Abstract
The Eph/ephrin receptor ligand system is known to play a role in inflammation induced by infection, injury, and inflammatory diseases. The present study aimed to evaluate plasma EphA2 receptor levels in critically ill patients with sepsis. This study was a prospective cohort study evaluating samples and clinical data from the medical intensive care unit (MICU) of a 2000-bed university tertiary referral hospital in South Korea. Positive correlations of the plasma EphA2 receptor level with the acute physiology and chronic health evaluation (APACHE) II score and the sequential organ failure assessment (SOFA) score were observed. The area under the curve (AUC) for the plasma EphA2 receptor level on a receiver operating characteristic curve was 0.690 (95% confidence interval [CI], 0.608–0.764); the AUCs for the APACHE II score and SOFA scores were 0.659 (95% CI, 0.576–0.736) and 0.745 (95% CI, 0.666–0.814), respectively. A Cox proportional hazard model identified an association between an increased plasma EphA2 receptor level (>51.5 pg mL−1) and increased risk of 28-day mortality in the MICU (hazard ratio = 3.22, 95% CI, 1.709–6.049). An increased plasma EphA2 receptor level was associated with sepsis severity and 28-day mortality among sepsis patients.
Similar content being viewed by others
Introduction
The progression of infection-induced sepsis leads to organ dysfunction and is associated with a high rate of mortality1. Although the sepsis mortality rate has significantly decreased over the years, this condition remains difficult to treat2. Furthermore, the reported incidence of sepsis has increased because of several factors, including an increase in elderly populations with more comorbidities3,4,5,6. In addition, half of all sepsis patients are managed in intensive care units (ICUs), and more than 25% of cases end in death7,8.
Vascular endothelial dysfunction plays a key role in the organ failure and mortality associated with sepsis2. Sepsis alters the endothelial barrier function, thus enhancing the passage of water, soluble proteins, and cellular components from the blood to the tissues9. In addition to conventional treatments such as early antibiotics and conservative management, the use of several anti-inflammatory or immunomodulatory therapies has been studied to combat the pro-inflammatory stage of sepsis10. However, several attempts to target tumor necrosis factor (TNF)-α, interleukin (IL)-1 β, toll-like receptors, and endotoxin have not been effective in clinical trials10,11,12.
Eph receptors comprise the largest family of tyrosine kinase receptors and, with ephrin ligands, are involved in cell-to-cell communication. Interactions between Eph receptors and ephrin ligands affect the pathogeneses of many conditions including wound healing, ischemia reperfusion injury, nerve injury, endothelial injury, and epithelial injury13,14,15,16. Eph receptors and ephrin ligands also regulate important processes during embryonic neuronal development, angiogenesis, and oncogenesis13,16. Recently, many studies have focused on the complex roles of Eph and ephrin in malignancy17,18. According to several studies, the EphA2 receptor and ephrinA1 ligand also affect inflammation via vascular endothelial injury. In an in vivo study in rats, ephrin A1 induced histological endothelial disruption in the lung and blocking EphA2 signaling markedly reduced leakage of albumin13,16,19. Another study showed that EphA2 activation induced expression of vascular cell adhesion molecule-1 and E-selectin20. The EphA2 receptor may be associated with sepsis due to the important role of endothelial injury in sepsis21,22. However, clinical studies investigating the association between the EphA2 receptor and sepsis are currently lacking.
We have found that the plasma EphA2 receptor level is elevated in sepsis patients, and therefore, we investigated the clinical implications of the plasma EphA2 receptor level with regard to sepsis. The primary outcome of the study was the association between the plasma EphA2 receptor level and all-cause 28-day mortality among sepsis patients. The secondary outcomes were an evaluation of the correlations between plasma EphA2 receptor levels and severity of sepsis as determined by the acute physiology and chronic health evaluation (APACE) II score and the sequential organ failure assessment (SOFA) score23,24. Because EphA2 receptor blocking antibodies are already undergoing clinical trials for cancer25,26,27,28, we also investigated the plasma EphA2 receptor level as a potential marker for therapeutic monitoring in sepsis patients.
Results
Demographic characteristics of the overall study population
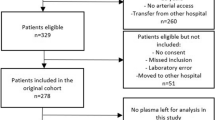
A total of 262 patients were admitted to our medical ICU (MICU) between March 2015 and August 2015. Of these, 145 patients presented with systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, or septic shock and agreed to participate in this study (Fig. 1). The baseline characteristics of the study population are represented in Table 1. The patient population mostly consisted of older individuals (median age, 70 years) with a higher proportion of men than women (65.5% vs. 35.5%). Patients with severe sepsis and septic shock accounted for 73.1% of the study population. The median score on the Charlson Comorbidity Index was 3 (interquartile range [IQR], 2–5), and only 6 patients did not present with comorbidities. The incidence of acute respiratory distress syndrome (ARDS) and positive blood culture were 18.6% and 25.5%, respectively. The most common site of infection was the lung (59.3%), followed by the gastrointestinal tract (10.3%). The median acute physiology and chronic health evaluation (APACHE) II score was 25 (IQR, 18–31), and the median sequential organ failure assessment (SOFA) score was 10 (IQR, 7–13). Overall, 43 patients (29.7%) died within 28 days of admission to the MICU.
Comparison of characteristics between 28-day survivors and 28-day non-survivors
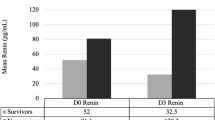
This study divided patients into two groups according to 28-day mortality: patients in the case group died within 28 days of admission to the MICU, whereas those in the control group survived longer than 28 days. The results of this inter-group comparison are shown in Table 2. The groups did not differ significantly with regard to sex, median age, and median body mass index (BMI). Regarding clinical parameters, the groups did not differ in terms of the rate of positive blood cultures and median scores on the Charlson Comorbidity Index. However, the non-survivor group had higher rates of ARDS (32.6% vs. 12.7% for survivors, P = 0.005) and a sepsis status above severe (86.0% vs. 67.6% for survivors, P = 0.022). The median APACHE II score (29 vs. 24, P = 0.002) and median SOFA score (13 vs. 8, P < 0.001) were also significantly higher in the non-survivor group relative to the survivor group. Regarding laboratory parameters, no differences were observed between survivors and non-survivors in the median levels for C-reactive protein (CRP) (73.5 mg mL−1 vs. 97 mg mL−1, P = 0.347) and procalcitonin (0.5 ng mL−1 vs. 1.8 ng mL−1, P = 0.181) on the day of admission. However, the median plasma EphA2 receptor level was significantly higher in the non-survivor group than in the survivor group (78.5 pg mL−1 vs. 33.3 pg mL−1, P < 0.001).
Association between the plasma EphA2 receptor level and severity or mortality
Positive correlations were observed between the plasma EphA2 receptor level and the APACHE II (r = 0.291, P < 0.001) and SOFA (r = 0.341, P < 0.001 Fig. 2) scores. Stratification of patients according to the IQRs for SOFA scores revealed an increasing trend in SOFA scores with increasing plasma EphA2 receptor levels (SOFA score <6 vs. SOFA score >13, P < 0.001; Fig. 3). The Area under the curve (AUC) for the plasma EphA2 receptor level on a receiver operating characteristic (ROC) curve was 0.690 (95% confidence interval [CI], 0.608–0.764); the AUCs for the APACHE II score and SOFA score were 0.659 (95% CI, 0.576–0.736) and 0.745 (95% CI, 0.666–0.814), respectively. In ROC curve comparisons, the AUC of the plasma EphA2 receptor level and SOFA score (0.690 vs. 0.745, P = 0.256) and APACHE II score (0.690 vs. 0.659, P = 0.605) did not differ significantly. However, the AUC of the SOFA score and APACHE II score differed significantly (0.745 vs. 0.659, P = 0.034; Fig. 4a).
(a) In ROC curve comparisons, the AUC of the plasma EphA2 receptor level and SOFA scores (0.690 vs. 0.745, P = 0.256) and APACHE II scores (0.690 vs. 0.659, P = 0.605) did not differ significantly. (b) Kaplan-Meier survival analysis showed that the 28-day mortality of patients with plasma EphA2 receptor level ≥51.5 pg mL−1 was higher than that of patients with plasma EphA2 receptor level <51.5 pg mL−1.
A cut-off value of >51.5 pg mL−1 for the plasma EphA2 receptor level was determined to predict 28-day mortality on the ROC (sensitivity, 62.8%; specificity, 71.6%). Patients were divided into two groups according to this plasma EphA2 receptor cut-off level of 51.5 pg mL−1 and subjected to a Cox proportional hazard model analysis involving several demographic and clinical characteristics and substituted plasma EphA2 receptor levels (Table 3). In the univariate analysis, an increased plasma EphA2 receptor level (>51.5 pg mL−1; hazard ratio, 3.41; 95% CI, 1.750–6.636) was associated with 28-day mortality. In the multivariate analysis, sex, age, BMI, positive blood culture and sepsis status higher than severe were not found to be associated with 28-day mortality; in contrast, ARDS (hazard ratio, 2.02; 95% CI, 1.051–3.389) and an increased plasma EphA2 receptor level (>51.5 pg mL−1; hazard ratio, 3.22; 95% CI, 1.709–6.049) were associated with increased 28-day mortality in the MICU. Figure 4b shows the survival rates of the groups classified according to plasma EphA2 receptor levels; these survival rates differed significantly (P < 0.001).
Discussion
In the current study, we investigated potential associations of the plasma EphA2 receptor level with sepsis severity and 28 day-mortality among sepsis patients in the MICU. The main finding of this study was that the plasma EphA2 receptor level correlates with sepsis severity scores and increased mortality among sepsis patients.
The APACHE II score and SOFA score are commonly used for initial severity assessments in sepsis patients23,29. The plasma EphA2 receptor level was comparable to these scores. The results of this analysis consistently indicated that an increased plasma EphA2 receptor level was related to the initial severity of sepsis. A higher SOFA score is associated with increased patient mortality24. In this study, plasma EphA2 receptor levels were evaluated in groups according to interquartile ranges of SOFA scores (Fig. 3). The result showed that a higher plasma EphA2 receptor level was associated with mortality in sepsis patients. The AUC of the ROC curve and results of the Cox hazard proportional model supported this association. Furthermore, we used a plasma EphA2 receptor cut-off value to discriminate mortality risk according to the Cox hazard proportional model. As a result, a plasma EphA2 receptor level >51.5 pg mL−1 was associated with a significant increase in mortality (hazard ratio, 3.22).
As mentioned previously, many studies have focused on the complex roles of the Eph receptor and ephrin ligand in malignancy30,31. Several studies have reported associations of EphA2 with multiple oncogenic signaling pathways such as MAP/ERK, phosphoinositide 3-kinase, E-cadherin, and integrin/FAK/paxillin30,32,33,34. In the field of oncology, the EphA2 receptor has been studied as a therapeutic target and is being investigated in an ongoing clinical trial in patients with advanced malignancies25,26,27,28. Tumor development, progression, and therapeutic responses may be affected by inflammation17. Cancer and infectious disease have many similarities. For example, inhibitors of programmed cell death ligand 1, which were developed for patients with cancer, have also been actively investigated as therapeutic agents for chronic infection and/or sepsis18. Accordingly, it is noteworthy that the Eph2 receptor and ephrin ligand also affect inflammation through vascular endothelial injury13,16,19. EphA2 and other inflammatory mediators, such as TNF-α and interferon (IFN)-γ, upregulate NF-κB, thus increasing intercellular adhesion molecule-1 expression and facilitating leukocyte migration and attachment19,35. Several emerging studies have shown a link between the EphA2 receptor and inflammation36,37,38,39,40. Animal models of lipopolysaccharide (LPS)-induced pneumonia have demonstrated an increase in the EphA2 receptor level after LPS exposure37. Another study reported the increased expression of EphA2 receptor with lung injury and found that EphA2 receptor blockade reduced lung injury and the passage of fluids and inflammatory cells41. EphA2 receptor signaling is associated with Src family kinases, mitogen activated protein kinase, p-21 activated kinase, post-synaptic density protein 95-dependent pathways, chemokine pathways, heterotrimeric G-protein pathways, and integrin-mediated pathways19,22. A recent study reported that the p-21 activated kinase was strongly associated with endothelial barrier in a sepsis murine model22. Given the results of this study and others, EphA2 receptor blockade may be a reasonable treatment target for reducing endothelial injury in sepsis patients. Unlike malignancy, however, sepsis does not involve a target lesion. Therefore, an appropriate blood marker with which to assess the therapeutic drug effect is needed if EphA2 receptor blocking is to be used as a therapeutic target. The plasma EphA2 receptor level may be useful to evaluate the effect of treatment in this setting.
The strengths of this study include the confirmed measurement of blood plasma EphA2 receptor levels and the identification of an association between human plasma EphA2 receptor levels and sepsis. These results provide clinical evidence for the use of EphA2 receptor as a therapeutic target in sepsis and provide a potential tool for monitoring responses to EphA2 receptor blocker treatment in sepsis patients.
However, our study has several limitations. First, this was a small study of plasma EphA2 receptor levels in sepsis patients at a single center without pediatric patients. However, this study is the first clinical study to use human blood samples to evaluate the relationship between plasma EphA2 receptor levels and sepsis. Previously, other studies investigated the relationship between the EphA2 receptor and inflammation. Most of these studies used in vitro or animal models36,37,39,42. However, no clinical proof of sepsis was provided. Second, the plasma EphA2 receptor level measurements have not been validated. The importance of proteases in regulating the Eph receptor and the ephrin ligand family has been shown43; it is thought that the proteases that activate the Eph receptor and the ephrin ligand family also degrade the Eph A2 receptor into the cleaved form, which in turn increases EphA2 receptors in the plasma. However, it is unclear whether the measured EphA2 receptor reflects the soluble or membrane-bound form due to limitation of the kit used in this study. Furthermore, in vitro and in vivo evidence that clearly supports an association between plasma EphA2 receptor signaling and sepsis is rare. Third, we did not analyze serial EphA2 receptor levels because we were unable to collect serial blood samples from the patients. Therefore, additional studies investigating the measurement of plasma EphA2 receptor levels in sepsis patients and evidence of an association between EphA2 receptor signaling and sepsis are needed.
Conclusion
An increased plasma EphA2 receptor level was associated with sepsis severity and 28-day mortality among sepsis patients. The plasma EphA2 receptor may be considered a potential therapeutic target in sepsis patients. The EphA2 receptor level may be used for treatment monitoring in sepsis patients treated with an EphA2 receptor blocker.
Methods
Study design, patients, and clinical setting
This was a prospective cohort study of patients and collected samples from the MICU of Severance Hospital, a 2000-bed (30-bed MICU) university tertiary referral hospital in Seoul, South Korea. The study considered 262 consecutive patients aged >19 years who were admitted to the MICU between March 2015 and August 2015. The study ultimately enrolled a total of 145 patients who presented with SIRS, sepsis, severe sepsis, or septic shock as defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference5,44. All patients presenting with SIRS, sepsis, severe sepsis, or septic shock were treated according to the guidelines of the Surviving Sepsis Campaign5.
Clinical data and blood sample collection
Data of all patients admitted to the MICU were collected from the hospital electronic medical records and their blood samples. The severity of each patient’s condition was classified according to two different scoring systems: The SOFA and APACHE II scores were calculated23,29. In addition, the Charlson Comorbidity Index was used to evaluate patients’ comorbidities45. Clinical parameters such as the development of ARDS, blood culture positivity, 28-day mortality, and other demographic characteristics were evaluated.
Venous blood samples were collected within 24 hours after admission to the MICU though central lines into tubes containing ethylenediaminetetraacetic acid. Simultaneously, we determined the CRP, procalcitonin, creatinine, and albumin levels, blood culture results, white blood cell and platelet counts, and APACHE II and SOFA scores. Plasma was prepared by centrifugation for 15 minutes at 800 × g and 4 °C. Supernatants from centrifuged blood were immediately aliquoted and stored at −80 °C until the analysis was performed. Plasma levels of the EphA2 receptor and other cytokines (IL-1β, IL-10, IL-18, IL-6, TNF-α, and interferon gamma induced protein-10) were measured using the Human Magnetic Luminex® Screening Assay Kit (R&D Systems, Inc., Minneapolis, MN, USA). The kit detects all forms of the EphA2 receptor including the soluble, membrane-bound, phosphorylated and non-phosphorylated forms All samples and standards were assayed in duplicate using the Luminex 200TM System (Merck Millipore, Darmstadt, Germany).
Ethical approval
The study protocol was submitted as an ICU cohort and approved by the Institutional Review Board (IRB) of Severance Hospital (IRB number: 4-2013-0585). All study procedures were performed in accordance with the relevant guidelines and regulations. In addition, this study was performed in compliance with the principles set forth in the Declaration of Helsinki.
Statistical analysis
Data are described as medians with IQRs. The chi-squared and Fisher’s exact tests or the Mann–Whitney U test was used to assess differences between the two groups. The Kruskal–Wallis test was used to compare three or more groups of a qualitative parameter. Pearson correlation analyses were performed to estimate associations between the biomarkers and the APACHE II and SOFA scores. AUC analyses of ROC curves were performed to compare the plasma EphA2 receptor level, APACHE II score, and SOFA score. Subsequently, the 28-day survivor and non-survivor groups were analyzed using a Cox proportional hazard model with a plasma EphA2 receptor level cut-off value to predict 28-day mortality according to the ROC and several variables. The Kaplan–Meier method was used to report survival curves that were analyzed using the log-rank test. In all cases, a p-value of <0.05 was considered statistically significant. SPSS version 20 (IBM, Armonk, NY, USA) was used for statistical analyses; AUC analyses of ROC curves were performed using MedCalc software (version 16.4.3; MedCalc, Oostende, Belgium).
References
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N Engl J Med 369, 840–851 (2013).
Iwashyna, T. J., Cooke, C. R., Wunsch, H. & Kahn, J. M. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 60, 1070–1077 (2012).
Gaieski, D. F., Edwards, J. M., Kallan, M. J. & Carr, B. G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41, 1167–1174 (2013).
Dellinger, R. P. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41, 580–637 (2013).
Rhee, C., Gohil, S. & Klompas, M. Regulatory mandates for sepsis care–reasons for caution. N Engl J Med 370, 1673–1676 (2014).
Rangel-Frausto, M. S. et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273, 117–123 (1995).
Heron, M. Deaths: Leading Causes for 2011. Natl Vital Stat Rep 64, 1–96 (2015).
Opal, S. M. & van der Poll, T. Endothelial barrier dysfunction in septic shock. J Intern Med 277, 277–293 (2015).
Hutchins, N. A., Unsinger, J., Hotchkiss, R. S. & Ayala, A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med 20, 224–233 (2014).
Abraham, E. et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA 277, 1531–1538 (1997).
Opal, S. M. et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309, 1154–1162 (2013).
Pasquale, E. B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008).
Egea, J. & Klein, R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol 17, 230–238 (2007).
Pasquale, E. B. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6, 462–475 (2005).
Kullander, K. & Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol 3, 475–486 (2002).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Hotchkiss, R. S. & Moldawer, L. L. Parallels between Cancer and Infectious Disease. N Engl J Med 371, 380–383 (2014).
Coulthard, M. G. et al. Eph/Ephrin signaling in injury and inflammation. Am J Pathol 181, 1493–1503 (2012).
Funk, S. D. et al. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb Vasc Biol 32, 686–695 (2012).
Cohen, J. et al. Sepsis: a roadmap for future research. Lancet Infect Dis 15, 581–614 (2015).
Li, Y. et al. Sepsis-induced elevation in plasma serotonin facilitates endothelial hyperpermeability. Sci Rep 6, 22747 (2016).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: a severity of disease classification system. Crit Care Med 13, 818–829 (1985).
Vincent, J. L. et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26, 1793–1800 (1998).
Chakar, S., Scott, A. & Gan, H. Safety and Bio-Imaging Trial of DS 8895a in Patients With Advanced EphA2 Positive Cancers (LUD2014-002). Available at: https://clinicaltrials.gov. Accessed July (2017).
Tao, H., Chen, J. & Niu, L. CAR-T Cell Immunotherapy for EphA2 Positive Malignant Glioma Patients. Available at: https://clinicaltrials.gov. Accessed July (2017).
Colrman, C. EphA2 Gene Targeting Using Neutral Liposomal Small Interfering RNA Delivery. Available at: https://clinicaltrials.gov. AccessedJuly (2017).
Askoxylakis, V. A Study Evaluating MM-310 in Patients With Solid Tumors. Available at: https://clinicaltrials.gov. AccessedJuly (2017).
Minne, L., Abu-Hanna, A. & de Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 12, R161 (2008).
Beauchamp, A. & Debinski, W. Ephs and ephrins in cancer: ephrin-A1 signalling. Semin Cell Dev Biol 23, 109–115 (2012).
Tandon, M., Vemula, S. V. & Mittal, S. K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin Ther Targets 15, 31–51 (2011).
Wykosky, J. & Debinski, W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 6, 1795–1806 (2008).
Pratt, R. L. & Kinch, M. S. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene 21, 7690–7699 (2002).
Miao, H. et al. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol 2, 62–69 (2000).
Chan, B. & Sukhatme, V. P. Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res 123, 745–752 (2009).
Ivanov, A. I. & Romanovsky, A. A. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life 58, 389–394 (2006).
Ivanov, A. I., Steiner, A. A., Scheck, A. C. & Romanovsky, A. A. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics 21, 152–160 (2005).
Cheng, N. & Chen, J. Tumor necrosis factor-alpha induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-kappa B-independent mechanism. J Biol Chem 276, 13771–13777 (2001).
Carpenter, T. C., Schroeder, W., Stenmark, K. R. & Schmidt, E. P. Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am J Respir Cell Mol Biol 46, 40–47 (2012).
Schruefer, R. et al. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique and by measurement of neovascularization in wounded skin of CD18-deficient mice. J Vasc Res 43, 1–11 (2006).
Cercone, M. A., Schroeder, W., Schomberg, S. & Carpenter, T. C. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 297, L856–863 (2009).
Hong, J. Y. et al. Inhibition of EphA2/EphrinA1 signal attenuates lipopolysaccharide-induced lung injury. Clin Sci (Lond) 130, 1993–2003 (2016).
Atapattu, L., Lackmann, M. & Janes, P. W. The role of proteases in regulating Eph/ephrin signaling. Cell Adh Migr 8, 294–307 (2014).
Levy, M. M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29, 530–538 (2003).
Quan, H. et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173, 676–682 (2011).
Acknowledgements
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (NRF-2014R1A1A1038278). This study was also supported by a grant from the Korea Health Technology R&D Project of the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1324).
Author information
Authors and Affiliations
Contributions
Su Hwan Lee contributed to the study design, analysis and interpretation of data, and writing of the manuscript. Ju Hye Shin, Joo Han Song, Ah Young Leem, Moo Suk Park, Young Sam Kim and Joon Chang contributed to the study design, analysis and interpretation of data, and review of the manuscript. Kyung Soo Chung contributed to the study design, analysis and interpretation of data, and writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.H., Shin, J.H., Song, J.H. et al. Clinical implications of the plasma EphA2 receptor level in critically ill patients with septic shock. Sci Rep 7, 17612 (2017). https://doi.org/10.1038/s41598-017-17909-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17909-7
This article is cited by
-
NADPH oxidase 4 signaling in a ventilator-induced lung injury mouse model
Respiratory Research (2022)
-
Biological correlates before esophageal cancer screening and after diagnosis
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.