Abstract
In this analysis, we identified febrile cancer patients with documented infections or neutropenia, whose procalcitonin levels are low at baseline or decrease on antibiotics. These patients had similar outcomes in terms of mortality and relapse of infection regardless of the duration of antimicrobial therapy (less or more than 7 days).
Similar content being viewed by others
Introduction
Clinical decisions are often complex and require serial patient evaluations to assess therapeutic responses and ensure favorable patient outcomes.
The management of febrile cancer patients could be particularly challenging. These patients frequently do not present with typical clinical syndromes and have multiple simultaneous sources of infectious and non-infectious causes of fever.
Because of their immunocompromised status due to the malignancy itself and treatment related toxicities, an empiric antimicrobial therapy is often begun in cancer patients presenting with fevers leading to a significant cost expenditure particularly in hospitalized patients1. However, increased antimicrobial use is associated with unwanted adverse effects and emergence of antimicrobial resistance leading to poor outcomes including increased mortality2,3. Serologic biomarkers might be useful adjunctive markers to guide antibiotic therapy. Procalcitonin (PCT) is a hormokine that has been studied in the general population to guide antimicrobial duration in the intensive care unit (ICU) as well as initiation of antibiotics in lower respiratory tract infections4,5,6. However, these studies excluded immunocompromised patients. Through several observational studies we have demonstrated that Procalcitonin could be a useful prognostic biomarker in febrile cancer patients with documented infections. However we did not study the role of PCT in guiding the duration of antimicrobial therapy7,8.
The objective of this study is to compare the outcomes of selected febrile cancer patients tested for procalcitonin based on their antimicrobial therapy duration. The patients were selected based on their PCT levels to help explore the applicability of a PCT based algorithm in guiding antimicrobial duration in cancer patients and febrile neutropenia.
Methods
This is study is a post-hoc analysis of a prospective observational cohort conducted at a tertiary care center between July 2009 and July 2010.
Febrile cancer patients admitted to MDACC with available PCT levels were eligible for the study.
PCT was measured in the research laboratory using the Kryptor bioanalyser via an immunofluorescent assay with a lower detection limit of 0.075ng/mL. The PCT results were not available to the treating physician and did not therefore dictate the course of the antibiotics.
For every febrile patient, PCT levels were obtained on residual patient sera upon their initial presentation to the hospital for fever and subsequently, at days 4 to 7.
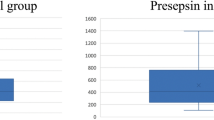
We included in this study febrile cancer patients who had clinically or microbiologically documented infection or presented with febrile neutropenia with no obvious source of infection. In addition, and based on cutoffs used in prior studies4, patients had to meet PCT response definition as follows: a PCT level less than 0.25 ng/ml on days 4–7 or a PCT level that has dropped by 80% on days 4–7 from baseline. Patients who received a short course of IV antibiotic of 7 days or less (group A) were compared to those who received a longer course with an antibiotic duration of more than 7 days (group B).
Categorical variables were compared using Chi-square or Fisher’s exact test, as appropriate. Continuous variables were compared using Wilcoxon rank-sum test. All tests were two-sided tests with a significance level of 0.05. The data analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
From July 2009 until June 2011, we identified 575 febrile cancer patients with available PCT levels that presented to the Emergency department or were admitted to MD Anderson Cancer Center.
Of the 196 patients who met the PCT response criteria, 121 had either febrile neutropenia or a documented infection and received antimicrobial treatment. These patients were further divided in two groups based on their antibiotic therapy duration: 7 days or less (group A) versus more than 7 days (group B).
The baseline characteristics of the study population were comparable among the 2 groups in terms of sex, age, type and stage of malignancy, as well as rate of neutropenia (absolute neutrophil count (ANC) < 500) and active cancer therapy (Table 1).
The sites of infection were also comparable among both groups. Half the patients had microbiologically documented infections. The most common sources of infection were bacteremia (18 vs 23%) and pneumonia (18% in both groups). No identifiable source was documented in 30% and 32% of groups A and B respectively (Table 1).
The outcomes were similar among both groups including resolution of the infection, rate of relapse, infection related mortality and 30 day- mortality, regardless of the antimicrobial duration received. They were also similar in terms of microbiologic eradication and time to defervescence (Table 1).
Discussion
Our study shows that a selected group of febrile cancer patients, particularly those with a low baseline PCT or a PCT that decreases with appropriate therapy might not require prolonged antimicrobial therapy beyond 7 days. These patients have similar rates of infection resolution, relapse and mortality compared with those receiving a prolonged course of antimicrobial therapy. This is the first study exploring the use of procalcitonin as a theragnostic marker in febrile cancer patients with documented infections or neutropenia.
A specific challenge to the cancer population is the elevation in inflammatory markers driven by the tumor and mucositis8,9. However, previous studies in the cancer population, showed that PCT levels were higher in cancer patients with fever compared to those without fever. In addition, PCT levels were the highest in febrile cancer patients with sepsis or bacteremia8. Furtheremore, in patients with suspected or documented bacterial infections, PCT levels decreased on appropriate therapy10.
Another important aspect is the potential role of PCT in guiding antimicrobial therapy duration in febrile neutropenia. The practice of keeping cancer patients, particularly neutropenic patients on prolonged duration of antimicrobials is based on literature going back to 1969. During that time, Pizzo et al.11 showed a higher rate of recurrent fevers when antimicrobial therapy was discontinued before the resolution of neutropenia in patients with no clear source of infection. In this particular group, laboratory markers may have a role in identifying patients who could safely receive a brief duration of therapy while simultaneously limiting unnecessary extended course of antimicrobial therapy. One small randomized controlled trial of PCT use for antimicrobial PCT duration in low risk febrile neutropenia failed to achieve significant reduction in antimicrobial therapy duration12. However, this may not apply to high risk profoundly neutropenic patients who are exposed to extensive antimocrobials for a prolonged duration.
Our study has several limitations: the sample size is small and the analysis is post-hoc, making specific subgroups related analyses statistically difficult. The study does not take into account the severity of illness of patients in both groups. In addition, PCT results were not made available to the treating physciain and it is unclear if more frequent PCT monitoring can further shorten the duration of antimicrobial therapy.
Conclusion
PCT might be an adjunctive biomarker in identifying patients who could be candidates for a short duration of antimicrobial therapy particularly cancer patients with a non-infectious cause for the fever. PCT guided algorithm may limit the duration of antibiotics, reduce adverse events and prevent the emergence of antimicrobial resistance.
Large randomized controlled trials comparing procalcitonin guided antimicrobial therapy vs. standard of care to limit unnecessary exposure to antimicrobials in immunocompromised cancer patients are warranted.
Change history
19 April 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Dela-Pena, J. et al. Top 1% of Inpatients Administered Antimicrobial Agents Comprising 50% of Expenditures: A Descriptive Study and Opportunities for Stewardship Intervention. Infect Control Hosp Epidemiol, 1–7, https://doi.org/10.1017/ice.2016.261 (2016).
Cosgrove, S. E. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 42(Suppl 2), S82–89, https://doi.org/10.1086/499406 (2006).
Huttner, A. et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2, 31, https://doi.org/10.1186/2047-2994-2-31 (2013).
Bouadma, L. et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet (London, England) 375, 463–474, https://doi.org/10.1016/s0140-6736(09)61879-1 (2010).
Schuetz, P. et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evidence-based child health: a Cochrane review journal 8, 1297–1371, https://doi.org/10.1002/ebch.1927 (2013).
de Jong, E. et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. The Lancet. Infectious diseases, https://doi.org/10.1016/s1473-3099(16)00053-0 (2016).
Debiane, L. et al. The utility of proadrenomedullin and procalcitonin in comparison to C-reactive protein as predictors of sepsis and bloodstream infections in critically ill patients with cancer*. Critical care medicine 42, 2500–2507, https://doi.org/10.1097/ccm.0000000000000526 (2014).
Chaftari, A. M. et al. Role of Procalcitonin and Interleukin-6 in Predicting Cancer, and Its Progression Independent of Infection. PloS one 10, e0130999, https://doi.org/10.1371/journal.pone.0130999 (2015).
Blijlevens, N. M., Donnelly, J. P., Meis, J. F., De Keizer, M. H. & De Pauw, B. E. Procalcitonin does not discriminate infection from inflammation after allogeneic bone marrow transplantation. Clinical and diagnostic laboratory immunology 7, 889–892 (2000).
Shomali, W. et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 118, 5823–5829, https://doi.org/10.1002/cncr.27602 (2012).
Pizzo, P. A. et al. Duration of empiric antibiotic therapy in granulocytopenic patients with cancer. The American journal of medicine 67, 194–200 (1979).
Lima, S. S. et al. Procalcitonin-guided protocol is not useful to manage antibiotic therapy in febrile neutropenia: a randomized controlled trial. Annals of hematology, https://doi.org/10.1007/s00277-016-2639-5 (2016).
Author information
Authors and Affiliations
Contributions
Hanine El Haddad, MD: Data collection, writing the manuscript. Anne-Marie Chaftari MD: Study design, data analysis, writing the manuscript. Ray Hachem, MD: Study design, data analysis, writing the manuscript. Majd Micheal, MD: Data collection. Ying Jiang, MS: Data analysis. Ammar Yousif, MD: Data collection. Sammy Raad, MS: Data collection and PCT measurement. Mary Jordan, MD: Data collection. Patrick Chaftari, MD: Results discussion and critical review of the manuscript. Issam Raad, MD: Study design, project supervision, data analysis, critical review of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haddad, H.E., Chaftari, AM., Hachem, R. et al. Procalcitonin Guiding Antimicrobial Therapy Duration in Febrile Cancer Patients with Documented Infection or Neutropenia. Sci Rep 8, 1099 (2018). https://doi.org/10.1038/s41598-018-19616-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19616-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.