Abstract
Age-related loss of vestibular function can result in decrements in gaze stabilization and increased fall risk in the elderly. This study was designed to see if low levels of electrical stochastic noise applied transcutaneously to the vestibular system can improve a gaze stabilization reflex in young and elderly subject groups. Ocular counter-rolling (OCR) using a video-based technique was obtained in 16 subjects during low frequency passive roll tilts. Consistent with previous studies, there was a significant reduction in OCR gains in the elderly compared to the young group. Imperceptible stochastic noise significantly increased OCR in the elderly (Mean 23%, CI: 17–35%). Increases in OCR gain were greatest for those with lowest baseline gain and were negligible in those with normal gain. Since stimulation was effective at low levels undetectable to subjects, stochastic noise may provide a new treatment alternative to enhance vestibular function, specifically otolith-ocular reflexes, in the elderly or patient populations with reduced otolith-ocular function.
Similar content being viewed by others
Introduction
The risk of vestibular dysfunction increases as a function of age1. Age-related vestibular loss has been demonstrated in multiple vestibular pathways2 and has been associated with increased falls3. Understanding age-related loss in vestibular function will therefore help characterize those who may be at greater risk of fall injury. More importantly, clinical interventions that can enhance vestibular function in the elderly would be expected to improve activity levels leading to better outcome measures of daily living4.
Ocular-counterroll (OCR) is one measure that has been shown to be sensitive to age-related loss in vestibular function5,6. OCR gain is a gaze stabilizing mechanism involving ocular torsion in a compensatory direction to lateral roll-tilt of the head7. At low frequencies of motion, OCR gain is predominantly mediated by the otoliths8. A recent study has demonstrated video-based measurement of OCR has high diagnostic value to detect bilateral and unilateral otolith loss9. We previously demonstrated that reductions in OCR gain with aging were correlated with increased postural sway, suggesting OCR loss may also a useful predictor of fall risk5.
Based on these previous findings, we chose OCR gain as a marker of vestibular loss to evaluate a method of enhancing gain in the elderly using low levels of imperceptible electrical stimulation. Noise is generally thought to be detrimental to the detection and transmission of signals but, under certain conditions noise can enhance weaker signals in nonlinear systems through the phenomenon of stochastic resonance10. For example, while higher levels of electrical vestibular stimulation (e.g., ~5 mA) have been used to evoke eye movements11 or postural instability12, recent studies have been using low levels of “noisy” electrical stimulation to improve vestibular function13. We previously used low levels of electrical stochastic noise (SN) to enhance tactile perception in both young14 and older human subjects15. In this study, we extend this work to examine the effects of imperceptible electrical SN on otolith-ocular function in both young and elderly subjects.
Our study used a cross-sectional design to compare the effect of age using both young and older subject groups. We then used a repeated measures approach to examine the effects of the electrical SN on OCR in both groups. Since we expected the OCR gains to be lower in the elderly, we hypothesized this group would show greater improvements with the SN intervention.
Results
Effects of aging on OCR
For both young and elderly groups, the OCR gain increased with frequency (P = 0.03, Table 1). This gain increase can be attributed to increasing canal input due to both higher frequencies of tilt and increased tilt velocity. Across all frequencies, the baseline OCR gains were significantly reduced for the elderly compared to the young group (P = 0.005). The lower OCR gains in this elderly group are in accordance with previous findings from our laboratory (N > 150)5. This difference between young and elderly was greatest at 0.03125 Hz at which the OCR gain was reduced by 57% versus 38% at the other two frequencies.
Enhancement of OCR using SN
Use of imperceptible stochastic noise stimulation resulted in significant increases in OCR gain in elderly individuals. Figure 1 shows OCR from one elderly subject at baseline and with stimulation during a roll frequency of 0.125 Hz. Electrical SN stimulation significantly increased OCR gain at all frequencies in all elderly subjects (P = 0.007) while producing no significant change in OCR gain in young subjects (Fig. 2). Table 1 depicts mean OCR gain values for young and elderly participants at each of the roll frequencies tested both with and without stimulation.
Change in OCR for Young and Elderly subjects from control to stim trials. The top panel shows individual data at each of the three stimulus frequencies, while the bottom panel shows the mean (±SEM). Elderly subjects demonstrated a significant increase in OCR at all motion frequencies suggesting improved vestibular function. Young subjects showed no significant change at any frequency.
Interestingly, subjects with the lowest baseline OCR gain demonstrated the greatest increases in OCR gain with stimulation (Fig. 3). This relationship was significant using both linear (R2 = 0.26, P = 0.003) and second-order quadratic functions (R2 = 0.40, P < 0.001). There was a significant interaction between age group, tilt frequency and stimulus condition (p = 0.018). When examining the raw data in Fig. 3, there appears to be a ceiling effect in that providing electrical SN in subjects with normal gain has limited or no effect. Thus, electrical SN appears to improve function in those with impaired otolith responses without inducing hypersensitivity or other adverse effects in those with normal function.
Discussion
This study had two main findings. First, we observed an age-related loss in vestibular (otolith-ocular) function when comparing OCR gain in elderly subjects compared to a young subject group. This is consistent with previous studies that have shown age-related loss in otolith-ocular reflexes5,6. Second, we observed that imperceptible electrical SN significantly enhanced OCR gain in the elderly group. Since the increase in OCR was inversely proportional to baseline OCR gain, this type of an intervention should be generalizable to other patient populations with reduced otolith-ocular function13.
To our knowledge, this is the first study that has shown a positive effect of electrical SN on a gaze stabilization measure in subjects with reduced otolith-ocular function. While high levels of galvanic stimulation can elicit ocular torsion11, our thresholding technique ensured that our stimulus amplitudes did not provide a confounding additive effect but more likely enhanced the sensory transduction processing of tilt orientation. Our results are consistent with a recent study by Iwasaki et al.16 that demonstrated noisy galvanic stimulation can increase the amplitude of ocular VEMPs in healthy subjects. Ocular VEMPs assess the utricular-ocular reflex pathways by stimulating the peripheral end organs and measuring the ocular muscle activity17. Given that galvanic vestibular stimulation can mediate both otolith and canal responses18, the extent to which the improvement we observed was otolith mediated or both canal and otolith mediated is a topic for future research.
Our study was limited to subjects who were healthy with no recent neurological dysfunction or history of falls. While we observed reduced otolith-ocular function in the elderly group, a further extension of this work would be to test electrical SN patients with vestibular hypofunction as established using a larger battery of tests. This would be a critical next step to extend this proof of concept toward clinical applications. We observed a significant improvement in OCR in our elderly group using one level of electrical SN (90% of threshold). Another extension of our study would be to use graded intensities to further characterize how different SN levels would affect the OCR.
Recent evidence suggests that this type of peripheral stimulation influences both hair cells and vestibular afferent fibers19. Irregular neurons may be preferentially activated at the lower currents used with our intervention20. While the underlying mechanisms of how electrical SN enhances vestibular function remain unclear, one possibility is that addition of electrical noise causes fluctuations in membrane potentials, thus occasionally reducing depolarization necessary for neurons to fire. We would expect that this stimulation would improve tilt thresholds as well as other functions mediated by the vestibular system. Electrical SN has indeed been recently shown to improve other vestibular reflexes, such as posture21,22 and locomotion23.
Vestibular rehabilitation has shown efficacy in the treatment of the elderly24. Electrical SN may provide an enhancement to existing rehabilitation strategies. One key advantage of this type of non-invasive intervention is that it is both well tolerated by the elderly and does not increase cognitive load by requiring re-training. In addition, since the stimulation is noise based, neural systems theoretically will not adapt to the stimulus and thus long-term treatment should be more feasible. This study demonstrates that transcutaneous application of imperceptible stochastic noise stimulation can be used to enhance reduced otolith-ocular function in an elderly population.
Methods
Participants
The effects of aging in this study were inferred by comparing two subject groups. The young group consisted of seven subjects (four females, three males) with a mean age of 26 yrs (range 21–39 yrs). The older group consisted of nine subjects (seven females, two males) with a mean age of 71 yrs (range of 62–82 yrs). Based on a brief medical history questionnaire, all subjects were healthy with no recent neurological dysfunction or history of falls. This study protocol was approved by the Beth Israel Deaconess Medical Centre Institutional Review Board. Informed consent was obtained from each subject prior to participation. All experiments were performed in accordance with relevant guidelines and regulations.
Measurement of OCR
OCR measurements were performed using a procedure we have previously published5. In summary, subjects were passively roll-tilted in darkness while fixating a small LED target at 1.4 m. This far target distance has been more sensitive to age-related OCR loss than near targets6. Subjects were tilted about their naso-occipital axis at eye level, with the head and torso stabilized using adjustable straps and padding to move en bloc with the chair. Each subject was tilted ±25 degrees at three frequencies: 0.03125 Hz (3.125 degrees/second), 0.125 Hz (12.5 degrees/second) and 0.2 Hz (20 degrees/second) for both control and stochastic noise trials (Fig. 2). Previous work has demonstrated that ocular reflexes in the 0.03125 Hz frequency are primarily otolith mediated while higher frequencies involve increasing canal inputs8. Stimulus conditions were random and counterbalanced across subjects.
Torsional, horizontal and vertical eye positions were derived from a near-infrared 3D videography system using custom software to allow recording in darkness25. Changes in horizontal and vertical eye position and pupil radius were negligible due to constant fixation on the wall target. Changes in torsional eye movements derived from natural landmarks in the iris represented the OCR response during roll tilt (Fig. 1). OCR gain was calculated as ratio of deg of torsion to tilt using least-squared regression. Effects of stochastic noise on OCR were expressed as a percentage change from baseline.
Electrical stimulation
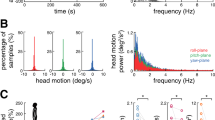
Stochastic noise signals were generated using Labview (National Instruments, Texas, USA) and applied bilaterally through a current isolator to large surface electrodes (5 × 5 cm) located on each mastoid process. The electrode site was cleaned with alcohol and skin abrasive to ensure low impedance to minimize pinprick tactile cues. The spectrum of stochastic noise was generated using an equation modified from that described by Collins et al.10 where the majority of the power of the signal falls below 2 Hz (Fig. 4). Stimulus amplitude was set at 90% of a threshold value determined for each subject by applying a constant stimulus starting at 0 mA and increasing in 0.1 mA increments. The threshold was the lowest stimulus value at which either the subjects reported an awareness of the galvanic stimulus (8 of 16 subjects) or we observed the onset of at least 3 consecutive nystagmus beats in a consistent direction (the remaining 8 subjects). To ensure input noise was sub-perceptual threshold, we confirmed that subjects reported no awareness of stimulus during the SN trials. Stimulus amplitudes were 1.6 ± 0.9 mA for elderly and 1.1 ± 0.5 mA for younger subjects.
Sample of the stochastic noise signal over time for one subject (top graph) with the corresponding power spectral density curve for the same signal (bottom graph). Note that while the stimulus amplitude was customized for each subject (see text), the frequency bandwidth was maintained consistently across subjects so that the stimulus was predominantly banded in the low frequency range (95% < 2 Hz).
Statistical Analysis
All statistical tests were performed using SPSS 24 using a GLM repeated measures analysis with age as a between-subjects factor (Young vs Elderly) and frequency (0.03125 vs 0.125 vs 0.2 Hz) and stochastic noise (Sham vs Stim) as within-subjects repeated measures. This mixed-model multivariate ANOVA used Wilks’ lambda as the critical statistic with an alpha significance level of 0.05. Post-hoc analyses to compare stimulus frequencies used Bonferroni correction to reduce Type I error. Least squares linear and quadratic fits were used to evaluate the effect of initial OCR on percent change between sham and stim, with coefficient of determination as the key outcome measure. All data are described using SEM to facilitate comparison of means.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C. & Minor, L. B. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Archives of internal medicine 169, 938–944, https://doi.org/10.1001/archinternmed.2009.66 (2009).
Arshad, Q. & Seemungal, B. M. Age-related vestibular loss: Current understanding and future research directions. Front Neurol 7, 231, https://doi.org/10.3389/fneur.2016.00231 (2016).
Herdman, S. J., Blatt, P., Schubert, M. C. & Tusa, R. J. Falls in patients with vestibular deficits. Am J Otol 21, 847–851 (2000).
Harun, A., Li, C., Bridges, J. F. & Agrawal, Y. Understanding the experience of age-related vestibular loss in older individuals: A qualitative study. Patient 9, 303–309, https://doi.org/10.1007/s40271-015-0156-6 (2016).
Serrador, J. M., Lipsitz, L. A., Gopalakrishnan, G. S., Black, F. O. & Wood, S. J. Loss of otolith function with age is associated with increased postural sway measures. Neuroscience letters 465, 10–15, https://doi.org/10.1016/j.neulet.2009.08.057 (2009).
Goltz, H. C. et al. Effects of age, viewing distance and target complexity on static ocular counterroll. Vision Res 49, 1848–1852, https://doi.org/10.1016/j.visres.2009.04.021 (2009).
Douglas, S. B., Clement, G., Denise, P. & Wood, S. J. Ocular reflex phase during Off-Vertical Axis Rotation in humans is modified by head-turn-on-trunk position. Sci Rep 7, 42071, https://doi.org/10.1038/srep42071 (2017).
Merfeld, D. M., Park, S., Gianna-Poulin, C., Black, F. O. & Wood, S. J. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol 94, 186–198 (2005).
Otero-Millan, J. et al. The video ocular counter-roll (vOCR): a clinical test to detect loss of otolith-ocular function. Acta Otolaryngol 137, 593–597, https://doi.org/10.1080/00016489.2016.1269364 (2017).
Collins, J. J. et al. Noise-enhanced human sensorimotor function. IEEE Eng Med Biol Mag 22, 76–83 (2003).
MacDougall, H. G., Brizuela, A. E., Burgess, A. M., Curthoys, I. S. & Halmagyi, G. M. Patient and normal three-dimensional eye-movement responses to maintained (DC) surface galvanic vestibular stimulation. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 26, 500–511 (2005).
Wood, S. J., Black, F. O., MacDougall, H. G. & Moore, S. T. Electrotactile feedback of sway position improves postural performance during galvanic vestibular stimulation. Annals of the New York Academy of Sciences 1164, 492–498, https://doi.org/10.1111/j.1749-6632.2009.03768.x (2009).
Wuehr, M., Decker, J. & Schniepp, R. Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol, https://doi.org/10.1007/s00415-017-8481-4 (2017).
Breen, P. P. et al. A new paradigm of electrical stimulation to enhance sensory neural function. Medical engineering & physics 36, 1088–1091, https://doi.org/10.1016/j.medengphy.2014.04.010 (2014).
Breen, P. P. et al. Peripheral tactile sensory perception of older adults improved using subsensory electrical noise stimulation. Medical engineering & physics 38, 822–825, https://doi.org/10.1016/j.medengphy.2016.05.015 (2016).
Iwasaki, S. et al. Effect of Noisy Galvanic Vestibular Stimulation on Ocular Vestibular-Evoked Myogenic Potentials to Bone-Conducted Vibration. Front Neurol 8, 26, https://doi.org/10.3389/fneur.2017.00026 (2017).
Curthoys, I. S., Vulovic, V. & Manzari, L. Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol Ital 32, 41–45 (2012).
Fitzpatrick, R. C. & Day, B. L. Probing the human vestibular system with galvanic stimulation. J Appl Physiol (1985) 96, 2301–2316, https://doi.org/10.1152/japplphysiol.00008.2004 (2004).
Gensberger, K. D. et al. Galvanic vestibular stimulation: Cellular substrates and response patterns of neurons in the vestibulo-ocular network. J Neurosci 36, 9097–9110, https://doi.org/10.1523/JNEUROSCI.4239-15.2016 (2016).
Curthoys, I. S. & Macdougall, H. G. What galvanic vestibular stimulation actually activates. Front Neurol 3, 117, https://doi.org/10.3389/fneur.2012.00117 (2012).
Iwasaki, S. et al. Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 82, 969–975, https://doi.org/10.1212/WNL.0000000000000215 (2014).
Mulavara, A. P. et al. Improving balance function using vestibular stochastic resonance: optimizing stimulus characteristics. Experimental brain research 210, 303–312 (2011).
Wuehr, M. et al. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86, 2196–2202, https://doi.org/10.1212/WNL.0000000000002748 (2016).
Martins, E. S. D. C. et al. Effects of vestibular rehabilitation in the elderly: a systematic review. Aging Clin Exp Res 28, 599–606, https://doi.org/10.1007/s40520-015-0479-0 (2016).
Wood, S. J. Human otolith-ocular reflexes during off-vertical axis rotation: effect of frequency on tilt-translation ambiguity and motion sickness. Neuroscience letters 323, 41–44 (2002).
Acknowledgements
The authors would like to thank Paul Breen, Alex Reisner, Ryan Hodgeman, Timothy Kita and Brittany Algerie for their assistance in data collection and analysis. We would also like to thank Dr. Jim Collins for his helpful input. This data was previously presented at the 19th International Symposium on the Autonomic Nervous System, in Kauai, Hawaii. This work was supported by NASA (grant NNJ04HI13G), the Science Foundation Ireland Walton Visiting Fellow Award.
Author information
Authors and Affiliations
Contributions
J.S. and S.W. conceived the study design, J.S., M.G. and B.D. conducted the experiment and analyzed the results. All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serrador, J.M., Deegan, B.M., Geraghty, M.C. et al. Enhancing vestibular function in the elderly with imperceptible electrical stimulation. Sci Rep 8, 336 (2018). https://doi.org/10.1038/s41598-017-18653-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18653-8
This article is cited by
-
Cellular shortening and calcium dynamics are improved by noisy stimulus in a model of cardiomyopathy
Scientific Reports (2023)
-
Effect of noisy galvanic vestibular stimulation on dynamic posture sway under visual deprivation in patients with bilateral vestibular hypofunction
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.