Abstract
Aspergillus fumigatus is one of the major causes of invasive pulmonary aspergillosis in immunocompromised patients. Novel antifungal therapy is in urgent need due to emerging resistance and adverse toxicity of current antifungal drugs. Gene products that are essential for Aspergillus viability during infection are attractive drug targets. To characterize these genes in vivo we developed a Tet-Off gene expression system in A. fumigatus, whereby the administration of doxycycline resulted in down regulation of the gene whose expression is under the control of the Tet-Off promoter. We tested the system on two potential drug targets, inosine 5′-monophosphate dehydrogenase (IMPDH) and L-ornithine N5-oxygenase (sidA) in a murine invasive pulmonary aspergillosis model. We show that depletion of IMPDH attenuated but did not completely abolish virulence in vivo whereas turning off the expression of sidA, which is required for iron acquisition, resulted in avirulence. We also investigated whether sidA expression could be controlled in a time-dependent manner in mice. Our results demonstrated that timing of doxycycline administration dramatically affects survival rate, suggesting that this genetic system can be used for testing whether an antifungal drug target is critical for fungal growth post-infection.
Similar content being viewed by others
Introduction
Aspergillus fumigatus is one of the most common filamentous fungi associated with severe invasive infections. Despite the availability of four major classes of anti-fungal therapies – polyenes, pyrimidine analogs, echinocandins and triazoles – invasive pulmonary aspergillosis still results in high mortality rate in immunocompromised patients. Polyenes, such as amphotericin B, bind to egosterol and destabilize cell membrane. Toxicity of amphotericin B has been reduced by using liposome formulation. Pyrimidine analogs, such as 5-fluorocytosine, inhibits pyrimidine biosynthesis. Echinocandins inhibit the β-1, 3 glucan synthase and therefore block cell wall biosynthesis. Triazoles inhibit specifically egosterol biosynthesis. Due to the side effects of these drugs and rising resistance, more efficacious antifungal drugs with novel therapeutic modalities or mechanisms of action are urgently needed1.
Genes essential for A. fumigatus viability or pathogenesis serve as potential drug targets. Conditional gene expression is a central strategy for characterizing the functions of essential genes. In addition, conditional gene expression systems allow for the level of a target gene to be regulated in a dose-dependent and/or in a time-dependent manner, which is important for discovery of gene function in physiologically relevant conditions.
Several conditional gene expression systems have been developed for A. fumigatus, including regulation by the Aspergillus nidulans alcA promoter2, A. fumigatus NiiA promoter3, and Escherichia coli tetracycline-controlled promoter4. A. nidulans alcA gene encodes alcohol dehydrogenase I, whose expression can be induced by ethanol or threonine and repressed by the presence of glucose. Tight regulation is achievable in vitro, however, controlling the activity of the alcA promoter is not possible in animal models. Recently, a nitrogen-regulated A. fumigatus NiiA promoter was used to identify essential genes in a large-scale functional study3. The NiiA promoter can be turned on in the absence of ammonium and the presence of nitrate, and turned off in the presence of ammonium regardless of the other nitrogen. As mouse serum and tissues naturally contain ammonium, expression of a given NiiA promoter driven gene can be sufficiently repressed in vivo 3. Similar to the alcA promoter, the drawback of the NiiA promoter is the inability to turn on/off a target gene in an animal model once infection is established because controlling the level of nitrate and ammonium in the mouse is not trivial. By contrast, the tetracycline-controlled transcription activation system provides an attractive opportunity to use non-native molecules such as tetracycline or doxycycline to regulate fungal gene expression. Indeed, the Tet-On and Tet-Off system has been successfully adopted in various eukaryotic cells. In the Tet-Off system, tetracycline-controlled transactivator (tTA) activates transcription by binding to the tet operator sequences (tetO) in the absence of tetracycline or doxycycline. Addition of tetracycline or doxycycline prevents tTA from binding tetO and therefore blocks transcription. Conversely, in the Tet-On system, a ‘reverse’ tetracycline transactivator (rtTA) only binds to tetO in the presence of tetracycline or doxycycline5,6. The Tet-On and Tet-Off system was first introduced in A. fumigatus on two separate plasmids, which contain tetO and tTA/rtTA, respectively4. However, the leakiness of the promoter compromises the reliability of phenotypic analyses7. More recently, the Tet-On system was improved and upgraded to one module that can be integrated to A. fumigatus genome7. A following study validated this Tet-On system in both murine pulmonary and systemic infection of A. fumigatus. Under the Tet-On promoter, genes required for aromatic amino acid biosynthesis were turned on by supplementing drinking water with doxycycline, resulting in infection of A. fumigatus 8,9. Subsequently, a Tet-Off gene expression system was created by substituting rtTA2S-M2 with a tTA2 transactivator in the Tet-On cassette described above. Under this system, gene expression tested was effectively inhibited in the presence of doxycycline in vitro10.
In this study, we created a Tet-Off gene expression system in A. fumigatus and used it to investigate whether two potential target genes are essential for virulence of A. fumigatus in a murine invasive pulmonary aspergillosis model. We further tested the utility of this system to repress target gene expression in vivo in a time-dependent manner.
Results and Discussion
IMPDH is conditionally essential for growth of A. fumigatus in vitro
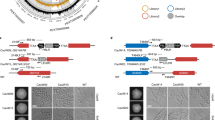
To construct a Tet-Off expression system for A. fumigatus, we modified a previously published Tet-On expression cassette7. We replaced the reverse tetracycline transactivator rtTA2 S -M2 with a tetracycline transactivator tTA-Advanced (tTA2 S) which was optimized for tight binding to tetO during induction (Clontech) (Fig. 1a). To test whether our Tet-Off cassette can effectively turn off expression of a given target gene, we integrated this Tet-Off cassette at the promoter region of A. fumigatus inosine 5′-monophosphate dehydrogenase (AfIMPDH) by homologous recombination in a non-homologous end-joining-deficient strain11.
AfIMPDH is conditionally essential for A. fumigatus in vitro. (a) Schematic representation of the Tet-Off cassette. The Tet-Off cassette is composed of the pyrithiamine resistance cassette (PtrA R), the tpiA promoter of A. nidulans (P tpiA )33, the tetracycline transactivator tTA2 S (Clontech), the terminating region of cgrA from A. fumigatus (T cgrA )34, and the chimeric tetO-P min promoter. (b) Doxycycline-dependent growth of the P Tet-Off -AfIMPDH strain. Approximate 500, 50 and 5 conidia of wild type (A1151) and the P Tet-Off -AfIMPDH strain were spotted on the AMM agar plates supplemented with the indicated compounds. PT: pyrithiamine, 0.5 μg/mL; Dox: doxycycline, 1 μg/mL; Guanine: 500 μM, HygB: hygromycin B, 200 μg/mL. The plates were subsequently incubated at 37 °C for 36–48 hours and imaged. (c) Growth of the AfIMPDHΔ strain. Conidia of the wild type and the mutant strains were spotted and grown as described in (b).
IMPDH catalyzes the first step in de novo guanine biosynthesis by converting inosine 5'-monophosphate (IMP) to xanthosine 5′-monophosphate (XMP). A specific inhibitor to human IMPDH, mycophenolic acid (MPA), is used as an immunosuppressant drug to prevent rejection in organ transplantation by inhibiting proliferation of T cells and B cells. Several features of IMPDH make it an attractive anti-microbial drug target12. While microbes can take up purine bases in the environment by salvage pathways, concentrations of purines in human plasma and other extracellular fluids are insufficient for microbial survival13, suggesting that IMPDH might be essential for virulence in vivo. Furthermore, structural differences between microbial and human IMPDHs suggest that an inhibitor that is specific to the fungal enzyme could be attained. Among fungal species, deletion of IMPDH results in avirulence of Cryptococcus neoformans 14. MPA inhibited in vitro growth of Candida albican, whereas overexpression of IMPDH confers resistance to MPA15, suggesting that enzymatic activity of IMPDH is critical for growth of C. albicans. In addition, another key enzyme in the de novo guanine biosynthesis, guanosine monophosphate (GMP) synthase that catalyzes XMP to GMP, has been shown essential for fungal viability and virulence in A. fumigatus and C. albicans 16,17. However, it remains to be ascertained if IMPDH is essential for virulence of A. fumigatus.
To determine whether IMPDH is essential for A. fumigatus viability, we created the AfIMPDHΔ and the Tet-Off promoter driven AfIMPDH strains (P Tet-Off -AfIMPDH) by homologous recombination. As expected, AfIMPDHΔ failed to grow in the absence of guanine, whereas addition of guanine restored its growth (Fig. 1c). Addition of doxycycline to the guanine-free media, ranging from 1 to 50 μg/mL, greatly inhibited, but did not completely abolish the growth of P Tet-Off -AfIMPDH strain. In addition, the growth of P Tet-Off -AfIMPDH strain was restored by supplementing guanine in the media (Fig. 1b, data not shown).
IMPDH is not essential for virulence of A. fumigatus in vivo
We next tested whether loss of AfIMPDH affects virulence of the pathogen using an invasive pulmonary aspergillosis mouse model18. Seven-week-old female BALB/c mice were immunosuppressed and then intranasally infected with 5 × 104 conidia of wild type, AfIMPDHΔ or P Tet-Off -AfIMPDH strains. Mortality and body weight were monitored daily. Surprisingly, deletion of AfIMPDH attenuated, but did not completely abolish virulence of A. fumigatus. While mice infected with the wild type conidia showed the median survival time of 4 days, mice infected with the AfIMPDHΔ conidia showed the median survival time of 7 days (Fig. 2a) (p < 0.0001).
Deletion of AfIMPDH attenuates but does not completely abolish virulence of A. fumigatus. (a) Survival curves of mice with invasive pulmonary aspergillosis (Kaplan-Meier plot). Mice were immunocompromised by injection of cyclophosphamide intraperitoneally and cortisone acetate subcutaneously before infection. Freshly prepared conidia (5 × 104) of wild type (A1151), AfIMPDHΔ, and P Tet-Off -AfIMPDH strains were intranasally inoculated under anesthesia. Doxycycline was administered at day −4 (4 days before infection) to turn off expression of AfIMPDH. Each group includes more than eight mice. (b) Body weight change after infection. The body weights of each day after infection are compared to the body weights at day −1. Averages and the standard errors of the mean are presented.
We further tested whether doxycycline treatment could recapitulate the attenuated virulence of the P Tet-Off -AfIMPDH strain in vivo. Similar to wild type, infection with P Tet-Off -AfIMPDH conidia had the median survival time of 5 days. To repress the expression of AfIMPDH, doxycycline was administered by oral gavage twice daily to mice from 4 days prior to infection for a total 10 days. We chose this dosage regimen because it has been shown to effectively induce Tet-inducible gene expression in a mouse subcutaneous xenograft model19. At this given dose, the serum steady state doxycycline level could reach to about 10 μg/mL in mice20, which is 10 fold the in vitro doxycycline concentration (1 μg/mL). We observed that mice given doxycycline survived 2 days longer than mice without doxycycline treatment (Fig. 2a) (p = 0.0008). Doxycycline-treated mice had the same median survival time (7 days) as the mice infected with the AfIMPDHΔ conidia, suggesting that the expression of AfIMPDH is effectively repressed. We also note that doxycycline treatment caused body weight reduction (Fig. 2b), as observed previously19.
Taken together, our data show that our Tet-off expression system can repress expression of AfIMPDH in vivo. Additionally, our results demonstrate that AfIMPDH is not essential for virulence and is therefore not a good drug target for A. fumigatus. A previous study showed that GMP synthase, an enzyme downstream of AfIMPDH in the de novo guanine biosynthesis, is essential for virulence16. We noted that a murine model of systemic aspergillosis was used in the study. Since the growth of A. fumigatus mutants defective in de novo guanine biosynthesis is solely dependent on concentration of guanine in the tissue, we postulate that difference could be due to the fact that extracellular concentration of purines in lung tissue is relatively higher than that in blood and kidney and may be sufficient to bypass the lack of AfIMPDH.
sidA is conditionally essential in vitro and essential in vivo for A. fumigatus
We next tested sidA, a gene that is known to be essential for A. fumigatus virulence21,22, to further validate our Tet-Off system. Iron is essential for growth of A. fumigatus but is poorly available in animal hosts. A. fumigatus depends on siderophore, an iron-specific chelator, to obtain iron in vivo. The sidA gene encodes L-ornithine N5-oxygenase that catalyzes the first committed step in hydroxamate siderophore biosynthesis23. Deletion of sidA leads to growth defect in iron-limited media and complete avirulence in murine models of invasive aspergillosis21,22. We created sidA null and Tet-Off promoter driven sidA (P Tet-Off -sidA) mutants and compared their growth. In the absence of iron, growth of sidAΔ was completely abolished (Fig. 3b). Likewise the P Tet-Off -sidA mutant did not grow in the presence of doxycycline at the concentration of 1 μg/mL (Fig. 3a), indicating that sidA expression was effectively repressed by doxycycline. Moreover, addition of ferrous ion (Fe2+) restored the growth of sidA null mutants (Fig. 3a). A previously reported Tet-Off gene expression system for A. fumigatus inhibited gene expression significantly in vitro in the presence of doxycycline at a concentration of 50 μg/mL10. In comparison, we found that our Tet-Off system greatly inhibited gene expression of AfIMPDH and sidA at 1 μg/mL concentration of doxycycline and increased doxycycline concentration did not enhance the inhibition. Future studies will be needed for direct comparison of these two Tet-Off systems.
Growth of the sidAΔ and P Tet-Off -sidA mutants in vitro. (a) Doxycycline-dependent growth of the P Tet-Off -sidA strain. (b) Growth of the sidAΔ strain. Conidia of the wild-type and the mutant strains were spotted and grown as described in Fig. 1.
We next tested virulence of the sidAΔ and P Tet-Off -sidA mutants. In contrast to the wild type conidia, the sidAΔ conidia were avirulent (p = 0.0001), consistent with previous reports21,22. The P Tet-Off -sidA conidia demonstrated similar virulence to the wild type in the absence of doxycycline, while the conidia were completely avirulent in the mice administered with doxycycline (Fig. 4a) (p < 0.0001), validating the effectiveness of the Tet-Off gene expression system for controlling gene expression in vivo. We again observed that doxycycline treatment caused body weight reduction (Fig. 4b).
Depletion of sidA abolishes virulence of A. fumigatus. (a) Survival curves for mice with invasive pulmonary aspergillosis (Kaplan-Meier plot). Mice were immunocompromised as previously described and were infected with conidia of wild type (A1151), sidAΔ, and P Tet-Off -sidA strains. Doxycycline was administered at day −4 to turn off expression of sidA. Each group includes more than eight mice. (b) Body weight change after infection. The body weights of each day were monitored and presented as described in Fig. 2.
Control of sidA expression in a time-dependent manner in vivo
We sought to test whether the Tet-Off system can regulate expression of a target gene in a time-dependent manner in vivo. Previously, we administered doxycycline 4 days prior to infection for continuously 10 days and observed 100% survival of mice (Figs 4a and 5). The critical factor to turn off the expression of sidA is the concentration of doxycycline in mouse lung tissue. It has been reported that when given to human patients orally doxycycline reached at relatively stable concentration in bronchial secretions about 24 hours after the first dose24. Therefore we administered the first dose of doxycycline to mice at −1, 0, 1, 2 and 3 days after infection to mimic the dosing regimen that patients receive with antifungal therapy in the clinic at 0, 1, 2, 3 and 4 days after infection, respectively. We observed that administering doxycycline at day −1 is very effective, and rescued 75% of mice (Fig. 5) (p < 0.0001); and administering doxycycline at day 0 also significantly prolonged the medial survival time from 5 days of control to 9 days (p = 0.0005). In contrast, administering doxycycline after day 1 (day 1, 2 and 3) showed very little effect on the survival rate of mice (Fig. 5). These results demonstrate that the timing of doxycycline administration dramatically affects survival rates.
Control of sidA expression in host animals in a time-dependent manner. Mice were immunocompromised and were infected with conidia of the P Tet-Off -sidA strain. Doxycycline was administered at day −4, −1, 0, +1, + 2, + 3 to regulate expression of sidA. The control group (No Dox) represents the mice without doxycycline treatment.
Previous animal studies reported that delayed antifungal therapy is ineffective for invasive pulmonary aspergillosis due to an extensive hyphal invasion in the first 48 hours post infection25,26. Unfortunately delayed antifungal treatment can often be the case in the clinic. Therefore it is of great interest to develop antifungal therapy that is effective after conidia enter hyphal growth phase. Our Tet-Off system provides a potential way to test targets for such therapy. Moreover, molecular mechanism underlying conidia transition from dormancy to germination is still largely unknown. Transcriptome and proteome studies revealed that over one third of Aspergillus genome is differentially expressed when conidia enter germination in vitro 27,28,29,30,31. This Tet-Off system can be used to verify if a given gene is required for germination and/or hyphal growth in vivo.
Methods
Strains and Media
The Aspergillus fumigatus strains used in this study are listed in Table 1. Conidia were collected from cultures grown on potato dextrose agar (Sigma-Aldrich) at 37 °C. The growth of the wild type and mutant conidia were tested on Aspergillus Minimal Media (AMM) plates supplemented with 200 μg/mL hygromycin B (HygB), 0.2 μg/mL pyrithiamine (PT), 1 μg/mL doxycycline (Dox), FeSO4 (Fe2+), or 500 μM guanine at 37 °C.
Plasmid construction
The Tet-Off gene expression cassette was constructed based on previously published Tet-On gene expression cassette in pCH0087. The reverse tetracycline transactivator rtTA2 S -M2 was replaced with the tetracycline transactivator tTA-Advanced (tTA2 S) (Clontech) using Gibson Assembly strategy (NEB). The final Tet-Off cassette is composed of PtrA resistance cassette, promoter of tpiA, tTA2 S, terminator of cgrA, tetO promoter and a minimal promoter.
Strain construction
Using a method based on homologous recombination described previously11, the target genes were deleted with a hygromycin B resistance cassette or the promoters of the target genes were replaced with the Tet-Off gene expression cassette in a non-homologous end-joining-deficient strain (A1151, Fungal Genetics Stock Center). First, approximately 1 kb upstream and downstream of the targeted chromosome region were amplified by PCR from genomic DNA of A1151. These two DNA fragments were assembled with the hygromycin B cassette or Tet-Off gene expression cassette in pUC19 using NEB Gibson assembly kit. Second, the resultant deletion cassette or Tet-Off cassette flanked with the targeted gene fragments were PCR amplified and purified. A. fumigatus was transformed by electroporation as described previously32. The transformants were selected on AMM supplemented with HygB (for deletion) or PT (for Tet-Off promoter replacement). The mutant strains were confirmed by colony PCR.
A. fumigatus virulence studies
Seven-week-old female BALB/c mice weighing 18–20 g (The Jackson Laboratory) were used for the invasive pulmonary aspergillosis model18. Immunosuppression was achieved by injection of cyclophosphamide (Baxter Healthcare) intraperitoneally 4 days at 150 mg/kg and 1 day at 100 mg/kg before inoculation and injection of cortisone acetate at 250 mg/kg (Sigma) subcutaneously 1 day before inoculation. On inoculation day, 40 μL of A. fumigatus conidial suspension containing 5 × 104 freshly collected conidia in sterile PBS + 0.02% Tween 20 were intranasally inoculated under anesthesia. Additional doses of cyclophosphamide (100 mg/kg) were given on days 2 and 6 after inoculation to maintain neutropenia. Mortality was assessed daily until day 14 after inoculation. Animals displaying signs of morbidity were euthanized, and their death was recorded as occurring 12 hours later19. 200 μL of 10 mg/mL doxycycline were treated twice a day via gastric lavage. Statistical analyses for comparison of survival rate between WT and knockout, or between P Tet-Off -target gene with doxycycline and without doxycycline were conducted using Prism 6 and p values from the Log-rank (Mantel-Cox) test were presented.
References
Sanglard, D. Emerging Threats in Antifungal-Resistant FungalPathogens. Front Med (Lausanne) 3, 11, https://doi.org/10.3389/fmed.2016.00011 (2016).
Romero, B., Turner, G., Olivas, I., Laborda, F. & De Lucas, J. R. The Aspergillus nidulans alcA promoter drives tightly regulated conditional gene expression in Aspergillus fumigatus permitting validation of essential genes in this human pathogen. Fungal Genet Biol 40, 103–114 (2003).
Hu, W. et al. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog 3, e24, https://doi.org/10.1371/journal.ppat.0030024 (2007).
Vogt, K., Bhabhra, R., Rhodes, J. C. & Askew, D. S. Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol 5, 1, https://doi.org/10.1186/1471-2180-5-1 (2005).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89, 5547–5551 (1992).
Helmschrott, C., Sasse, A., Samantaray, S., Krappmann, S. & Wagener, J. Upgrading fungal gene expression on demand: improved systems for doxycycline-dependent silencing in Aspergillus fumigatus. Appl Environ Microbiol 79, 1751–1754, https://doi.org/10.1128/AEM.03626-12 (2013).
Sasse, A., Hamer, S. N., Amich, J., Binder, J. & Krappmann, S. Mutant characterization and in vivo conditional repression identify aromatic amino acid biosynthesis to be essential for Aspergillus fumigatus virulence. Virulence 7, 56–62, https://doi.org/10.1080/21505594.2015.1109766 (2016).
Dümig, M. & Krappmann, S. Controlling Fungal Gene Expression Using the Doxycycline-Dependent Tet-ON System in Aspergillus fumigatus. 2 (Springer International Publishing, Switzerland, 2015; 131–138.
Wanka, F. et al. Tet-on, or Tet-off, that is the question: Advanced conditional gene expression in Aspergillus. Fungal Genet Biol 89, 72–83, https://doi.org/10.1016/j.fgb.2015.11.003 (2016).
da Silva Ferreira, M. E. et al. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell 5, 207–211, https://doi.org/10.1128/EC.5.1.207-211.2006 (2006).
Shu, Q. & Nair, V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med Res Rev 28, 219–232, https://doi.org/10.1002/med.20104 (2008).
Traut, T. W. Physiological concentrations of purines and pyrimidines. Molecular and cellular biochemistry 140, 1–22 (1994).
Morrow, C. A. et al. De novo GTP biosynthesis is critical for virulence of the fungal pathogen Cryptococcus neoformans. PLoS Pathog 8, e1002957, https://doi.org/10.1371/journal.ppat.1002957 (2012).
Kohler, G. A., White, T. C. & Agabian, N. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol 179, 2331–2338 (1997).
Rodriguez-Suarez, R. et al. Mechanism-of-action determination of GMP synthase inhibitors and target validation in Candida albicans and Aspergillus fumigatus. Chem Biol 14, 1163–1175, https://doi.org/10.1016/j.chembiol.2007.09.009 (2007).
Jiang, L. et al. Functional characterization and virulence study of ADE8 and GUA1 genes involved in the de novo purine biosynthesis in Candida albicans. FEMS Yeast Res 10, 199–208, https://doi.org/10.1111/j.1567-1364.2009.00600.x (2010).
Lewis, R. E. & Wiederhold, N. P. Murine model of invasive aspergillosis. Methods Mol Med 118, 129–142, https://doi.org/10.1385/1-59259-943-5:129 (2005).
Cawthorne, C., Swindell, R., Stratford, I. J., Dive, C. & Welman, A. Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech 18, 120–123 (2007).
Prall, A. K. et al. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg 35, 923–929 (2002).
Schrettl, M. et al. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 200, 1213–1219, https://doi.org/10.1084/jem.20041242 (2004).
Hissen, A. H., Wan, A. N., Warwas, M. L., Pinto, L. J. & Moore, M. M. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun 73, 5493–5503, https://doi.org/10.1128/IAI.73.9.5493-5503.2005 (2005).
Schrettl, M. & Haas, H. Iron homeostasis–Achilles’ heel of Aspergillus fumigatus? Curr Opin Microbiol 14, 400–405, https://doi.org/10.1016/j.mib.2011.06.002 (2011).
Hartnett, B. J. & Marlin, G. E. Doxycycline in serum and bronchial secretions. Thorax 31, 144–148 (1976).
Hope, W. W. et al. The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1−>3) beta-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother 54, 4879–4886, https://doi.org/10.1128/AAC.00673-10 (2010).
Barchiesi, F. et al. Delay of antifungal therapy influences the outcome of invasive aspergillosis in experimental models of infection. J Antimicrob Chemother, https://doi.org/10.1093/jac/dkw111 (2016).
Oh, Y. T. et al. Proteomic analysis of early phase of conidia germination in Aspergillus nidulans. Fungal Genet Biol 47, 246–253, https://doi.org/10.1016/j.fgb.2009.11.002 (2010).
Teutschbein, J. et al. Proteome profiling and functional classification of intracellular proteins from conidia of the human-pathogenic mold Aspergillus fumigatus. J Proteome Res 9, 3427–3442, https://doi.org/10.1021/pr9010684 (2010).
Novodvorska, M. et al. Trancriptional landscape of Aspergillus niger at breaking of conidial dormancy revealed by RNA-sequencing. BMC Genomics 14, 246, https://doi.org/10.1186/1471-2164-14-246 (2013).
van Leeuwen, M. R. et al. Germination of conidia of Aspergillus niger is accompanied by major changes in RNA profiles. Stud Mycol 74, 59–70, https://doi.org/10.3114/sim0009 (2013).
Hagiwara, D. et al. Comparative transcriptome analysis revealing dormant conidia and germination associated genes in Aspergillus species: an essential role for AtfA in conidial dormancy. BMC Genomics 17, 358, https://doi.org/10.1186/s12864-016-2689-z (2016).
Weidner, G., d’Enfert, C., Koch, A., Mol, P. C. & Brakhage, A. A. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr Genet 33, 378–385 (1998).
Upshall, A. et al. Secretion of active human tissue plasminogen activator from the filamentous fungus Aspergillus nidulans. Biotechnology 5, 1301–1304 (1987).
Dichtl, K., Helmschrott, C., Dirr, F. & Wagener, J. Deciphering cell wall integrity signalling in Aspergillus fumigatus: identification and functional characterization of cell wall stress sensors and relevant Rho GTPases. Mol Microbiol 83, 506–519, https://doi.org/10.1111/j.1365-2958.2011.07946.x (2012).
Acknowledgements
We thank Dr. Sven Krappmann for sharing the plasmid pCH008. We thank Dr. Eric Kofoed for discussion on IMPDH and Dr. Hany Girgis for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Y.P., H.Z., M.X. and M.-W.T. designed and/or conducted the experiments. Y.P. and M.-W.T. wrote the main text of the manuscript. Y.P. prepared Figs 1 and 3; H.Z. prepared Figs 2, 4 and 5. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
Y.P., H.Z., M.X. and M.-W.T. are employees of Genentech, Inc.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, Y., Zhang, H., Xu, M. et al. A Tet-Off gene expression system for validation of antifungal drug targets in a murine invasive pulmonary aspergillosis model. Sci Rep 8, 443 (2018). https://doi.org/10.1038/s41598-017-18868-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18868-9
This article is cited by
-
Inducible promoters and functional genomic approaches for the genetic engineering of filamentous fungi
Applied Microbiology and Biotechnology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.