Abstract
We investigated the association between von Willebrand factor (VWF) and exudative age-related macular degeneration (AMD) in 114 Japanese patients. Intravitreal injection of vascular endothelial growth factor (VEGF) inhibitor is the most effective therapy for AMD. Therefore, we analyzed changes of VWF antigen (VWF:Ag) and VWF multimers (VWFMs) after intravitreal injection of aflibercept, an anti-VEGF antagonist. The relationship between polymorphisms in complement factor H (p.Y402H and p.I62V) and AMD was previously reported. In our patients, p.I62V, but not p.Y402H, was significantly associated with an increased risk of AMD. Pre-treatment plasma levels of VWF:Ag in patients with AMD were significantly higher than those in controls. Unusually large VWFMs (UL-VWFMs) were detected in the majority of AMD patients with concurrent vitreous or subretinal hemorrhage. After intravitreal injection of aflibercept, plasma levels of VWF:Ag and VEGF-A were significantly decreased. UL-VWFMs disappeared after aflibercept injection in three cases, but persisted even 1 month after injection in the other five cases. In conclusion, plasma VWF:Ag levels were significantly elevated in patients with AMD, and decreased after intravitreal aflibercept injection. VWF may play an important role in the pathophysiology of AMD, and aflibercept might improve AMD by reducing plasma levels of VWF in addition to VEGF-A.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a chorioretinal degenerative disease that occurs mainly in the elderly, and is the leading cause of severe vision loss in industrialized countries1,2. Most cases of AMD with vision loss result from pathologic choroidal neovascularization (CNV) in the exudative form of the disease. Vascular endothelial growth factor (VEGF), a key mediator of angiogenesis3, was previously shown to be elevated in the eyes of patients with AMD4. Intravitreal injection of a VEGF inhibitor is currently the most effective means of arresting CNV in AMD5,6. In particular, the intravitreal injection of aflibercept, the latest VEGF antagonist, significantly decreased plasma VEGF concentrations 1 month after injection7,8. However, the pathogenesis of AMD is still poorly understood.
Recent association studies determined that the coding variant p.Y402H in the complement factor H (CFH) gene was strongly associated with AMD in numerous Caucasian populations9,10,11. The Y402H variant, however, has not shown a significant association with AMD in Asian populations12,13. Meanwhile, some studies found that the CFH I62V variant was associated with AMD in both Caucasian and Asian populations14,15.
von Willebrand factor (VWF) is a multimeric plasma glycoprotein consisting of a single 250-kD subunit, linked by disulfide bonds in a head-to-head and tail-to-tail fashion, that plays an essential role in primary hemostasis through anchoring platelets onto denuded subendothelial matrices16. Plasma VWF is synthesized in vascular endothelial cells and released into the circulation as unusually large VWF multimers (UL-VWFMs), the most biologically active form of VWF17. In the normal circulation, UL-VWFMs are rapidly degraded under high shear stress into smaller VWFMs by the metalloproteinase ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motifs 13)18. Deficiency of ADAMTS13 activity (ADAMTS13:AC) induces the accumulation of UL-VWFMs and leads to platelet hyperaggregation under high shear stress, resulting in thrombotic thrombocytopenic purpura (TTP), a life-threatening blood disease17.
Notably, recent studies have indicated that CFH reduces VWF multimer sizes through its action as a disulfide-bond reductase or by conferring a heightened susceptibility to ADAMTS13 by binding to VWF19,20. Further, Randi and Laffan21 more recently reported that VWF controls angiogenesis and vascular maturation through multiple pathways. In 2001, Lips et al.22 demonstrated increased plasma levels of VWF in patients with exudative AMD, but no further information has been published since then. In this study, therefore, we extensively analyzed plasma VWF and its multimer forms, as well as ADAMTS13, VEGF-A, and CFH single nucleotide polymorphisms (SNPs) in Japanese patients with AMD.
Results
Characteristics of Study Subjects
One hundred and fourteen patients with exudative AMD, aged between 70.2 and 82.0 years old, and 105 age-matched control subjects with cataracts were enrolled in the study. The characteristics of AMD patients and controls are summarized in Table 1. Exudative AMD was classified into three subtypes, including typical AMD, polypoidal choroidal vasculopathy (PCV), and retinal angiomatous proliferation (RAP). In this study, 70 patients were diagnosed with typical AMD, and the remaining 44 patients were diagnosed with PCV. There were no patients with RAP in this study. Sex and cigarette smoking (past or current) were found to be significantly associated with AMD, consistent with a previous study showing that these were putative risk factors for the disease23. However, no significant association was found between AMD and body mass index, dyslipidemia, or hypertension. Further, although it was reported that plasma levels of VWF antigen (VWF:Ag) in individuals with blood group O were lower than in those with non-O blood groups24, no significant difference in the distribution of ABO blood groups was found between AMD patients and controls in this study.
Plasma levels of VEGF-A, VWF:Ag, and ADAMTS13:AC
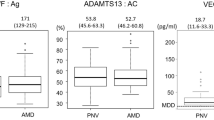
In contrast to previous reports7,22, plasma levels of VEGF-A in untreated patients with AMD were significantly lower than those in controls (p = 0.01) (Fig. 1A). The median age of patients in both the AMD and control groups was over 70 years, and plasma levels of VWF:Ag in both groups were higher than those in healthy individuals (aged between 20 and 40 years old). Plasma levels of VWF:Ag in untreated patients with AMD were significantly higher than those in controls (p < 0.001) (Fig. 1B). However, plasma levels of ADAMTS13:AC were almost the same in AMD patients and controls (Fig. 1C) and were low compared with those in young, healthy individuals (53.4% and 55.7% vs 100%, respectively).
Plasma levels of VEGF-A, VWF antigen, and ADAMTS13 activity in patients with untreated exudative AMD and controls. (A) Plasma levels of VEGF-A in patients with AMD were significantly lower than those in controls (**p < 0.01). The minimum detectable dose (MDD) of VEGF-A defined by the manufacturer was 9 pg/ml. (B) Plasma levels of VWF antigen (:Ag) in patients with age-related macular degeneration (AMD) were significantly higher than those in controls (***p < 0.001). (C) Plasma ADAMTS13 activity (:AC) levels in patients with AMD were almost the same as those in controls.
VWF multimer analysis in untreated AMD patients
As shown in Figs 2 and 3, S1, and S2, VWF multimer analysis was performed in all 114 patients with AMD. Each patient number (No.) enclosed by a square indicates a patient with PCV. Three patients (Nos. 1, 6, and 21) had vitreous hemorrhage (VH), 11 patients (Nos. 10, 11, 15, 19, 23, 27, 33, 34, 36, 53, and 55) had subretinal hemorrhage (SH) ≥ 1 disc area (DA), and 4 patients (Nos. 14, 17, 28, and 38) had SH < 1 DA. Both VH and SH cause severe vision loss.
VWF multimer analysis in patients with untreated exudative AMD. Fifty-two out of 114 patients with untreated exudative AMD, patient number (Nos.) 1–52, were shown in this figure. Each patient number enclosed by a square indicates a patient with polypoidal choroidal vasculopathy (PCV). UL-VWFMs were found in 18 patients with AMD, including 9 patients with PCV and 9 with typical AMD. Of these, 3 patients (Nos. 1, 6, and 21) had vitreous hemorrhage (VH) and 6 (Nos. 11, 15, 23, 33, 34, and 36) had subretinal hemorrhage (SH) ≥1 disc area (DA). Asterisks indicate UL-VWFM positivity. NP: normal pool plasma.
VWF multimer analysis before and after intravitreal injection of aflibercept. We performed VWF multimer analysis in 22 patients with exudative AMD received an intravitreal injection of aflibercept before and at 1 day, 1 week, and 1 month after injection. Of these, VWF multimer analysis of 8 patients (patient Nos. 53–60) were shown in this figure. Each patient number enclosed by a square indicates a patient with polypoidal choroidal vasculopathy (PCV). Patient Nos. 53 and 55 had subretinal hemorrhage and UL-VWFMs before treatment. Patient Nos. 54 and 56 also showed UL-VWFMs before treatment. Patient Nos. 55 and 56 had UL-VWFMs even at 1 month after injection. Asterisks indicate UL-VWFM positivity. NP: normal pool plasma.
UL-VWFMs were found in 31 patients (27.2%), including 15 of 44 PCV patients (34.1%) and 16 of 70 typical AMD patients (22.8%). Furthermore, all 3 patients with VH (100%), 8 out of 11 patients with SH ≥ 1 DA (72.4%), 2 out of 4 patients with SH < 1 DA (50.0%) and 18 of 96 patients without ocular hemorrhage (18.7%) had UL-VWFMs. By contrast, UL-VWFMs were not detected in 40 age-matched controls (patients with cataracts) (Supplementary Fig. S3).
Influence of CFH polymorphisms on plasma levels of VWF:Ag, ADAMTS13:AC, and VEGF-A
Previous studies have demonstrated a significant association between the incidence of AMD and CFH polymorphisms (p.I62V and p.Y402H)9,10,11,14. As mentioned above, it is now thought that CFH down-regulates VWF multimer sizes via disulfide-bond reductase activity or by increasing VWF susceptibility to ADAMTS1319,20.
To further investigate this issue, we first analyzed the SNPs of CFH in our patients, and found that the GG genotype in the p.I62V polymorphism was significantly associated with an increased risk of AMD [Odds ratio (OR) = 6.88, 95% confidence interval (CI) = 2.03–23.29], whereas no association was found with the p.Y402H polymorphism (Supplementary Table S1). Further, after adjusting for age, gender, and smoking (past or current), significant associations were still found for p.I62V, but not for p.Y402H (Supplementary Table S1).
Next, we analyzed the relationship between plasma VWF:Ag and CFH polymorphisms. There was no association between plasma levels of VWF:Ag and either the p.I62V or p.Y402H mutations (Supplementary Table S2). Further, the CFH polymorphisms (p.I62V and p.Y402 H) were not associated with plasma levels of either ADAMTS13:AC or VEGF-A (Supplementary Table S2).
Changes of plasma levels of VEGF-A, VWF:Ag, and ADAMTS13:AC after the intravitreal injection of aflibercept
In 82 patients with AMD received intravitreal injections of aflibercept, 22 patients consented to the follow-up analysis after the injection. Plasma levels of VEGF-A, VWF:Ag, and ADAMTS13:AC were analyzed in these 22 patients with AMD at 1 day, 1 week, and 1 month after the intravitreal injection of aflibercept. As shown in Fig. 4A, the mean plasma level of VEGF-A was significantly decreased in all patients at 1 day after the injection (<9.0 pg/ml) compared with before the injection (15.4 pg/ml) (p < 0.001). Levels of VEGF-A then gradually recovered, but at 1 week after the injection, 18 of 22 patients still had levels below 9.0 pg/ml (p < 0.001). At 1 month after the injection, 11 of 22 patients had levels below the detection limit (p = 0.01).
Interestingly, plasma VWF:Ag levels were highly influenced by the intravitreal injection of aflibercept, and thereafter significantly decreased over time (p = 0.004 at 1 week after, p = 0.003 at 1 month after) (Fig. 4B). However, no significant changes were observed in plasma levels of ADAMTS13:AC during the same period (Fig. 4C).
Plasma levels of VWF antigen, ADAMTS13 activity, and VEGF-A before and after intravitreal injection of aflibercept in patients with exudative AMD. (A) Plasma levels of VEGF-A were significantly decreased at 1 day after injection (***p < 0.001), and this reduction continued until 1 month after injection (*p < 0.05). (B) Plasma VWF:Ag levels gradually decreased after injection, with a significant reduction starting at 1 week (**p < 0.01). (C) No significant change was observed in ADAMTS13:AC after injection.
Changes of VWF multimers after the intravitreal injection of aflibercept
As shown in Fig. 3 and Supplementary Fig. S2, we investigated VWF multimers in all 22 patients with AMD who were followed up after the intravitreal injection of aflibercept. Eight (Nos. 53, 54, 55, 56, 101, 102, 104, and 109) of the patients had UL-VWFMs before the injection, and 2 (Nos. 53 and 55) of these had current subretinal hemorrhage. In 3 patients (Nos. 53, 54, and 102), UL-VWFMs were disappeared at 1 month after injection. In the other 5 patients (Nos. 55, 56, 101, 104, and 109), UL-VWFMs were still present at 1 month after the injection. One patient (No. 110) had UL-VWFMs at 1 day and 1 week after injection. The remaining 13 patients exhibited no UL-VWFMs at any point during the same 1-month period.
Discussion
In this study, we showed that pre-treatment plasma levels of VWF:Ag were elevated in patients with AMD compared with controls, and for the first time demonstrated the presence of UL-VWFMs in patients with severe vitreous or subretinal hemorrhage.
VWF is produced in vascular endothelial cells. It is either constitutively secreted or stored in intracellular organelles, termed Weibel-Palade bodies (WPB), from which it is released into the circulation upon vessel injury or in response to various stimuli, including cytokines such as VEGF, epinephrine, and vasopressin25. Several studies showed that patients with exudative AMD have severe vascular disturbance22,26, which might be related to elevated plasma levels of VWF:Ag as well as enhanced exocytosis of VWF from WPB due to inflammatory cytokines. Thus, accumulation of UL-VWFMs in plasma may induce thrombus formation even in the eye, resulting in vascular injuries and bleeding, both of which may contribute to the development of CNV. However, there is also possibility that both elevated VWF:Ag and the presence of UL-VWFMs in plasma were the consequences of AMD itself.
Several studies have shown that CFH p.Y402H is significantly associated with AMD in Caucasian patients9,10,11, but not in Japanese patients12,27. This study confirmed the lack of association in a Japanese population, and was also consistent with previous findings showing a correlation between CFH p.I62V and Japanese AMD14,28.
Nolasco et al.20 showed that recombinant human CFH functions as a disulfide bond reductase, thereby decreasing the multimeric size of VWF. Hence, we investigated the relationship between CFH polymorphisms (p.Y402H and p.I62V) and VWF multimer size, but no clear relationship was found. This, however, might be due to the fact that no patients with CFH p.Y402H had the CC allele and only 4 patients with CFH p.I62V had the AA allele, indicating that many more patients need to be recruited in future studies before a final conclusion can be reached.
Curiously, in contrast with the findings of Lip et al.22, pre-treatment plasma levels of VEGF-A in our patients with exudative AMD were significantly lower than those in controls. We are currently unable to explain this discrepancy. Of note, one group also reported that plasma levels of VEGF-A in patients with untreated exudative AMD were lower than those in controls29.
In this study, however, intravitreal injection of aflibercept dramatically decreased plasma levels of VEGF-A in AMD patients and also gradually decreased levels of VWF:Ag. The reduction of both VWF:Ag and VEGF-A levels persisted up to 1 month after the injection. Rondaiji et al.25 reported that VEGF activated the exocytosis of WPBs, which releases VWF from endothelial cells. Aflibercept was shown to have the highest affinity of all VEGF inhibitors to date30. Interestingly, local intravitreal injection decreased the systemic levels of both VWF:Ag and VEGF-A in this study. Thus, targeted ocular anti-VEGF therapy appears to reduce plasma levels of VWF:Ag and prevent the development of CNV.
We found that plasma UL-VWFMs disappeared after the intravitreal injection of aflibercept in 3 cases (patient Nos. 53, 54, and 102). However, in the other 5 patients (Nos. 55, 56, 101, 104, and 109) with UL-VWFMs before treatment, these molecules were still present even at 1 month after aflibercept injection. The existence of UL-VWFMs appears to be a potential risk factor for intraocular thrombosis/bleeding or generalized thrombosis in such patients. Fortunately, the abnormal ophthalmological findings in the latter 5 patients improved at 1 month after the injection, but they were still at risk of conditions involving systemic thrombosis or bleeding, such as cerebral infarction or hemorrhage. Notably, such adverse complications of aflibercept have been reported in the literature31,32; therefore, further study of this issue is required in conjunction with UL-VWFM analysis.
This study has several limitations. First, it used a cross-sectional design with a relatively small sample size. We will continue this study and evaluate larger numbers of patients to better analyze the pathophysiology of AMD. Second, we sequentially evaluated only patients who were treated with intravitreal injections of aflibercept. It is necessary to compare plasma VWF in patients with and without intravitreal injections to verify the effect of aflibercept. However, it is difficult to analyze AMD patients who have received no prior treatment, because blood tests are not performed in such patients. Finally, we did not evaluate patients for longer than 1 month after the intravitreal injection of aflibercept. Long-term follow-up is necessary to confirm the efficacy of this treatment.
In conclusion, we found an association between plasma levels of VWF:Ag and exudative AMD. Plasma levels of VEGF-A and VWF:Ag were significantly decreased after the intravitreal injection of aflibercept. VWF regulates angiogenesis and could be a new biomarker for AMD. These findings can help expand our knowledge of the pathogenesis of AMD and may be relevant to the potential treatment of this disease.
Methods
Subjects
Two hundred and nineteen participants, including 114 treatment-naïve patients with exudative AMD and 105 age-matched controls who were scheduled for cataract surgeries were enrolled. This study was conducted in accordance with the Declaration of Helsinki and was performed after approval from the Institutional Review Board Committee of Nara Medical University. Written informed consent was obtained from all participants.
Diagnosis of exudative AMD
Exudative AMD was diagnosed primarily based on the ophthalmological findings, spectral-domain optical coherence tomography (SD-OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA) according to the definition of the Japanese Study Group guidelines33. Briefly, exudative AMD was diagnosed in patients who were 50 years or older with abnormalities in the macular area or retinal pigment epithelium (RPE), or with associated neurosensory detachment, retinal hemorrhage, or retinal fibrous scarring without other retinal disorders. Exudative AMD was classified into three types (typical AMD, PCV, and RAP). Eyes with typical neovascular AMD exhibited classic- or occult-type CNV in FA without polypoidal lesions in ICGA and SD-OCT findings of CNV either in the subretinal space or beneath the RPE line. Eyes with PCV exhibited clusters of polypoidal dilation of the vessels with or without abnormal vascular networks in the superficial choroid in ICGA and irregularly elevated RPE line in SD-OCT images. Eyes with RAP exhibited retinal–retinal or retinochoroidal anastomosis in FA or ICGA and retinal swelling with or without RPE detachment in SD-OCT images.
Injection technique
Eighty-two of 114 patients received intravitreal injections of aflibercept (Eylea; Bayer HealthCare Pharmaceuticals, Berlin, Germany) at a dose of 2.0 mg/0.05 ml. A compounding pharmacy using aseptic methods packaged the medication in a 1.0-ml syringe with an integrated 30-gauge needle. The medication was administered by injection 3.5–4.0 mm posterior to the limbus.
Blood sampling
Blood samples were collected in tubes containing sodium citrate. Centrifugation was performed at 3,000 g for 15 minutes immediately after sampling, and the obtained plasma was frozen in polypropylene tubes and stored at −80 °C in aliquots until needed for the appropriate assays.
Genetic analysis
Genomic DNA was extracted from leukocytes using a commercially available genomic DNA extraction and purification kit (QIAmpDNA; Qiagen, Valencia, CA, USA). SNPs of p.Y402H (rs1061170) and p.I62V (rs800292) in CFH were genotyped. Polymorphic sites were amplified by polymerase chain reaction (PCR) with specific primers34. PCR products were used as the templates for direct DNA sequencing (Applied Biosystems, Foster City, CA, USA) on an automated sequencer (3730xl DNA analyser; Applied Biosystems).
Assay of VWF antigen (:Ag), ADAMTS13 activity (:AC), and VEGF-A
Plasma VWF:Ag levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using rabbit anti–human VWF polyclonal antiserum (DAKO, Glostrup, Denmark)35. Plasma levels of ADAMTS13 activity (ADAMTS13:AC) were measured by chromogenic ADAMTS13 activity ELISA (Kinos Laboratories, Tokyo, Japan)36. The 100% reference value was defined as the amount of VWF:Ag and ADAMTS13:AC in pooled normal plasma from 20 normal volunteers (10 males and 10 females) aged between 20 and 40 years old. Plasma VEGF-A concentrations were measured by ELISA (Quantikine VEGF ELISA kit; R&D Systems, Minneapolis, MN, USA). The minimum detectable dose (MDD) defined by the manufacturer was 9.0 pg/ml.
VWF multimer analysis
Multimeric analysis of plasma VWF was performed according to the method of Ruggeri and Zimmerman37, with modifications as reported by Warren et al.38. The experimental conditions, including those for western blotting with luminographic detection, were as previously described by Budde et al.39. High molecular weight bands that were not detected in normal plasma were defined as UL-VWFMs. The blots were scanned and subjected to densitometric analysis using ImageJ (National Institute of Health, Bethesda, MD, USA). For quantitative analyses, we caluculated the ratio of the densities of UL-VWFMs relative to total VWFM densities. In this study, we judged the positive of UL-VWFM when the ratio of UL-VWFM was over 0.1%.
Statistical analyses
All statistical analyses were performed using the SPSS 24.0 (IBM Corporation, Armonk, NY, USA) statistical software package. The chi-squared test was used to assess whether the genotype distribution of the controls was in agreement with the Hardy-Weinberg equilibrium. Comparison of genotype frequency between cases and controls was conducted by chi-squared analysis. The logistic regression model was used to analyze the association between AMD and age, gender, smoking status, CFH p.Y402H, and CFH p.I62V. Odds ratios with 95% confidence intervals were calculated by the chi-squared test to evaluate the association between risk of AMD and CFH polymorphisms. Continuous data are given as median and interquartile range (IQR) in parentheses and qualitative data are given as percentages. The Kolmogorov-Smirnov test was used to test for normal distribution. VEGF-A measurements were not normally distributed, and exact values were not given partially because the values were below the minimum detectable dose of 9 pg/ml. Therefore, non-parametric testing was applied and a conservative value of 8 pg/ml was imputed for each value below MDD when performing the calculations. Comparisons between cases and controls were performed using the chi-squared test or Mann-Whitney U test as appropriate. Within-treatment-group comparisons were performed with the Friedman and Wilcoxon signed-rank tests. P values < 0.05 were considered to be statistically significant.
References
Bressler, N. M. Age-related macular degeneration is the leading cause of blindness. JAMA 291, 1900–1901 (2004).
Lim, L. S., Mitchell, P., Seddon, J. M., Holz, F. G. & Wong, T. Y. Age-related macular degeneration. Lancet 379, 1728–1738, https://doi.org/10.1016/S0140-6736(12)60282-7 (2012).
Aiello, L. P. et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92, 10457–10461 (1995).
Kliffen, M., Sharma, H. S., Mooy, C. M., Kerkvliet, S. & de Jong, P. T. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81, 154–162 (1997).
Ahn, J. K. & Moon, H. J. Changes in aqueous vascular endothelial growth factor and pigment epithelium-derived factor after ranibizumab alone or combined with verteporfin for exudative age-related macular degeneration. Am J Ophthalmol 148, 718–724 e711, https://doi.org/10.1016/j.ajo.2009.06.012 (2009).
Sawada, O. et al. Aqueous vascular endothelial growth factor after intravitreal injection of pegaptanib or ranibizumab in patients with age-related macular degeneration. Retina 30, 1034–1038, https://doi.org/10.1097/IAE.0b013e3181ce74c8 (2010).
Wang, X. et al. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol 158, 738–744 e731, https://doi.org/10.1016/j.ajo.2014.06.009 (2014).
Yoshida, I. et al. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 252, 1483–1489, https://doi.org/10.1007/s00417-014-2717-0 (2014).
Klein, R. J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389, https://doi.org/10.1126/science.1109557 (2005).
Haines, J. L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421, https://doi.org/10.1126/science.1110359 (2005).
Edwards, A. O. et al. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424, https://doi.org/10.1126/science.1110189 (2005).
Gotoh, N. et al. No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet 120, 139–143, https://doi.org/10.1007/s00439-006-0187-0 (2006).
Kim, N. R. et al. Association between complement factor H gene polymorphisms and neovascular age-related macular degeneration in Koreans. Invest Ophthalmol Vis Sci 49, 2071–2076, https://doi.org/10.1167/iovs.07-1195 (2008).
Yamashiro, K. et al. Association of elastin gene polymorphism to age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 52, 8780–8784, https://doi.org/10.1167/iovs.11-8205 (2011).
Ng, T. K. et al. Multiple gene polymorphisms in the complement factor h gene are associated with exudative age-related macular degeneration in chinese. Invest Ophthalmol Vis Sci 49, 3312–3317, https://doi.org/10.1167/iovs.07-1517 (2008).
Sadler, J. E. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 67, 395–424, https://doi.org/10.1146/annurev.biochem.67.1.395 (1998).
Moake, J. L. Thrombotic microangiopathies. N Engl J Med 347, 589–600, https://doi.org/10.1056/NEJMra020528 (2002).
Dong, J. F. et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 100, 4033–4039, https://doi.org/10.1182/blood-2002-05-1401 (2002).
Feng, S. et al. The interaction between factor H and Von Willebrand factor. PLoS One 8, e73715, https://doi.org/10.1371/journal.pone.0073715 (2013).
Nolasco, L., Nolasco, J., Feng, S., Afshar-Kharghan, V. & Moake, J. Human complement factor H is a reductase for large soluble von Willebrand factor multimers–brief report. Arterioscler Thromb Vasc Biol 33, 2524–2528, https://doi.org/10.1161/ATVBAHA.113.302280 (2013).
Randi, A. M. & Laffan, M. A. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost 15, 13–20, https://doi.org/10.1111/jth.13551 (2017).
Lip, P. L., Blann, A. D., Hope-Ross, M., Gibson, J. M. & Lip, G. Y. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology 108, 705–710 (2001).
Yasuda, M. et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology 116, 2135–2140, https://doi.org/10.1016/j.ophtha.2009.04.017 (2009).
Gill, J. C., Endres-Brooks, J., Bauer, P. J., Marks, W. J. Jr & Montgomery, R. R. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 69, 1691–1695 (1987).
Rondaij, M. G., Bierings, R., Kragt, A., van Mourik, J. A. & Voorberg, J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 26, 1002–1007, https://doi.org/10.1161/01.ATV.0000209501.56852.6c (2006).
Hogg, R. E. et al. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology 115, 1046–1052 e1042, https://doi.org/10.1016/j.ophtha.2007.07.031 (2008).
Okamoto, H. et al. Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol Vis 12, 156–158 (2006).
Aoki, A. et al. Risk Factors for Age-Related Macular Degeneration in an Elderly Japanese Population: The Hatoyama Study. Invest Ophthalmol Vis Sci 56, 2580–2585, https://doi.org/10.1167/iovs.14-16339 (2015).
Zehetner, C. et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 93, e154–159, https://doi.org/10.1111/aos.12604 (2015).
Papadopoulos, N. et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15, 171–185, https://doi.org/10.1007/s10456-011-9249-6 (2012).
Kitchens, J. W. et al. Comprehensive Review of Ocular and Systemic Safety Events with Intravitreal Aflibercept Injection in Randomized Controlled Trials. Ophthalmology 123, 1511–1520, https://doi.org/10.1016/j.ophtha.2016.02.046 (2016).
Schmid, M. K. et al. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: a trade-off analysis. Br J Ophthalmol 99, 141–146, https://doi.org/10.1136/bjophthalmol-2014-305149 (2015).
Takahashi, K., Ishibashi, T., Ogura, Y. & Yuzawa, M. Classification and diagnostic criteria of age-related macular Degeneration. J Jpn Ophtjalmol Soc 112, 1076–1084 (2008).
Ohkuma, Y. et al. Retinal angiomatous proliferation associated with risk alleles of ARMS2/HTRA1 gene polymorphisms in Japanese patients. Clin Ophthalmol 8, 143–148, https://doi.org/10.2147/OPTH.S56483 (2014).
Matsumoto, M. et al. Platelets treated with ticlopidine are less reactive to unusually large von Willebrand factor multimers than are those treated with aspirin under high shear stress. Pathophysiol Haemost Thromb 34, 35–40, https://doi.org/10.1159/000088546 (2005).
Kato, S. et al. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion 46, 1444–1452, https://doi.org/10.1111/j.1537-2995.2006.00914.x (2006).
Ruggeri, Z. M. & Zimmerman, T. S. Variant von Willebrand’s disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest 65, 1318–1325, https://doi.org/10.1172/JCI109795 (1980).
Warren, C. M., Krzesinski, P. R. & Greaser, M. L. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24, 1695–1702, https://doi.org/10.1002/elps.200305392 (2003).
Budde, U. et al. Luminographic detection of von Willebrand factor multimers in agarose gels and on nitrocellulose membranes. Thromb Haemost 63, 312–315 (1990).
Acknowledgements
This study was partially supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Takeda Science Foundation. The authors thank to Drs. Ayami Isonishi and Yoko Yoshida for their technical assistance.
Author information
Authors and Affiliations
Contributions
Conception and design: M.M., Y.F. and N.O., Writing the article: M.Y., M.M., Y.F., N.O., Analysis: M.Y., M.H., K.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamashita, M., Matsumoto, M., Hayakawa, M. et al. Intravitreal injection of aflibercept, an anti-VEGF antagonist, down-regulates plasma von Willebrand factor in patients with age-related macular degeneration. Sci Rep 8, 1491 (2018). https://doi.org/10.1038/s41598-018-19473-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19473-0
This article is cited by
-
Visual and anatomical outcomes associated with treat-and-extend administration of intravitreal aflibercept for neovascular age-related macular degeneration
International Journal of Retina and Vitreous (2021)
-
Analysis focusing on plasma von Willebrand factor in pachychoroid neovasculopathy and age-related macular degeneration
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.