and heat stress tolerance. To test this hypothesis, the ability to resist salt and heat was evaluated in OE, WT, and NC plants. During 7 days of continuous salt stress, a similar response was observed among OE, WT, and NC tobacco seedlings. The leaves of almost every plant turned from green to yellow, and no increased tolerance was observed after KdN41 over-expression (Supplementary Fig. S10a). For the heat stress treatment, OE, WT, and NC tobacco seedlings were compared under a constant high temperature of 50 °C. After 7 h of heat stress, almost all OE, WT, and NC plants showed similar responses, with the leaves beginning to turn brown. No significant difference was observed between samples of OE and samples of the two other groups (Supplementary Fig. S10b). However, OE tobacco seedlings of similar developmental stage under PEG drought stress displayed better resistance phenotypes than WT and NC plants (Supplementary Fig. S11).
KdN41 gene expression responded to drought stress signals, and over-expression of KdN41 gene improved tobacco drought resistance but failed to improve the resistance of tobacco plants to salt and heat stress. This indicates that this gene could play an indirect role in clearing harmful substances induced by stress. The lack of response of this gene during salt and heat stress indicates that systematic control of the specific response gene is crucial for the perception of certain environmental stresses.
The reduced leaf size of the KdN41-overexpressing plants is suggestive of several roles for this gene. According to the data (Supplementary Table S3), the leaves of KdN41-overexpressing plants were much shorter and narrower than those of WT and NC plants. These reduced leaf areas provide benefits for drought resistance. For example, lower work load for various physiologic traits such as leaf hydraulic conductance, photosynthesis, and nutrition consumption might be achieved18. In addition, lower membrane damage, lower levels of oxidant toxin levels and osmotic adjustment substances would be achieved19. The latter was consistent with the physiological parameters we observed (Fig. 6). In addition, tissue-specific expression of KdN41 gene in vascular tissue under drought stress (Fig. 3) reflects its possible role in communicating with other regulators in the leaf hydraulic conductance photosynthesis and nutrition consumption processes. The leaf vein is an essential structure in nutrition, water, and hormone transportation during the plant life span, and supports photosynthesis20. The leaf hydraulic conductance machinery can manipulate water transport and keep the stomata open to allow photosynthesis21. Previous study also confirmed that reduced cell size rather than cell number will determine the plasticity in vein and stomata density, which adjusts leaf transpiration in dealing with changing environment conditions22. The KdN41-overexpressing plants showed an obvious reduced decrease in photosynthetic rate compared to the WT (Supplementary Fig. S12b,e and h) and NC (Supplementary Fig. S12c,f and i) plants, especially under medium and severe drought stress (Supplementary Fig. S12d–f and g–i).
Thus, the reduced leaf size of KdN41-overexpressing tobacco might also serve as a dynamic leaf transpiration system to adapt to drought stress condition. In summary, the reduced size of leaves in KdN41-overexpressing tobacco may be an adaptive in response to drought stress.
Since the first report on the function of DREB transcription factor23, dwarfing plants has been a classic way to enhance stress tolerance24. However, some studies have confirmed improved stress tolerance in DREB1B-overexpressing plants without dwarfing, suggesting that ABA signaling is an independent stress response mechanism25. In this report, differences in ABA concentration between different kinds of transgenic plants and WT were much smaller than other physiological parameters, which might suggest that KdN41-overexpressing tobacco possess different methods of improving drought resistance.
During light to severe drought stress, the KdN41-overexpressing tobacco leaves showed high to low antioxidant enzyme activities compared to WT and NC plants (Fig. 6), indicative of hypersensitivity in detoxifying the oxidant substances. During drought stress, the KdN41-overexpressing plants continued to grow taller, but the leaf length and width were much lower than WT and NC plants (Supplementary Table S3). This could also be explained by the lower expression levels of auxin-related genes and higher expression of the ABA responsive element binding factor (ABF) gene, which endows KdN41-overexpressing plants with a slower growth rate than WT and NC plants under continuous drought stress (Supplementary Fig. S13). Therefore, the KdN41 gene in KdN41-overexpressing plants may be involved in balancing growth and drought stress resistance, and the low ratio of growth to drought stress resistance in KdN41-overexpressing plants is a result of “fine-tuning” of drought stress resistance.
The main approach in modern crop breeding is to cultivate a single species with multiple key agronomic traits (seed quality, biomass production, and pest and disease resistance) to cope with rapidly changing environmental conditions. For this purpose, low-input, space, and resource conservation, as well as biodiverse agriculture or horticulture, is essential26. K. daigremontiana is an ideal plant with rapid propagation and various environmental condition adaptation traits in agriculture and horticulture usage.
The KdN41 gene is an interesting example that reflects precise control between growth rate and drought stress resistance. In this study, the 35 S promoter was used to drive KdN41 gene expression in various tissues. KdN41 overexpression was found to confer improved drought resistance, but it could also decrease biomass production due to reduced leaf size in transgenic tobacco. However, an inducible or tissue-specific promoter to control KdN41 gene expression could be used to regulate expression of this gene in specific situations. One potential approach is to apply the RD29A promoter (a specific drought response promoter) to regulate KdN41 gene expression. During water shortages in the drought season, this gene may function in stressed crop tissues to enhance the survival rate. When the water availability returns to normal, the crop will provide better biomass production compared to non-breeding cultivars. Considering the early flowering phenomenon in KdN41-overexpressing tobacco, this could be beneficial for breeding based on the crop flowering time. Generating a crop with a controllable flowering time in a specific season would address the late flowering problem in agriculture and horticulture production. Future studies on other gene candidates for crop breeding may be possible by exploring the KdN41 gene network.
Methods
Plant materials and growth conditions
Tobacco and K. daigremontiana were grown in substrate (peat:perlite = 3:1) under a 16/8-h light (250 μmol m−2 s−1) cycle at 25 °C at 50–70% relative humidity in a growth room.
RACE assay, Genome-walking assay and bioinformatics analysis of KdN41 gene
DNA and RNA extractions from K. daigremontiana were performed as described previously27,28. Based on a partial cDNA sequence, TaKaRa 5′-Full-RACE (TaKaRa, Japan) and TaKaRa 3′-Full-RACE (TaKaRa, Japan) kits were used to amplify the 5′- and 3′-ends of the full length KdN41 cDNA sequence with cDNA template. According to a partial gDNA sequence of KdN41 gene, TaKaRa Genome Walking Kit (TaKaRa, Japan) was employed to amplify 5′ flanking sequence containing promoter. The KdN41 gene ORF was predicted using the ORF Finder program (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), and cis-acting elements in the promoter were predicted using the Plant Cis-Acting Regulatory Element (PlantCare; http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) database. Conserved domains were predicted using the NCBI Conserved Domain Search program (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), and CDS-encoding protein characteristics were predicted using the ProtParam (http://web.expasy.org/protparam/), iPSORT (http://ipsort.hgc.jp/), and PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/) programs.
Hormone treatments in K. daigremontiana
Three-month-old WT K. daigremontiana plants were divided into five groups. All groups were treated using the foliage spray method, as follows: 500 mL H2O (CK), 500 mL of 100 μM ABA (product number: A1049, Sigma, USA), 500 mL of 100 μM SA (product number: S7401, Sigma, USA), 500 mL of 100 μM GA3 (product number: G7645, Sigma, USA), and 500 mL of 100 μM MeJa (product number: 392707 ALDRICH, USA) respectively. RNA was extracted from the leaves of three individual plants (each representing a biological replicate) from each group after 2, 4, 6, and 10 h. cDNA synthesis and real-time PCR analyses were performed using the TransStart® Tip Green qPCR SuperMix (TransGen Biotech, Beijing), following the manufacturer’s instructions, and an ABI Step One PCR instrument (Applied Biosystems). The results were calculated using the 2−ΔΔCt method29. The assay for each gene used three biological replicates as templates (For each biological replicate, three technical replicates were used. Thus, a total of nine reactions were run.) The partial cDNA sequence of the KdActin gene was used as a reference gene. Because this gene indicated stable expression level among different plant organs including leaf, stem and root in previous study. Leaf of CK was treated as a reference tissue.
Vector construction and tobacco transformation
The complete KdN41 ORF cDNA sequence was introduced into the pBIN438 plasmid driven by the enhanced CaMV35S promoter as an overexpression construct (35S::N41); A tissue expression construct of the KdN41 gene (Promoter KdN41 ::GUS) was designed by using its 197-bp promoter sequence to replace the CaMV35s promoter in the pBI121 plasmid, to drive expression of the GUS gene. The empty plasmids pBIN438 (35S::None) and pBI121 (35S::GUS) were used as a NC and PC in further experiments. YFP was employed as a reporter. Recombinant pGTVII plasmids containing 35S::N41::YFP and 35S::YFP (as control) were constructed respectively. All plasmids mentioned above were transformed into the Agrobacterium tumefaciens strain LBA4404.
The tobacco plants were infected by A. tumefaciens strain LBA4404 with the constructs mentioned above through previous method30. Confirmation of positive transformation in tobacco T0 generation of each construct was performed by PCR amplification of the KdN41 gene ORF, KdN41 promoter, partial NPTII respectively (Supplementary Fig. S14–S17). Three primary transformed tobacco plants of each construct were selected randomly to provide T1 progeny. Seeds of primary transgenic plants were grown on Murashige and Skoog (MS) medium with a 50−mg L−1 kanamycin selection pressure for three weeks (WT seeds were grown on MS medium) (Supplementary Table S4).
Transgenic tobacco line stress treatments
Drought stress treatment
T1 seedlings from three individual lines of each construct were used, and for each line, three seedlings were employed (nine seedlings in total). The combination of Promoter KdN41 ::GUS, PC, and WT (for KdN41 tissue expression) and the combination of OE, NC, and WT (for KdN41 function identification) were grown in MS liquid medium with 20% PEG6000 for 10 h as a drought stress treatment (controls were grown in liquid MS medium) at 25 °C under a 16/8-h light (250 μmol m−2 s−1) cycle at 50–70% relative humidity (Supplementary Table S5).
Three-month-old T1 generation plants from three individual lines of each construct were used (three biological replicates for each individual line, and nine plants per construct). KdN41 OE, WT, and NC tobacco plants grown in peat substrate were watered to pot capacity and allowed to dry gradually. After the first sign of wilting was observed, soil water content was measured using a Delta T wet sensor (Delta-T Devices Ltd., UK). Three weeks after soil drying, LD was reached at 8–10% (8.34% average) soil water content; four weeks after soil drying, MD was reached at 3–5% (3.42% average) soil water content; five weeks after soil drying, severe SD was reached below 1% (0.21% average) soil water content. Each kind of transgenic plant and WT contained a non-treatment group (NT) that remained well-watered as a control (Supplementary Table S5).
Salt stress treatment
T1 seedlings from three individual lines of each construct were used, and for each line, fifteen seedlings were employed (forty-five seedlings per construct). The combination of Promoter KdN41 ::GUS, PC, and WT (for KdN41 tissue expression) and the combination of OE, NC, and WT (for KdN41 function identification) were treated as follows (Supplementary Table S5): grown on solid MS medium containing 400 mM NaCl at 25 °C under a 16/8-h light (250 μmol m−2 s−1) cycle at 50–70% relative humidity for 7 d for salt stress treatment (controls were grown on MS medium).
Heat stress treatment
T1 seedlings from three individual lines of each construct were used, and for each line, fifteen seedlings were employed (forty-five seedlings per construct). The combination of Promoter KdN41 ::GUS, PC, and WT (for KdN41 tissue expression) and the combination of OE, NC, and WT (for KdN41 function identification) were treated as follows (Supplementary Table S5): grown on MS medium at 50 °C for 7 h in a growth chamber for heat stress treatment (controls were grown on MS medium at 25 °C).
Histochemical staining assay
For GUS protein expression localization, tobacco leaves were stained as described previously31. H2O2 localization in tobacco leaves was detected using the DAB and NBT staining methods32. Images are representative of >10 observed samples stained in three independent experiments for each stress treatment.
YFP localization assay
One-month old tobacco leaves were injected with the LBA4404 strain containing one of the plasmids mentioned above, as described previously33. After 48 h, microscopic observations for YFP or KdN41-YFP fusion protein were performed using a Confocal Laser Scanning Platform Leica TCS SP8 (Leica, Germany).
Physiological traits and gene expression quantification
The photosynthetic activities of the three uppermost fully developed leaves were measured at three individual stress stages using a Li-6400 (LI-COR, USA). POD and CAT activities of leaves were measured following a previously described method34. EL of 0.1 g of fresh leaves from three individual stress stages was measured using a previously described method35. Proline, MDA, H2O2 and O2− contents were measured following a previously described method32. Total RNA was extracted from 0.1 g of tobacco leaves using the Eastep® Super Total RNA Extraction Kit (Promega, Shanghai) and cDNA was synthesized using the GoScript™ Reverse Transcription System (Promega, Shanghai) and real-time PCR was performed using the TransStart® Tip Green qPCR SuperMix (TransGen Biotech, Beijing), following the manufacturer instructions, using an ABI Step One PCR instrument (Applied Biosystems). The results were calculated using the 2−ΔΔCt method29. The reference gene used for Quantitative Real-time PCR was NtEF1α, which is a housekeeping gene (GenBank accession no. D63396). WT tobacco leaf of NT was chosen as a reference tissue. Three individual lines (three plants for each line as technical replicates, totally nine plants) of OE, WT, and NC were used as biological replicates.
Hormone detection in transgenic tobacco plants
Raw hormone mixtures were extracted from 0.1 g of tissue from the uppermost fully developed leaf with 1 mL of pH 7.0 PBS. ABA concentrations were measured using a colorimetric ELISA kit containing plates pre-coated with antibody specific to ABA. (Winter Song Boye Biotechnology Co. Ltd., Beijing). Samples were diluted six-fold prior to the tests. The standard curve was generated based on a series of known ABA standard sample reactions to 180, 120, 60, 30, 15, and 0 ng mL−1. Three individual lines from each transgenic plant and WT were used. Three biological replicates of each individual line of each kind of transgenic plant were used.
Bioinformatic analysis of SSH library
A SSH library from the leaves of K. daigremontiana induced by drought stress was obtained as previous described16. Different expressed genes (DEGs) as ESTs between normal watering and drought treatment samples were further annotated by Nr, Nt, Swiss-prot, and KEGG, and classified by COG and GO.
Statistical analyses
ANOVA and mean comparisons were performed using SPSS version 20.0 software. The value of 2−ΔΔCT was also proceeded with SPSS version 20.0 software. Error bars represent standard deviation. *And different letters indicate statistically significant differences at p < 0.05 based on Duncan’s multiple range test. Figures were created using SciDavis 0.2.4.
Primers
Primers were listed in supplementary materials (Supplementary Table S6).
References
Su, Z. et al. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25, 3785–807 (2013).
Breshears, D. D. et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102, 15144–8 (2005).
Chaves, M. M., Flexas, J. & Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103, 551–60 (2009).
Lawlor, D. W. & Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot 103, 561–79 (2009).
Xiong, L., Schumaker, K. S. & Zhu, J. K. Cell signaling during cold, drought, and salt stress. Plant Cell 14, Suppl S165–83 (2002).
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53, 247–73 (2002).
Hirayama, T. & Shinozaki, K. Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12, 343–51 (2007).
Klingler, J. P., Batelli, G. & Zhu, J. K. ABA receptors: the START of a new paradigm in phytohormone signalling. J Exp Bot 61, 3199–210 (2010).
Nambara, E. & Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56, 165–85 (2005).
Yin, F. et al. Genome-wide analysis of water-stress-responsive microRNA expression profile in tobacco roots. Funct Integr Genomics 14, 319–32 (2014).
Lepiniec, L. et al. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57, 405–30 (2006).
Yamaguchi, S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59, 225–51 (2008).
Garces, H. M. et al. Evolution of asexual reproduction in leaves of the genus Kalanchoe. Proc Natl Acad Sci USA 104, 15578–83 (2007).
Garces, H., and Sinha, N. The ‘mother of thousands’ (Kalanchoe daigremontiana): a plant model for asexual reproduction and CAM studies. Cold Spring Harb Protoc 2009, pdb.emo133 (2009a).
Winter, K., Garcia, M. & Holtum, J. A. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoe, and Opuntia. J Exp Bot 59, 1829–40 (2008).
Zhong, T., Zhu, C., Zeng, H. & Han, L. Analysis of gene expression in Kalanchoe daigremontiana leaves during plantlet formation under drought stress. Electronic Journal of Biotechnology 16, 4–4 (2013).
You, J. & Chan, Z. ROS Regulation during abiotic stress responses in crop plants. Front Plant Sci 6, 1092 (2015).
Zhang, N. et al. Treatment with spermidine protects chrysanthemum seedlings against salinity stress damage. Plant Physiol Biochem 105, 260–270 (2016).
Liu, X. et al. Repression of ARF10 by microRNA160 plays an important role in the mediation of leaf water loss. Plant Mol Biol. https://doi.org/10.1007/s11103-016-0514-3 (2016).
Kehr, J. & Buhtz, A. Long distance transport and movement of RNA through the phloem. J Exp Bot 59, 85–92 (2008).
Scoffoni, C., Rawls, M., McKown, A., Cochard, H. & Sack, L. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156, 832–43 (2011).
Carins, M. M., Jordan, G. J. & Brodribb, T. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ 37, 124–131 (2014).
Liu, Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, In Arabidopsis. Plant Cell 10, 1391–406 (1998).
Singh, D. & Laxmi, A. Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Front Plant Sci 6, 895 (2015).
Wei, T. et al. Arabidopsis DREB1B in transgenic Salvia miltiorrhiza increased tolerance to drought stress without stunting growth. Plant Physiol Biochem 104, 17–28 (2016).
Litrico, I. & Violle, C. Diversity in plant breeding: A new conceptual framework. Trends Plant Sci. 20, 604–613 (2007).
Garces, H. & Sinha, N. Extraction of DNA from the plant Kalanchoe daigremontiana. Cold Spring Harb Protoc 2009, pdb.prot5304 (2009b).
Garces, H. & Sinha, N. Extraction of RNA from the plant Kalanchoe daigremontiana. Cold Spring Harb Protoc 2009, pdb.prot5305 (2009c).
Reiner, A., Yekutieli, D. & Benjamini, Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19, 368–75 (2003).
Lai, B. et al. Two LcbHLH transcription factors Interacting with LcMYB1 in regulating late structural genes of anthocyanin biosynthesis in Nicotiana and Litchi chinensis during anthocyanin accumulation. Front Plant Sci 7, 166 (2016).
Kang, J. & Dengler, N. Cell cycling frequency and expression of the homeobox gene ATHB-8 during leaf vein development in Arabidopsis. Planta 216, 212–9 (2002).
Luo, P. et al. Overexpression of Rosa rugosa anthocyanidin reductase enhances tobacco tolerance to abiotic stress through increased ROS scavenging and modulation of ABA signaling. Plant science: an international journal of experimental plant biology 245, 35–49, https://doi.org/10.1016/j.plantsci.2016.01.007 (2016).
Fang, Y. & Spector, D.L. BiFC imaging assay for plant protein-protein interactions. Cold Spring Harb Protoc 2010, pdb.prot5380 (2010).
Zhang, Y. et al. Freezing resistance in Patagonian woody shrubs: the role of cell wall elasticity and stem vessel size. Tree Physiol. https://doi.org/10.1093/treephys/tpw036 (2016).
Roqueiro, G. et al. Effects of photooxidation on membrane integrity in Salix nigra seeds. Ann Bot 105, 1027–34 (2010).
Acknowledgements
This project was supported by Special Fund for Forest Scientific Research in the Public Welfare under Grant No. 201504704 and the Fundamental Research Funds for the Central Universities, No. BLYJ201506. We are grateful to Prof. Rajeev Arora (Iowa State University) for providing valuable revision comments.
Author information
Authors and Affiliations
Contributions
Li Wang, Chen Zhu and Luyi Ma conceived and designed research. Li Wang, Chen Zhu and and Lin Jin conducted experiments. Aihua Xiao and Jie Duan analysed data. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Zhu, C., Jin, L. et al. A novel gene of Kalanchoe daigremontiana confers plant drought resistance. Sci Rep 8, 2547 (2018). https://doi.org/10.1038/s41598-018-20687-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20687-5
This article is cited by
-
Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils
Environmental Science and Pollution Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.